Population-Specific Plant-To-Plant Signaling in Wild Lima Bean
Abstract
1. Introduction
2. Results
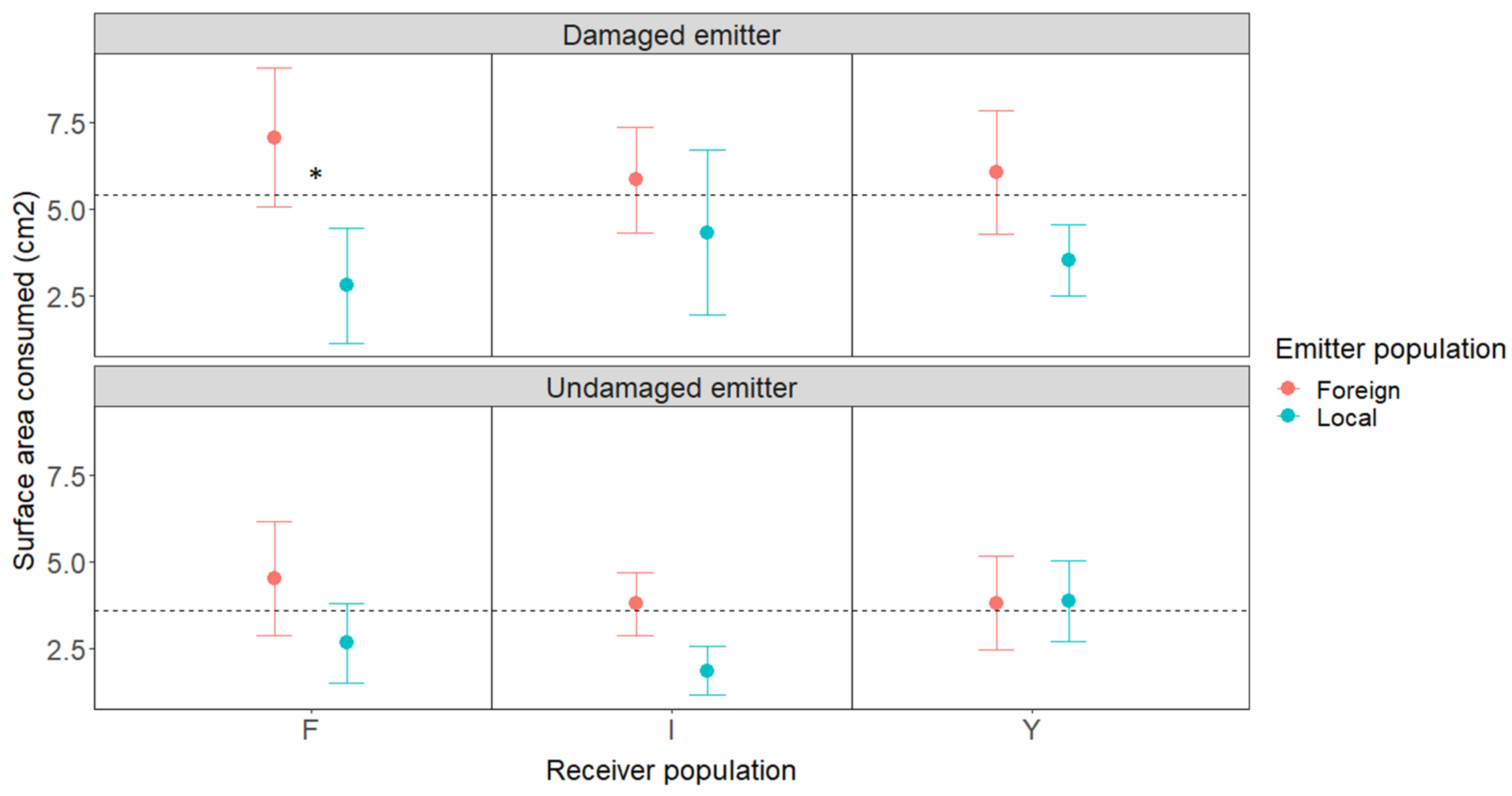
2.1. Volatile Exposure Experiment and Herbivore Choice Tests
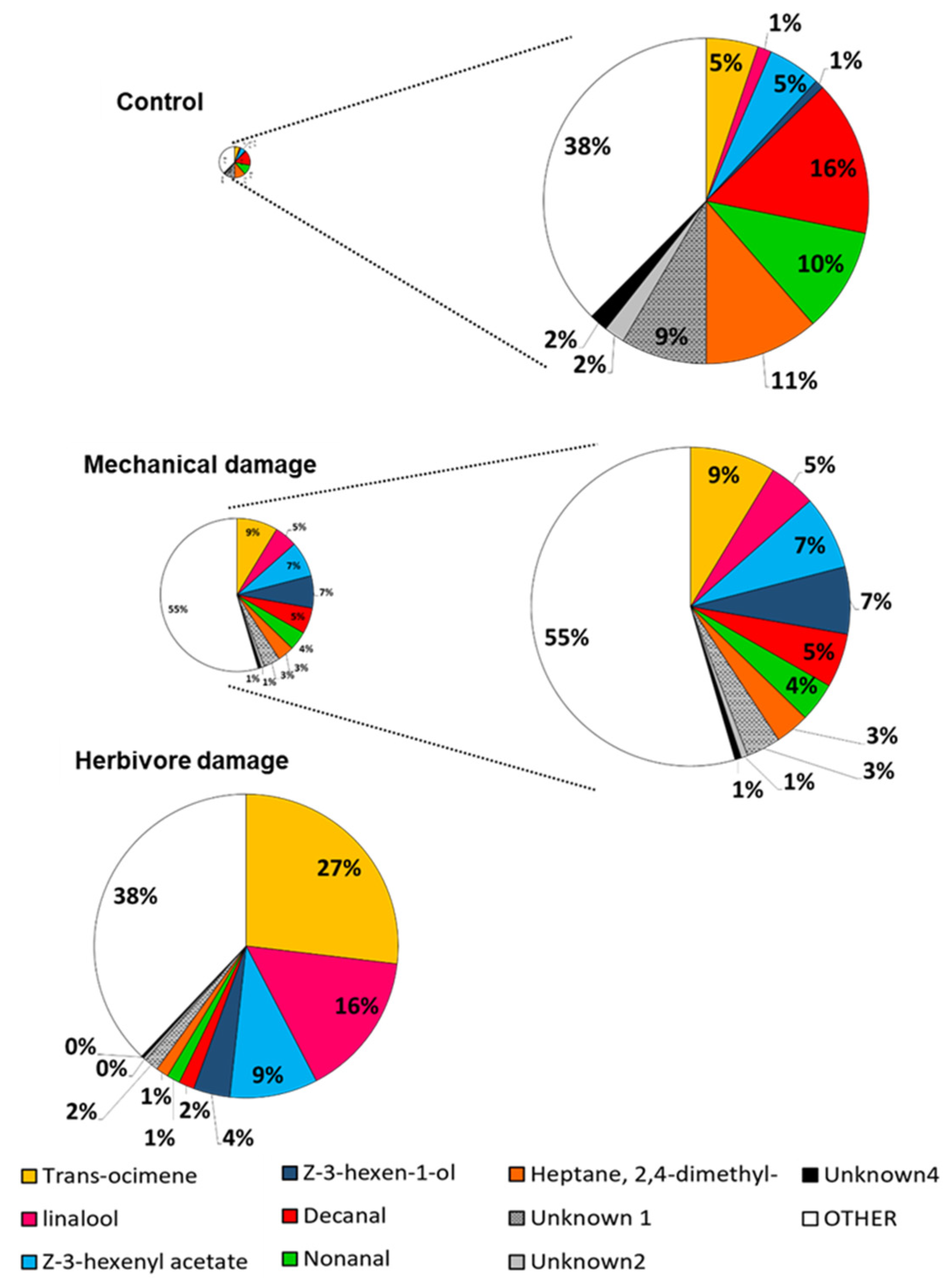
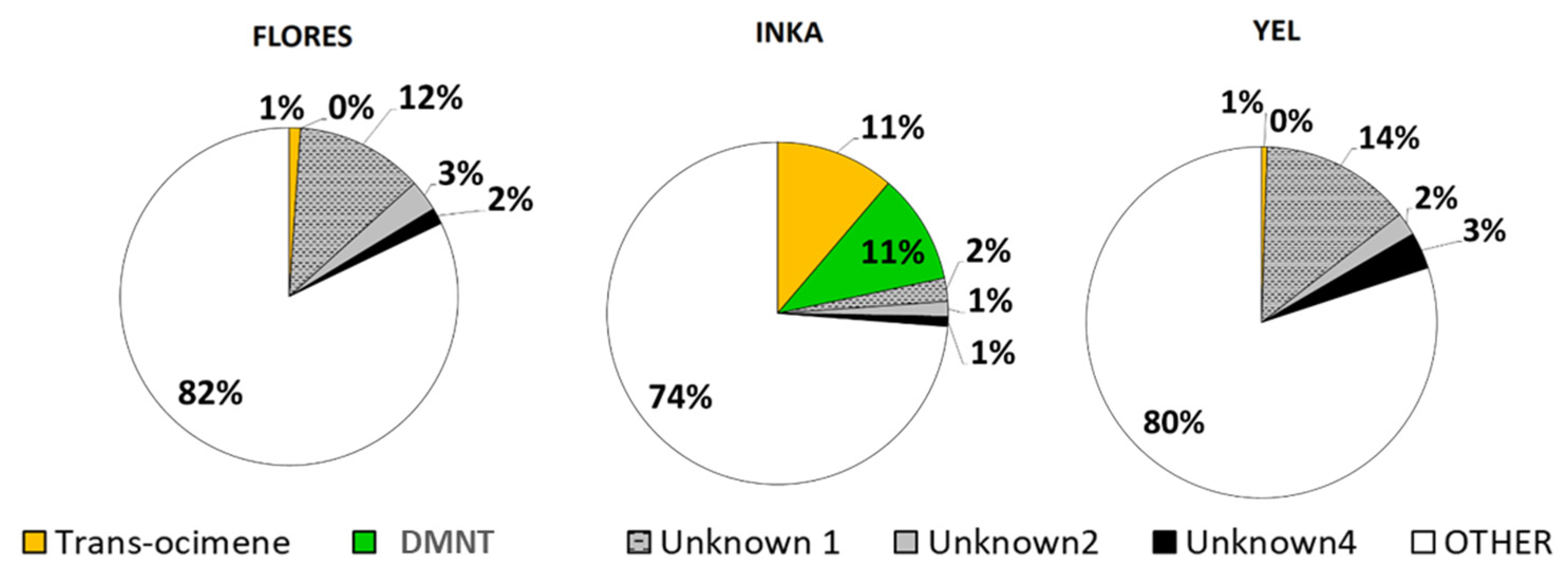
2.2. Volatile Collection and GS-MS Analysis
2.3. Effects of the Geographic Relationship and Chemotype on Damage
3. Discussion
4. Materials and Methods
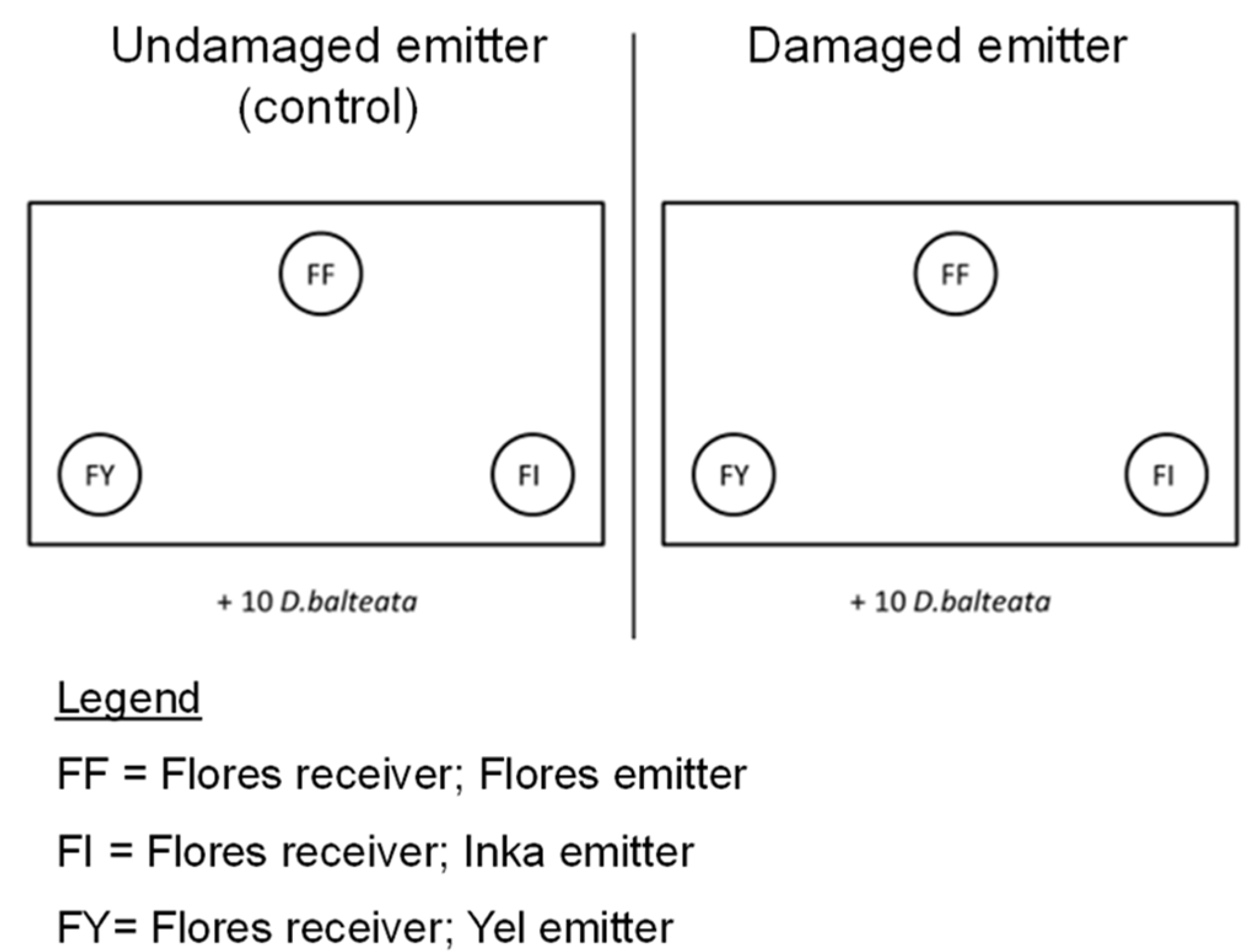
4.1. Plant Material
4.2. Herbivores
4.3. Volatile Exposure Experiment and Herbivore Choice Tests
4.4. Volatile Collection and GS-MS Analysis
4.5. Statistical Analysis
4.6. Effects of the Geographic Relationship and Chemotype on the Damage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Callaway, R.M. The Detection of Neighbors by Plants. Trends Ecol. Evol. 2002, 17, 104–105. [Google Scholar] [CrossRef]
- Dudley, S.A.; File, A.L. Kin Recognition in an Annual Plant. Biol. Lett. 2007, 3, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Karban, R. Plant Sensing and Communication; The University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. CRC Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological Role of Volatiles Produced by Plants in Response to Damage by Herbivorous Insects. Annu. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef]
- Rowen, E.; Kaplan, I. Eco-Evolutionary Factors Drive Induced Plant Volatiles: A Meta-Analysis. New Phytol. 2016, 210, 284–294. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The Evolutionary Context for Herbivore-Induced Plant Volatiles: Beyond the “Cry for Help”. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Heil, M.; Bueno, J.C.S. Within-Plant Signaling by Volatiles Leads to Induction and Priming of an Indirect Plant Defense in Nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-Induced Plant Volatiles: Targets, Perception and Unanswered Questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Heil, M.; Karban, R. Explaining Evolution of Plant Communication by Airborne Signals. Trends Ecol. Evol. 2010, 25, 137–144. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant Defense Priming against Herbivores: Getting Ready for a Different Battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef]
- Karban, R. The Ecology and Evolution of Induced Responses to Herbivory and How Plants Perceive Risk. Ecol. Entomol. 2020, 45, 1–9. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant Volatiles as Cues and Signals in Plant Communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Shiojiri, K. Self-Recognition Affects Plant Communication and Defense. Ecol. Lett. 2009, 12, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Pearse, I.S.; Hughes, K.; Shiojiri, K.; Ishizaki, S.; Karban, R. Interplant Volatile Signaling in Willows: Revisiting the Original Talking Trees. Oecologia 2013, 172, 869–875. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Ishizaki, S.; Wetzel, W.C.; Evans, R.Y. Kin Recognition Affects Plant Communication and Defence. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123062. [Google Scholar] [CrossRef]
- Crepy, M.A.; Casal, J.J. Photoreceptor-Mediated Kin Recognition in Plants. New Phytol. 2015, 205, 329–338. [Google Scholar] [CrossRef]
- Karban, R.; Wetzel, W.C.; Shiojiri, K.; Pezzola, E.; Blande, J.D. Geographic Dialects in Volatile Communication between Sagebrush Individuals. Ecology 2016, 97, 2917–2924. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Plant Root Exudates Mediate Neighbour Recognition and Trigger Complex Behavioural Changes. New Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole Is an Essential Herbivore-Induced Volatile Priming Signal in Maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef]
- Moreira, X.; Nell, C.S.; Meza-Lopez, M.M.; Rasmann, S.; Mooney, K.A. Specificity of Plant–Plant Communication for Baccharis Salicifolia Sexes but not Genotypes. Ecology 2018, 99, 2731–2739. [Google Scholar] [CrossRef]
