Effects of Exogenous Salicylic Acid Application to Aboveground Part on the Defense Responses in Bt (Bacillus thuringiensis) and Non-Bt Corn (Zea mays L.) Seedlings
Abstract
:1. Introduction
2. Results
2.1. Direct and Systemic Effect of Exogenous Salicylic Acid Application to the Aboveground Part on the Content of Defense Chemicals in the Leaves and Roots of Bt and Non-Bt Corn Seedlings
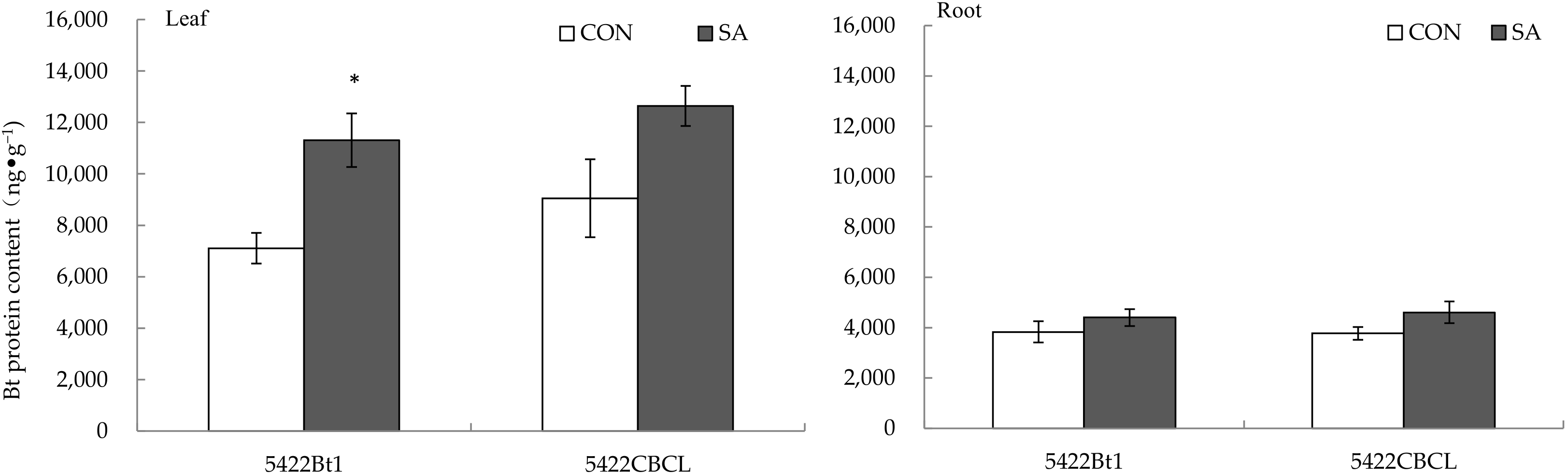
2.1.1. Bt Protein Content
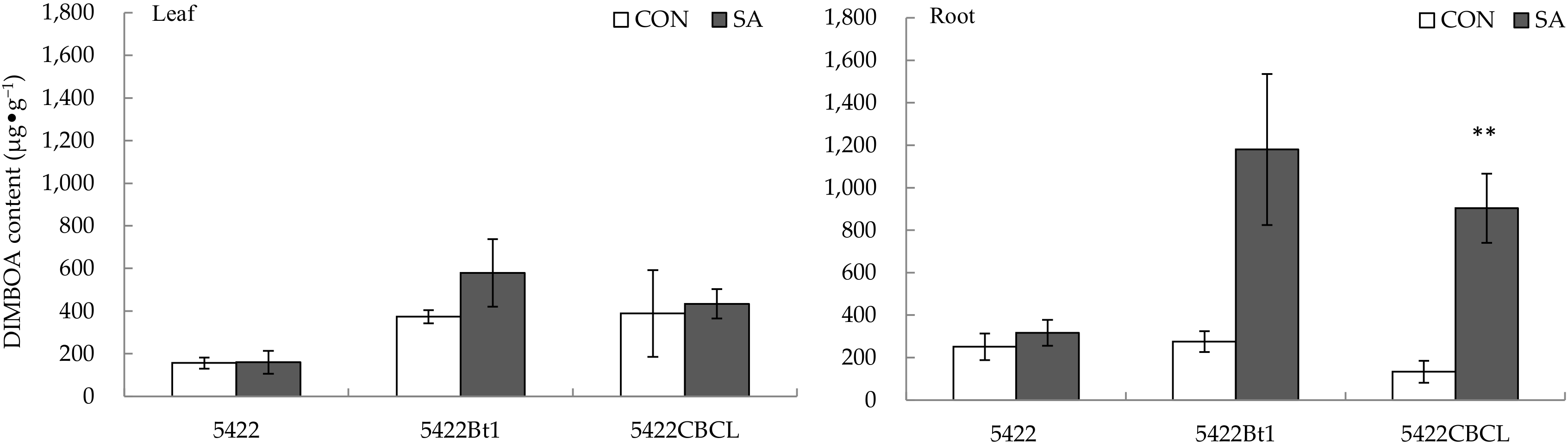
2.1.2. DIMBOA Content
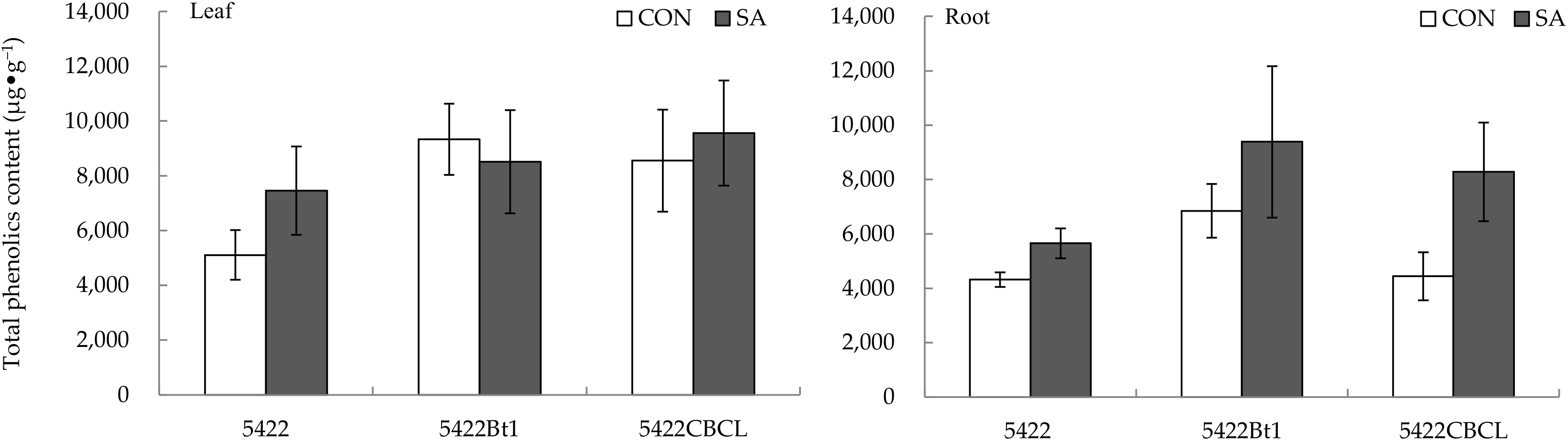
2.1.3. Total Phenolic Content
2.2. Direct and Systemic Effect of Exogenous Salicylic Acid Application to the Aboveground Part on the Activity of Defense Enzymes in the Leaves and Roots of Bt and Non-Bt Corn Seedlings
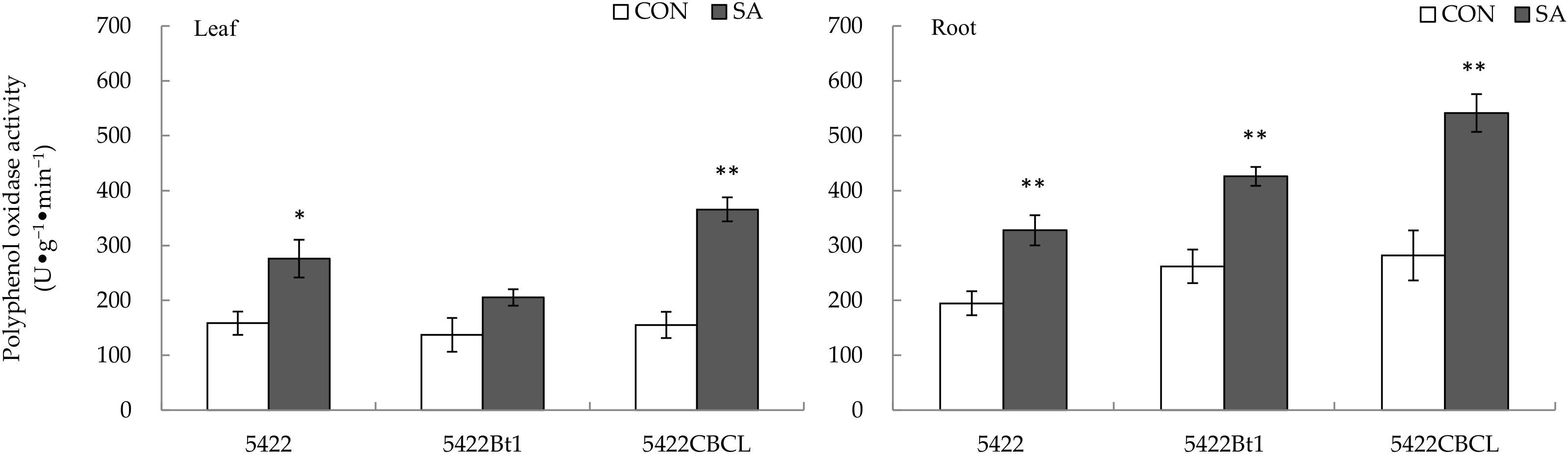
2.2.1. Polyphenol Oxidase Activity
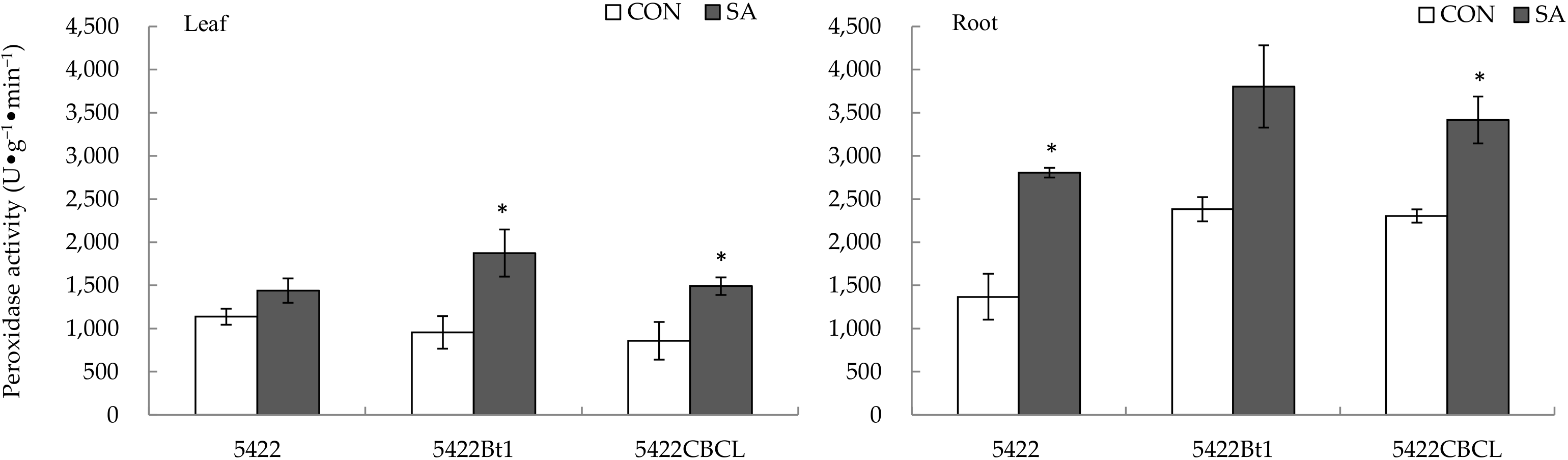
2.2.2. Peroxidase Activity
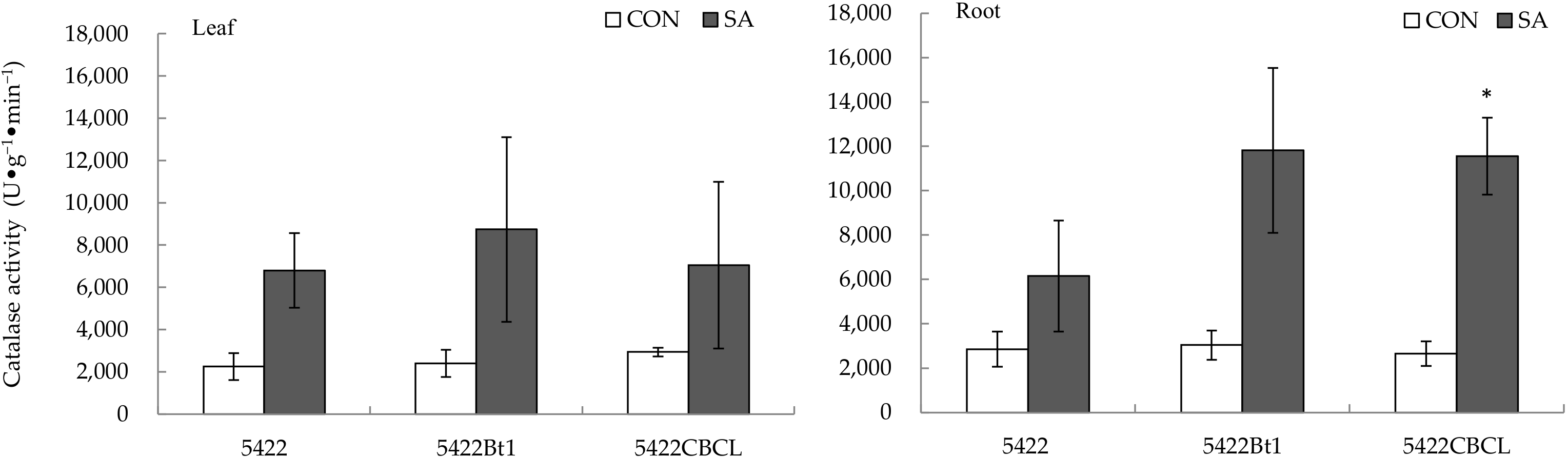
2.2.3. Catalase Activity
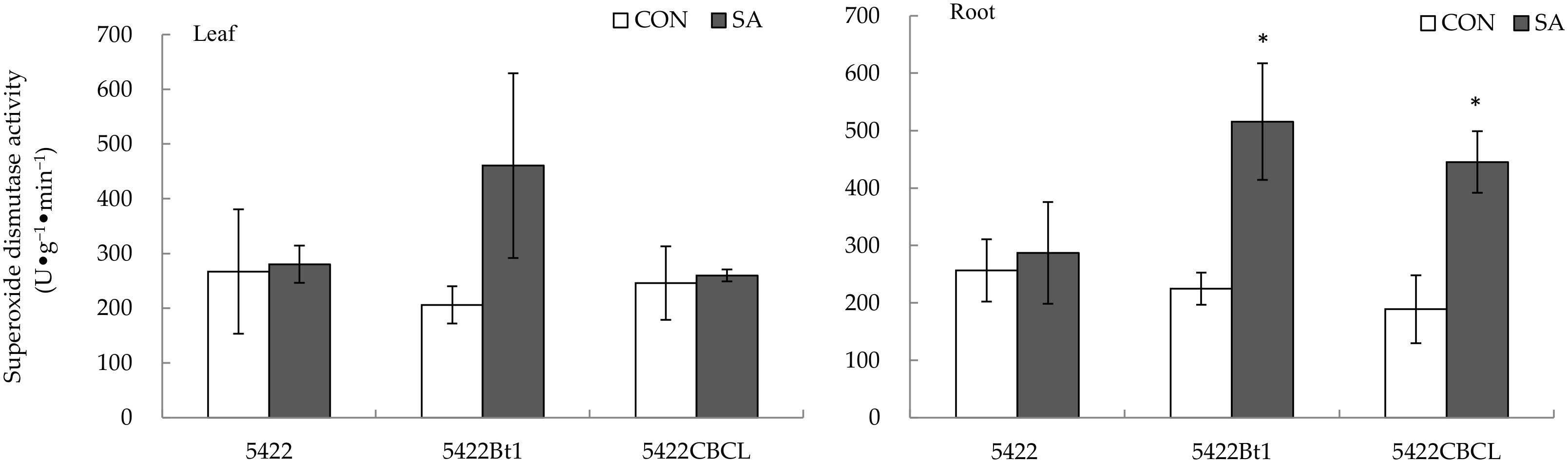
2.2.4. Superoxide Dismutase Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Analysis of Bt Protein
4.4. Analysis of DIMBOA
4.5. Analysis of Total Phenolics
4.6. Analysis of Polyphenol Oxidase Activity
4.7. Analysis of Peroxidase Activity
4.8. Analysis of Catalase Activity
4.9. Analysis of Superoxide Dismutase Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISAAA. Global status of commercialized biotech/GM crops: 2019. China Biotechnol. 2021, 411, 114–119. [Google Scholar]
- Nie, C.R.; Wang, J.W.; Luo, S.M.; Peng, Z.S.; Guo, J.W.; Xin, M.G. Spatial and temporal distribution of Bt proteins in Bt maize leaves. Food Agric. Immunol. 2021, 32, 450–459. [Google Scholar]
- Feng, Y.J.; Jin, Q.; Tan, F.X.; Wang, J.W. Jasmonic acid induced defence responses in Bt (Bacillus thuringiensis) and non-Bt corn (Zea mays L.) seedlings. Allelopath. J. 2018, 43, 203–206. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, Y.J.; Suo, W.F.; Shao, Y.T.; Wang, J.W. Effects of jasmonic acid application to belowground part on the content of defence chemicals and activity of defence enzymes in Bt corn (Zea mays L.) seedlings. Allelopath. J. 2018, 43, 239–254. [Google Scholar] [CrossRef]
