Effects of Polyploidization on Morphology, Photosynthetic Parameters and Sucrose Metabolism in Lily
Abstract
:1. Introduction
2. Results
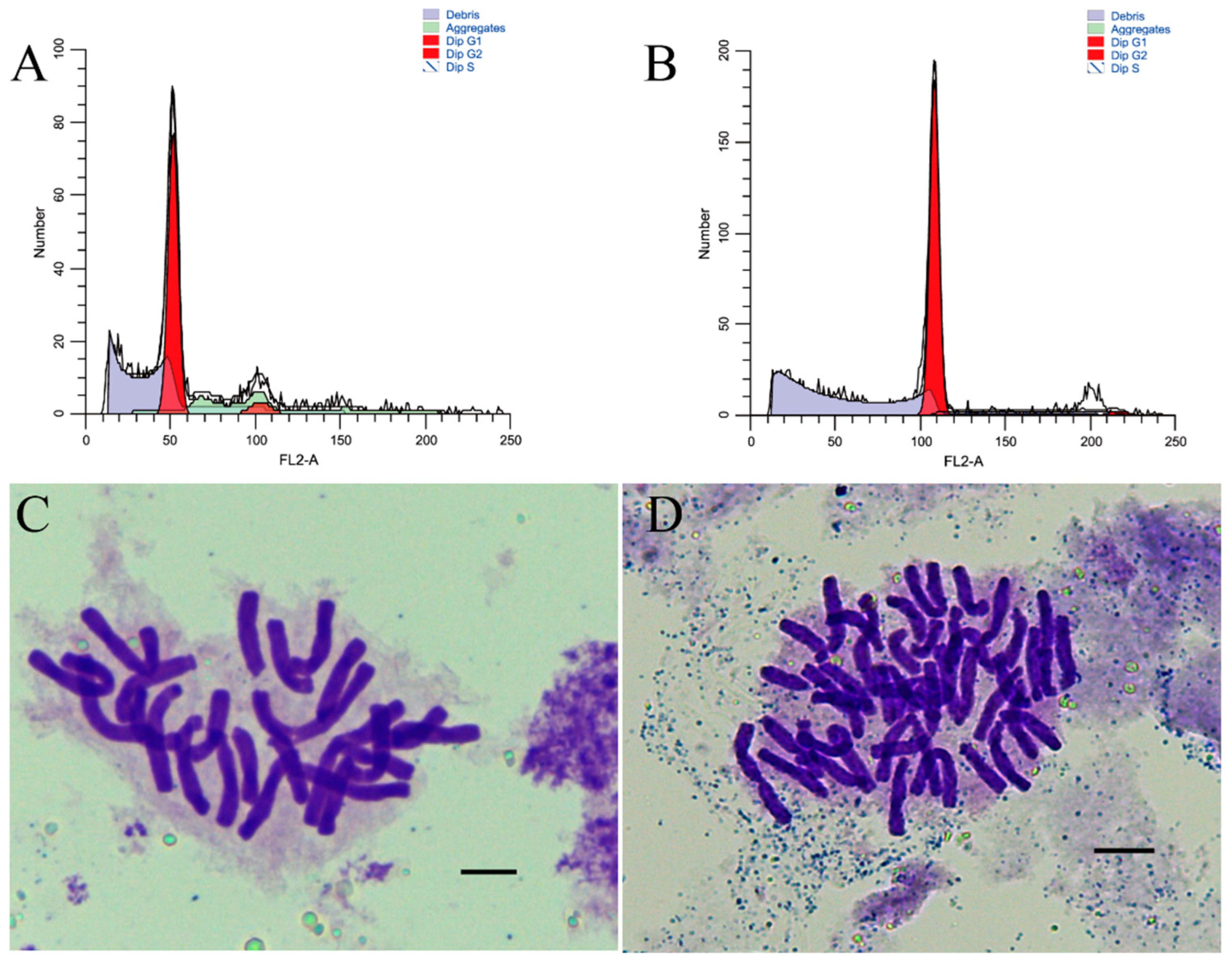
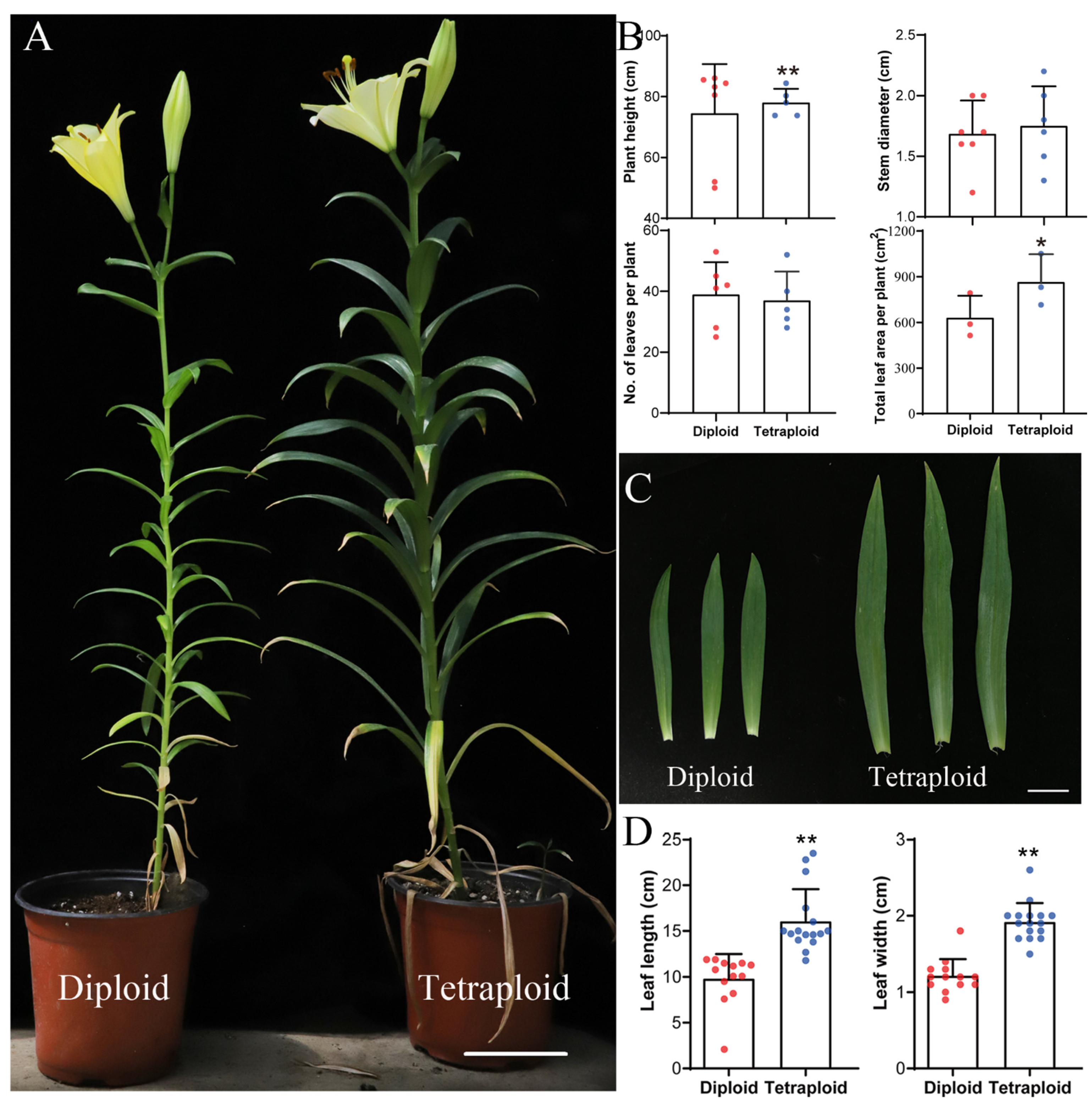