- Moreira, X.; Petry, W.K.; Hernández-Cumplido, J.; Morelon, S.; Benrey, B. Plant Defence Responses to Volatile Alert Signals Are Population-Specific. Oikos 2016, 125, 950–956. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Lion, U.; Heil, M. Trade-Offs between Direct and Indirect Defences of Lima Bean (Phaseolus lunatus). J. Ecol. 2008, 96, 971–980. [Google Scholar] [CrossRef]
- Podos, J.; Warren, P.S. The Evolution of Geographic Variation in Birdsong. Adv. Study Behav. 2007, 37, 403–458. [Google Scholar] [CrossRef]
- Karban, R.; Wetzel, W.C.; Shiojiri, K.; Ishizaki, S.; Ramirez, S.R.; Blande, J.D. Deciphering the Language of Plant Communication: Volatile Chemotypes of Sagebrush. New Phytol. 2014, 204, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rodriguez-Ramos, J.C.; Erbilgin, N. Spatial Characteristics of Volatile Communication in Lodgepole Pine Trees: Evidence of Kin Recognition and Intra-Species Support. Sci. Total Environ. 2019, 692, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Grof-Tisza, P.; Karban, R.; Pan, V.S.; Blande, J.D. Assessing Plant-to-Plant Communication and Induced Resistance in Sagebrush Using the Sagebrush Specialist Trirhabda Pilosa. Arthropod Plant Interact. 2020, 14, 327–332. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Lengwiler, U.B.; Bernasconi, M.L.; Wechsler, D. Timing of Induced Volatile Emissions in Maize Seedlings. Planta 1998, 207, 146–152. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H. Systemic Release of Chemical Signals by Herbivore-Injured Corn. Proc. Natl. Acad. Sci. USA 1992, 89, 8399–8402. [Google Scholar] [CrossRef]
- Himanen, S.J.; Blande, J.D.; Klemola, T.; Pulkkinen, J.; Heijari, J.; Holopainen, J.K. Birch (Betula spp.) Leaves Adsorb and Re-Release Volatiles Specific to Neighbouring Plants—A Mechanism for Associational Herbivore Resistance? New Phytol. 2010, 186, 722–732. [Google Scholar] [CrossRef]