- Horikoshi, R.J.; Vertuan, H.; Castro, A.A.; Morrell, K.; Griffith, C.; Evans, A.; Tan, J.; Asiimwe, P.; Anderson, H.; José, M.O.M.A.; et al. A new generation of Bt maize for control of fall armyworm (Spodoptera frugiperda). Pest Manag. Sci. 2021, 77, 3727–3736. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Alfageme, F.; Devos, Y.; Camargo, A.M.; Arpaia, S.; Messéan, A. Managing resistance evolution to transgenic Bt maize in corn borers in Spain. Crit. Rev. Biotechnol. 2022, 42, 201–219. [Google Scholar] [CrossRef]
- Wang, J.W.; Luo, S.M.; Feng, Y.J.; Cindy, N. The environmental distribution and ecological effects of Bt toxin in transgenic Crops. Acta Ecol. Sin. 2003, 4, 797–804. [Google Scholar]
- Liang, J.G.; Zhang, D.D.; Li, D.Y.; Zhao, S.Y.; Wang, C.Y.; Xiao, Y.T.; Xu, D.; Yang, Y.Z.; Li, G.P.; Wang, L.L.; et al. Expression profiles of Cry1Ab protein and its insecticidal efficacy against the invasive fall armyworm for Chinese domestic GM maize DBN9936. J. Integr. Agric. 2021, 20, 792–803. [Google Scholar] [CrossRef]
- Wang, Y.Q.; He, K.L.; Wang, Z.Y. Evolution of resistance to transgenic Bacillus thuringiensis maize in pest insects and a strategy for managing this. Chin. J. Appl. Entomol. 2019, 56, 12–23. [Google Scholar]
- Bilbo, T.R.; Reay-Jones, F.P.F.; Greene, J.K. Evaluation of Insecticide thresholds in late-planted Bt and Non-Bt Corn for management of fall Armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2020, 113, 814–823. [Google Scholar] [CrossRef]
- Li, G.P.; Feng, H.Q.; Ji, T.J.; Huang, J.R.; Tian, C.H. What type of Bt corn is suitable for a region with diverse lepidopteran pests: A laboratory evaluation. GM Crops Food 2020, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.S.; Santos-Amaya, O.F.; Oliveira, E.E.; Paula-Moraes, S.V.; Pereira, E.J.G. Facing Bt toxins growing up: Developmental changes of susceptibility to Bt corn hybrids in fall armyworm populations and the implications for resistance management. Crop Prot. 2021, 146, 105664. [Google Scholar] [CrossRef]