2.1. Comparisons of the Morphology of Allotetraploids and Allodiploids
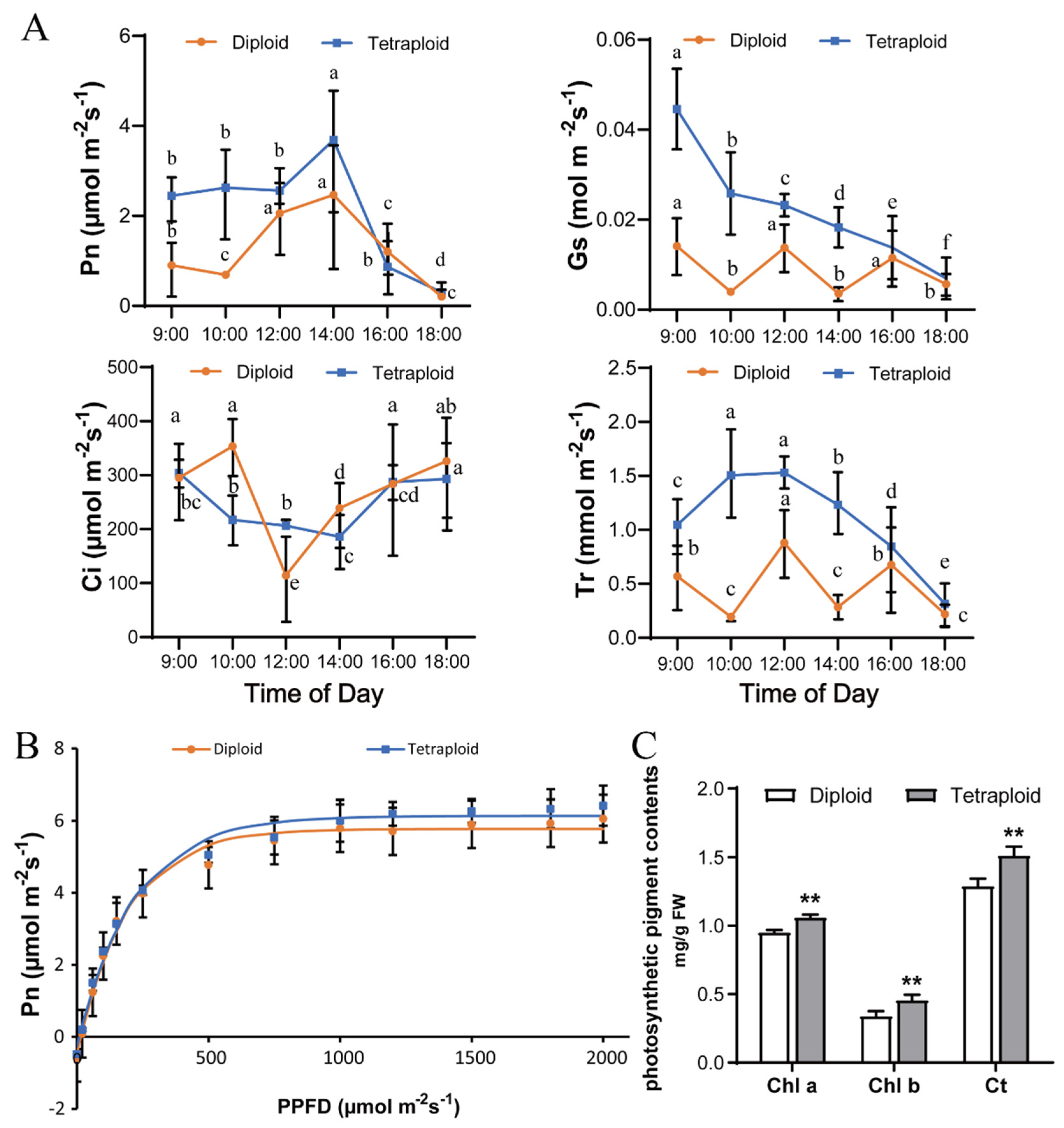
2.2. Changes in Photosynthetic Parameters
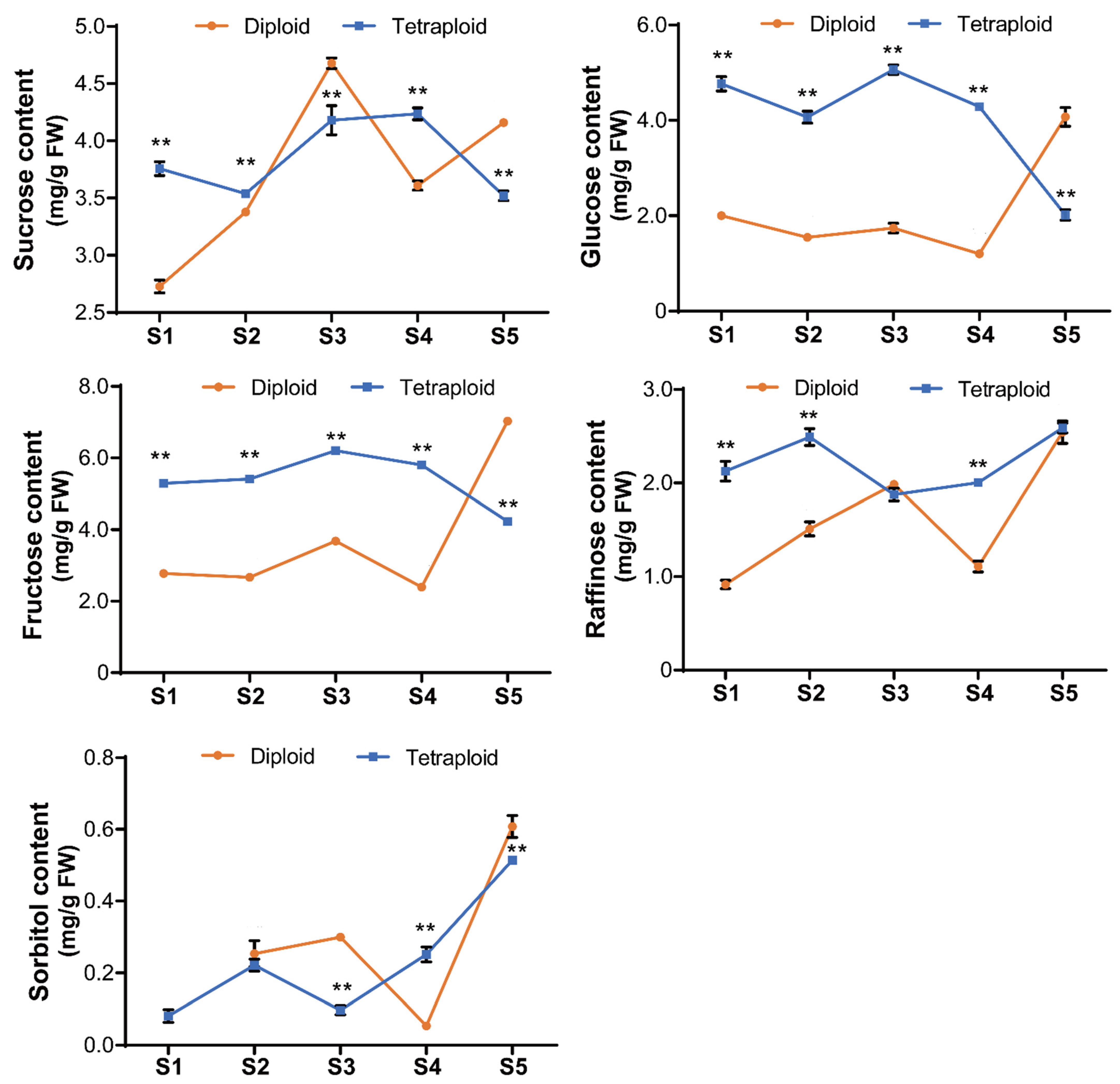