- Mofikoya, A.O.; Yli-Pirilä, P.; Kivimäenpää, M.; Blande, J.D.; Virtanen, A.; Holopainen, J.K. Deposition of α-Pinene Oxidation Products on Plant Surfaces Affects Plant VOC Emission and Herbivore Feeding and Oviposition. Environ. Pollut. 2020, 263, 114437. [Google Scholar] [CrossRef]
- Grof-Tisza, P.; Kruizenga, N.; Tervahauta, A.; Blande, J. Volatile-Mediated Induced and Passively Acquired Resistance in Sagebrush (Artemisia tridentata). J. Chem. Ecol. 2022. [Google Scholar] [CrossRef]
- Karban, R.; Yang, L.H.; Edwards, K.F. Volatile Communication between Plants that Affects Herbivory: A Meta-Analysis. Ecol. Lett. 2014, 17, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Maron, J.; Felton, G.W.; Ervin, G.; Eichenseer, H. Herbivore Damage to Sagebrush Induces Resistance in Wild Tobacco: Evidence for Eavesdropping between Plants. OIKOS 2003, 100, 325–332. [Google Scholar] [CrossRef]
- Arimura, G.I.; Ozawa, R.; Shimoda, T.; Nishloka, T.; Boland, W.; Takabayashi, J. Herbivory-Induced Volatiles Elicit Defence Genes in Lima Bean Leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New Roles for Cis-Jasmone as an Insect Semiochemical and in Plant Defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef]
- Engelberth, J.; Contreras, C.F.; Dalvi, C.; Li, T.; Engelberth, M. Early Transcriptome Analyses of Z-3-Hexenol-Treated Zea Mays Revealed Distinct Transcriptional Networks and Anti-Herbivore Defense Potential of Green Leaf Volatiles. PLoS ONE 2013, 8, e77465. [Google Scholar] [CrossRef]
- Bricchi, I.; Leitner, M.; Foti, M.; Mithöfer, A.; Boland, W.; Maffei, M.E. Robotic Mechanical Wounding (MecWorm) versus Herbivore-Induced Responses: Early Signaling and Volatile Emission in Lima Bean (Phaseolus lunatus L.). Planta 2010, 232, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Plant Methyl Salicylate Induces Defense Responses in the Rhizobacterium Bacillus Subtilis. Environ. Microbiol. 