- Eghrari, K.; Oliveira, S.C.; Nascimento, A.M.; Queiroz, B.; Fatoretto, J.; de Souza, B.H.S.; Fernandes, O.A.; Môro, G.V. The implications of homozygous vip3Aa20- and cry1Ab-maize on Spodoptera frugiperda control. J. Pest Sci. 2022, 95, 115–127. [Google Scholar] [CrossRef]
- Feng, Y.J.; Ling, L.; Fan, H.J.; Liu, Y.H.; Tan, F.X.; Shu, Y.H.; Wang, J.W. Effects of temperature, water content and pH on degradation of Cry1Ab protein released from Bt corn straw in soil. Soil Biol. Biochem. 2011, 43, 1600–1606. [Google Scholar] [CrossRef]
- Lv, X.; Feng, Y.J.; Wang, X.Y.; Wang, J.W. Research progress on effects of Bt crop straw return on soil ecosystem. Ecol. Sci. 2019, 38, 235–240. [Google Scholar]
- Shu, Y.H.; Du, Y.; Wang, J.W. Presence of Cry1Ab in the Bt maize–aphid (Rhopalosiphum maidis)–ladybeetle (Propylea japonica) system has no adverse effects on insect biological parameters. Entomol. Exp. Appl. 2019, 167, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.F.; Wu, F.C.; Yin, J.Q.; Jiang, Z.L.; Song, X.Y.; Reddy, G.V.P. Use of taxonomic and trait-based approaches to evaluate the effect of Bt maize expressing Cry1Ie protein on non-target Collembola: A Case Study in Northeast China. Insects 2021, 12, 88. [Google Scholar] [CrossRef]
- Yurchak, V.; Leslie, A.W.; Dively, G.P.; Lamp, W.O.; Hooks, C.R.R. Degradation of transgenic Bacillus thuringiensis proteins in corn tissue in response to post-harvest management practices. Transgenic Res. 2021, 30, 851–865. [Google Scholar] [CrossRef]
- Xing, Y.J.; Qin, Z.F.; Feng, M.Y.; Li, A.M.; Zhang, L.; Wang, Y.; Dong, X.H.; Zhang, Y.X.; Tan, S.Q.; Shi, W.P. The impact of Bt maize expressing the Cry1Ac protein on non-target arthropods. Environ. Sci. Pollut. Res. 2019, 26, 5814–5819. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, Y.D.; Cao, K.L.; Qin, Z.F.; Zhao, X.X.; Dong, X.H.; Shi, W.P. Impact assessment of Bt maize expressing the Cry1Ab and Cry2Ab protein simultaneously on non-target arthropods. Environ. Sci. Pollut. Res. Int. 2020, 27, 21552–21559. [Google Scholar] [CrossRef]