2015, 17, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Park, K.C. Methyl Salicylate, a Soybean Aphid-Induced Plant Volatile Attractive to the Predator Coccinella Septempunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of Jasmonate and Salicylate Signal Crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Keefover-Ring, K.; Thompson, J.D.; Linhart, Y.B. Beyond Six Scents: Defining a Seventh Thymus Vulgaris Chemotype New to Southern France by Ethanol Extraction. Flavour Fragr. J. 2009, 24, 117–122. [Google Scholar] [CrossRef]
- Ishizaki, S.; Shiojiri, K.; Karban, R.; Ohara, M. Effect of Genetic Relatedness on Volatile Communication of Sagebrush (Artemisia tridentata). J. Plant Interact. 2011, 6, 193. [Google Scholar] [CrossRef]
- Grof-Tisza, P.; Karban, R.; Rasheed, M.U.; Saunier, A.; Blande, J.D. Risk of Herbivory Negatively Correlates with the Diversity of Volatile Emissions Involved in Plant Communication. Proc. R. Soc. B 2021, 288, 20211790. [Google Scholar] [CrossRef]
- Clavijo Mccormick, A.; Gershenzon, J.; Unsicker, S.B. Little Peaks with Big Effects: Establishing the Role of Minor Plant Volatiles in Plant-Insect Interactions. Plant Cell Environ. 2014, 37, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Lutmerding, A.; Dudareva, N.; Smith, B.H. Intensity and the Ratios of Compounds in the Scent of Snapdragon Flowers Affect Scent Discrimination by Honeybees (Apis mellifera). J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2005, 191, 105–114. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.; Brunner, V.; von Mérey, G.; Turlings, T.C.J. Strong Attraction of the Parasitoid Cotesia Marginiventris towards Minor Volatile Compounds of Maize. J. Chem. Ecol. 2009, 35, 999–1008. [Google Scholar] [CrossRef][Green Version]
- Arora, M.; Zambrzycki, S.C.; Levy, J.M.; Esper, A.; Frediani, J.K.; Quave, C.L.; Fernández, F.M.; Kamaleswaran, R. Machine Learning Approaches to Identify Discriminative Signatures of Volatile Organic Compounds (VOCs) from Bacteria and Fungi Using SPME-DART-MS. Metabolites 2022, 12, 232. [Google Scholar] [CrossRef]