- Tan, F.X.; Wang, J.W.; Feng, Y.J.; Chi, G.L.; Kong, H.L.; Qiu, H.F.; Wei, S.L. Bt corn plants and their straw have no apparent impact on soil microbial communities. Plant Soil 2010, 329, 349–364. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.N.; Li, G.; Yang, D.L.; Wang, S.R.; Na, B.Q.; Na, R.S. Effects of transgenic Bt maize on soil available nutrients and enzyme activities. Soils 2012, 44, 167–171. [Google Scholar]
- Li, F.; Wang, M.; Sun, H.W.; Yang, S.K.; Lu, X.B. Dynamics of Cry1Ab protein content in the rhizosphere soil and straw debris of transgenic Bt corn. Chin. J. Appl. Ecol. 2013, 24, 1907–1913. [Google Scholar]
- Sun, H.W.; Li, F.; Yang, S.K.; Wang, M.; Lu, X.B. Effects of Bt transgenic maize on genetic diversity of rhizosphere soil bacteria. J. Maize Sci. 2013, 21, 10–16. [Google Scholar]
- Zeng, P.; Feng, Y.J.; Zhang, W.C.; Zhang, Y.F.; Dong, W.C.; Wang, J.W. Change of Bt protein in soil after growing Bt corns and returning corn straws to soil and its effects on soil nutrients. Chin. J. Appl. Ecol. 2014, 25, 1997–2003. [Google Scholar]
- Shu, Y.H.; Zhang, Y.Y.; Cheng, M.M.; Zeng, H.L.; Wang, J.W. Multilevel assessment of Cry1Ab Bt-maize straw return affecting the earthworm Eisenia Fetida. Chemosphere 2015, 137, 59–69. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, Y.J.; Yan, S.; Zeng, P.; Suo, W.F.; Wang, J.W. Effects of continuous planting Bt corn on Bt protein content and microbial quantity in soil. Ecol. Environ. Sci. 2016, 25, 1945–1952. [Google Scholar]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; The University of Chicago Press: Chicago, IL, USA, 1997; pp. 1–11. [Google Scholar]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef]
- Feng, Y.J.; Wang, J.W.; Su, Y.J.; Luo, S.M. The role of jasmonic acid in the systemic induced defense response of aboveground and underground in corn (Zea mays). Sci. Agric. Sin. 2009, 42, 2726–2735. [Google Scholar]
- Feng, Y.J.; Wang, J.W.; Luo, S.M.; Jin, Q.; Fan, H.Z.; Su, Y.J.; Liu, Y.H. Effects of exogenous application of jasmomic acid and salicylic acid on the leaf and root induction of chemical defence in maize (Zea mays L.). Allelopath. J. 2010, 25, 133–146. [Google Scholar]
- Feng, Y.J.; Wang, J.W.; Luo, S.M. Review on the induced defense response between plant aboveground and belowground parts. Ecol. Sci. 2010, 29, 292–297. [Google Scholar]