- Defossez, E.; Pitteloud, C.; Descombes, P.; Glauser, G.; Allard, P.M.; Walker, T.W.N.; Fernandez-Conradi, P.; Wolfender, J.L.; Pellissier, L.; Rasmann, S. Spatial and Evolutionary Predictability of Phytochemical Diversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2013344118. [Google Scholar] [CrossRef]
- Arce, C.M.; Besomi, G.; Glauser, G.; Turlings, T.C.J. Caterpillar-Induced Volatile Emissions in Cotton: The Relative Importance of Damage and Insect-Derived Factors. Front. Plant Sci. 2021, 12, 1503. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Huntzinger, M.; McCall, A.C. Damage-Induced Resistance in Sagebrush: Volatiles Are Key to Intra- and Interplant Communication. Ecology 2006, 87, 922–930. [Google Scholar] [CrossRef]
- Pitre, H.N.; Kantack, E.J. Biology of the Banded Cucumber Beetle, Diabrotica Balteata, in Louisiana. J. Econ. Entomol. 1962, 55, 904–906. [Google Scholar] [CrossRef]
- Marchioro, C.A.; Krechemer, F.S. Potential Global Distribution of Diabrotica Species and the Risks for Agricultural Production. Pest Manag. Sci. 2018, 74, 2100–2109. [Google Scholar] [CrossRef]
- Jaccard, C.; Marguier, N.T.; Arce, C.C.M.; Bruno, P.; Glauser, G.; Turlings, T.C.J.; Benrey, B. The Effect of Squash Domestication on a Belowground Tritrophic Interaction. Plant Environ. Interact. 2022, 3, 28–39. [Google Scholar] [CrossRef]
- Heil, M.; Adame-Álvarez, R.M. Short Signalling Distances Make Plant Communication a Soliloquy. Biol. Lett. 2010, 6, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Kost, C.; Heil, M. Herbivore-Induced Plant Volatiles Induce an Indirect Defence in Neighbouring Plants. J. Ecol. 2006, 94, 619–628. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R Package Version 0.3. 2020. Available online: http://florianhartig.github.io/DHARMa/ (accessed on 30 June 2022).