- Feng, Y.J.; Wang, J.W.; Luo, S.M.; Fan, H.Z.; Jin, Q. Costs of jasmonic acid induced defense in aboveground and belowground parts of corn (Zea mays L.). J. Chem. Ecol. 2012, 38, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Hemanth Kumar, N.K.; Kumari, M.S.; Singh, M.V.; Jagannath, S. Oxidative stress and antioxidant metabolic enzymes response of maize (Zea mays L.) seedlings to a biotic stress (Alachlor) condition. Int. J. Agric. 2016, 8, 2227–2231. [Google Scholar]
- Bates, S.L.; Zhao, J.Z.; Roush, R.T.; Shelton, A.M. Insect resistance management in GM crops: Past, present and future. Nat. Biotechnol. 2005, 23, 57–62. [Google Scholar] [CrossRef]
- Ferry, N.; Edwards, M.G.; Gatehouse, J.; Capell, T.; Christou, P.; Gatehouse, A.M. Transgenic plants for insect pest control: A forward looking scientific perspective. Transgenic Res. 2006, 15, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.J.; Wang, J.W.; Luo, S.M. Effects of exogenous jasmonic acid on concentrations of direct-defense chemicals and expression of related genes in Bt (Bacillus thuringiensis) corn (Zea mays). Agric. Sci. China 2007, 6, 1456–1462. [Google Scholar] [CrossRef]
- Feng, Y.J.; Jin, Q.; Wang, J.W. Systemic induced effects of mechanical wounding on the chemical defense of Bt corn (Zea mays). Chin. J. Plant Ecol. 2010, 34, 695–703. [Google Scholar]
- Feng, Y.J.; Wang, J.W.; Jin, Q. Asian corn borer (Ostrinia furnacalis) damage induced systemic response in chemical defence in Bt corn (Zea mays L.). Allelopath. J. 2010, 26, 101–112. [Google Scholar]
- Xu, H.; Wang, X.Y.; Chi, G.L.; Tan, B.C.; Wang, J.W. Effects of Bacillus thuringiensis genetic engineering on induced volatile organic compounds emission in maize and the attractiveness to a parasitic wasp. Front. Bioeng. Biotechnol. 2019, 7, 160. [Google Scholar] [CrossRef]
- Klessig, D.F.; Malam, Y.J. The salicylic acid signal in plants. Plant Mol. 1994, 26, 1439–1458. [Google Scholar] [CrossRef]