| Factor | X2 | DF | p |
|---|---|---|---|
| Receiver pop. | 0.248 | 2 | 0.883 |
| Emitter (Loc. vs. For.) | 0.578 | 2 | 0.016 |
| Treatment (Dam. Vs. Und.) | 4.890 | 1 | 0.027 |
| Volatile Compound | Undamaged | Mechanical Damage | Herbivore Damage | F Test | p Value |
|---|---|---|---|---|---|
| Trans-β-ocimene | 1.61 ± 0.78 | 13.57 ± 4.59 | 88.82 ± 9.93 | 53.51 | <0.001 |
| DMNT 1 | 1.36 ± 0.83 | 21.23 ± 7.3 | 54.4 ± 11.3 | 26.88 | <0.001 |
| Linalool | 0.43 ± 0.32 | 7.52 ± 1.84 | 51.09 ± 8.98 | 22 | <0.001 |
| Z-3-hexenyl acetate | 1.65 ± 0.97 | 11.76 ± 2.54 | 30.96 ± 4.75 | 27.46 | <0.001 |
| Indole | 1.76 ± 0.58 | 13.24 ± 2.72 | 15.31 ± 2.66 | 13.56 | <0.001 |
| Z-3-hexen-1-ol | 0.27 ± 0.15 | 10.66 ± 2.32 | 12.83 ± 1.96 | 19.27 | <0.001 |
| TMTT 2 | 0.33 ± 0.19 | 3.44 ± 0.9 | 9.61 ± 1.82 | 6.22 | <0.001 |
| Methyl salicylate | 0.72 ± 0.22 | 6.98 ± 2.16 | 4.11 ± 1.27 | 9.58 | <0.001 |
| Trans-caryophyllene | 0.11 ± 0.05 | 1.7 ± 0.64 | 3.92 ± 0.85 | 9.13 | <0.001 |
| Cis-jasmone | 0.21 ± 0.11 | 1.85 ± 0.59 | 3.8 ± 0.87 | 8.29 | <0.001 |
| Cis-3-hexenyl isovalerate | 0.28 ± 0.13 | 1.4 ± 0.51 | 3.24 ± 0.51 | 20.48 | <0.001 |
| Trans-nerolidol | 0.03 ± 0.03 | 1.29 ± 0.5 | 2.71 ± 0.33 | 16.02 | <0.001 |
| Cis-β-ocimene | 0.33 ± 0.19 | 1.36 ± 0.43 | 2.66 ± 0.34 | 12.92 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grof-Tisza, P.; Morelon, S.; Desurmont, G.A.; Benrey, B. Population-Specific Plant-To-Plant Signaling in Wild Lima Bean. Plants 2022, 11, 2320. https://doi.org/10.3390/plants11182320
Grof-Tisza P, Morelon S, Desurmont GA, Benrey B. Population-Specific Plant-To-Plant Signaling in Wild Lima Bean. Plants. 2022; 11(18):2320. https://doi.org/10.3390/plants11182320
Chicago/Turabian StyleGrof-Tisza, Patrick, Stéphanie Morelon, Gaylord A. Desurmont, and Betty Benrey. 2022. "Population-Specific Plant-To-Plant Signaling in Wild Lima Bean" Plants 11, no. 18: 2320. https://doi.org/10.3390/plants11182320
APA StyleGrof-Tisza, P., Morelon, S., Desurmont, G. A., & Benrey, B. (2022). Population-Specific Plant-To-Plant Signaling in Wild Lima Bean. Plants, 11(18), 2320. https://doi.org/10.3390/plants11182320