- Cueto-Ginzo, I.A.; Serrano, L.; Sin, E.; Rodríguez, R.; Morales, J.G.; Lade, S.B.; Medina, V.; Achon, M.A. Exogenous salicylic acid treatment delays initial infection and counteracts alterations induced by maize dwarf mosaic virus in the maize proteome. Physiol. Mol. Plant Pathol. 2016, 96, 47–59. [Google Scholar] [CrossRef]
- Belt, K.; Huang, S.; Thatcher, L.F.; Casarotto, H.; Singh, K.; Aken, O.V. Salicylic acid-dependent plant stress signalling via mitochondrial succinate dehydrogenase. Plant Physiol. 2017, 173, 2029–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, N.; Fekry, M.; Bishr, M.; Zalabani, S.E.; Salama, O. Foliar spraying of salicylic acid induced accumulation of phenolics, increased radical scavenging activity and modified the composition of the essential oil of water stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef]
- Naz, R.; Sarfraz, A.; Anwar, Z.; Yasmin, H.; Nosheen, A.; Keyani, R.; Roberts, T.H. Combined ability of salicylic acid and spermidine to mitigate the individual and interactive effects of drought and chromium stress in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 159, 285–300. [Google Scholar] [CrossRef]
- Parveen, A.; Arslan, A.M.; Hussain, I.; Perveen, S.; Rasheed, R.; Mahmood, Q.; Hussain, S.; Ditta, A.; Hashem, A.; Al-Arjani, A.F.; et al. Promotion of growth and physiological characteristics in water-stressed Triticum aestivum in relation to foliar-application of salicylic acid. Water 2021, 13, 1316. [Google Scholar] [CrossRef]
- Sultan, I.; Khan, I.; Chattha, M.U.; Hassan, U.M.; Barbanti, L.; Calone, R.; Ali, M.; Majeed, S.; Ghani, M.A.; Batool, M.; et al. Improved salinity tolerance in early growth stage of maize through salicylic acid foliar application. Ital. J. Agron. 2021, 16, 1810. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Ahmed, N.; Saha, T.; Rahman, M.; Rahman, K.; Alam, M.M.; Rohman, M.M.; Nahar, K. Exogenous salicylic acid and kinetin modulate reactive oxygen species metabolism and glyoxalase system to confer waterlogging stress tolerance in soybean (Glycine max L.). Plant Stress 2022, 3, 100057. [Google Scholar] [CrossRef]
- Pan, L.W.; Xiang, C.Y.; Ding, J.W.; Liu, J.B.; Du, J.; Cao, G.Y. Physiological response of salicylic acid on physiological characteristics of rape seeding under salt stress. J. Tianjin Agric. Univ. 2022, 29, 22–26. [Google Scholar]
- Van Dam, N.M.; Harvey, J.A.; Wäckers, F.L.; Bezemer, T.M.; Van der Putten, W.H.; Vet, L.E.M. Interactions between aboveground and belowground induced responses against phytophages. Basic Appl. Ecol. 2003, 4, 63–77. [Google Scholar] [CrossRef]
- Xu, T.; Wang, J.W.; Luo, S.M. Cloning of the key genes in maize oxylipins pathways and their roles in herbivore induced defence. Chin. Sci. 2005, 50, 2457–2466. [Google Scholar]
- Ludwig-Müller, J.; Schubert, B.; Pieper, K.; Ihmig, S.; Hilgenberg, W. Glucosinolate content in susceptible and resistant Chinese cabbage varieties during development of clubroot disease. Phytochemistry 1997, 44, 407–417. [Google Scholar] [CrossRef]
- Du, Y.P.; Ji, X.L.; Jiang, E.S.; Cui, L.J.; Zhai, H. Phylloxera resistance induced by salicylic and jasmonic acids in Kyoho grapevine. Acta Entomol. Sin. 2014, 47, 443–448. [Google Scholar]
- Khanna, P.; Kaur, K.; Gupta, A.K. Salicylic acid induces differential antioxidant response in spring maize under high temperature stress. Indian J. Exp. Biol. 2016, 54, 386–393. [Google Scholar] [PubMed]
- Methenni, K.; Abdallah, M.B.; Nouairi, I.; Smaoui, A.; Ammar, W.B.; Zarrouk, M. Salicylic acid and calcium pretreatments alleviate the toxic effect of salinity in the Oueslati olive variety. Sci. Hortic. 2018, 233, 349–358. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates, Inc.: Sunderland, MA, USA, 1998. [Google Scholar]
- Kiddie, G.A.; Doughty, K.J.; Wallsgrove, R.M. Salicylic acid-induced accumulation of glucosinolates in oilseed rape (Brassica napus L.) leaves. J. Exp. Bot. 1994, 45, 1343–1346. [Google Scholar] [CrossRef]
- Song, Y.L.; Dong, Y.J.; Kong, J.; Tian, X.Y.; Bai, X.Y.; Xu, L.L. Effects of root addition and foliar application of nitric oxide and salicylic acid in alleviating iron deficiency induced chlorosis of peanut seedlings. J. Plant Nutr. 2017, 40, 63–81. [Google Scholar] [CrossRef]
- Colak, N.; Kurt-Celebi, A.; Fauzan, R.; Torun, H.; Ayaz, F.A. The protective effect of exogenous salicylic and gallic acids ameliorates the adverse effects of ionizing radiation stress in wheat seedlings by modulating the antioxidant defence system. Plant Physiol. Biochem. 2021, 168, 526–545. [Google Scholar] [CrossRef]
- Ni, X.; Quisenberry, S.S. Comparison of DIMBOA concentrations among wheat isolines and corresponding plant introduction lines. Entomol. Appl. 2000, 96, 275–279. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Developmental stimulation of total phenolics and related antioxidant activity in light-and dark-germinated corn by natural elicitors. Process Biochem. 2005, 40, 1724–1732. [Google Scholar] [CrossRef]
- Sivakumar, G.; Sharma, R.C. Induced biochemical changes due to seed bacterization by Pseudomonas fluorescens in maize plants. Indian Phytopathol. 2003, 56, 134–137. [Google Scholar]
- Gao, J.F. Plant Physiology Experimental Guidance; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Li, H.S. Experiments Principle and Technology of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Wang, X.; Du, T.; Shu, Y.; Tan, F.; Wang, J. Effects of Exogenous Salicylic Acid Application to Aboveground Part on the Defense Responses in Bt (Bacillus thuringiensis) and Non-Bt Corn (Zea mays L.) Seedlings. Plants 2022, 11, 2162. https://doi.org/10.3390/plants11162162
Feng Y, Wang X, Du T, Shu Y, Tan F, Wang J. Effects of Exogenous Salicylic Acid Application to Aboveground Part on the Defense Responses in Bt (Bacillus thuringiensis) and Non-Bt Corn (Zea mays L.) Seedlings. Plants. 2022; 11(16):2162. https://doi.org/10.3390/plants11162162
Chicago/Turabian StyleFeng, Yuanjiao, Xiaoyi Wang, Tiantian Du, Yinghua Shu, Fengxiao Tan, and Jianwu Wang. 2022. "Effects of Exogenous Salicylic Acid Application to Aboveground Part on the Defense Responses in Bt (Bacillus thuringiensis) and Non-Bt Corn (Zea mays L.) Seedlings" Plants 11, no. 16: 2162. https://doi.org/10.3390/plants11162162
APA StyleFeng, Y., Wang, X., Du, T., Shu, Y., Tan, F., & Wang, J. (2022). Effects of Exogenous Salicylic Acid Application to Aboveground Part on the Defense Responses in Bt (Bacillus thuringiensis) and Non-Bt Corn (Zea mays L.) Seedlings. Plants, 11(16), 2162. https://doi.org/10.3390/plants11162162