2.3. Increased NSC Contents in Allotetraploids
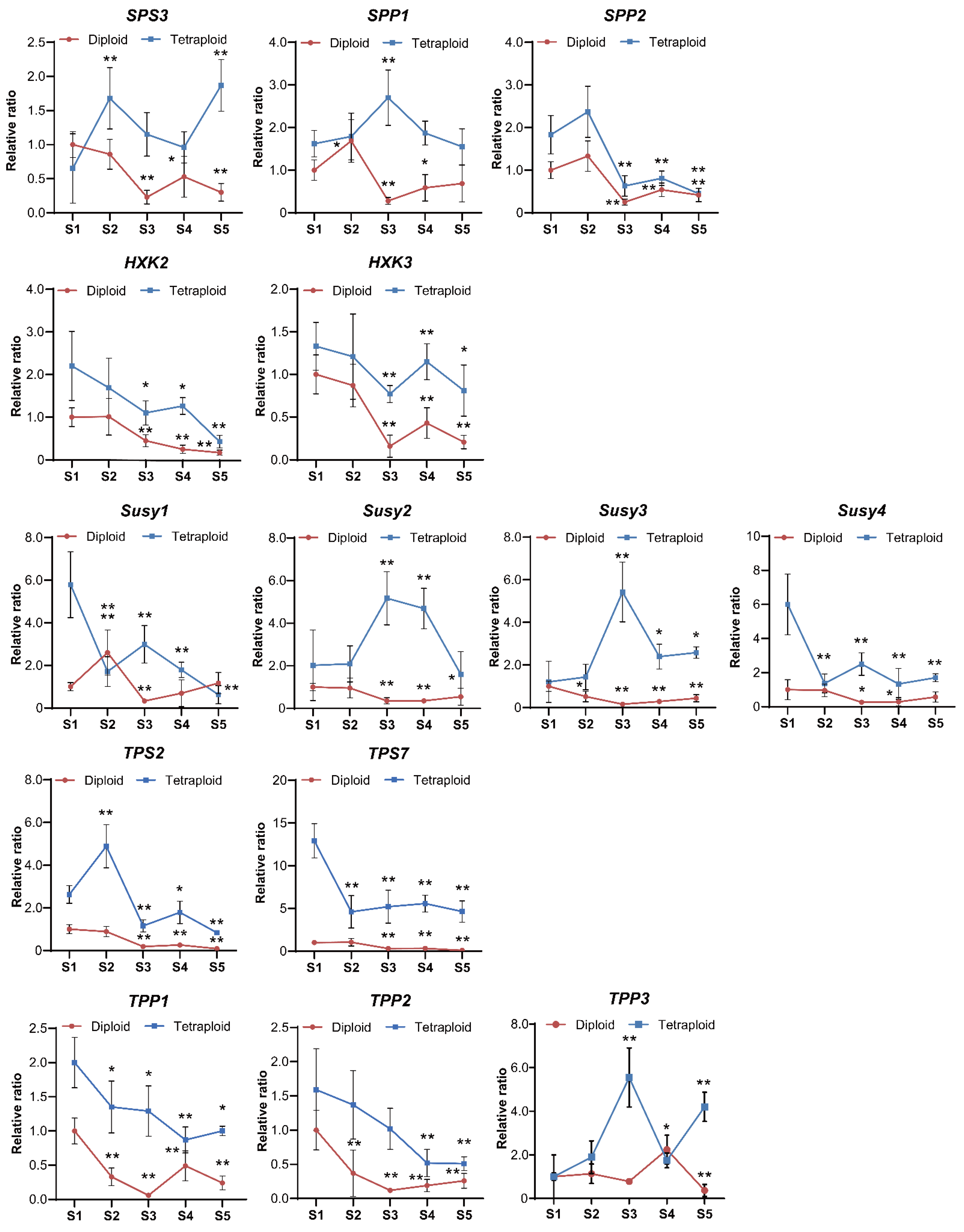
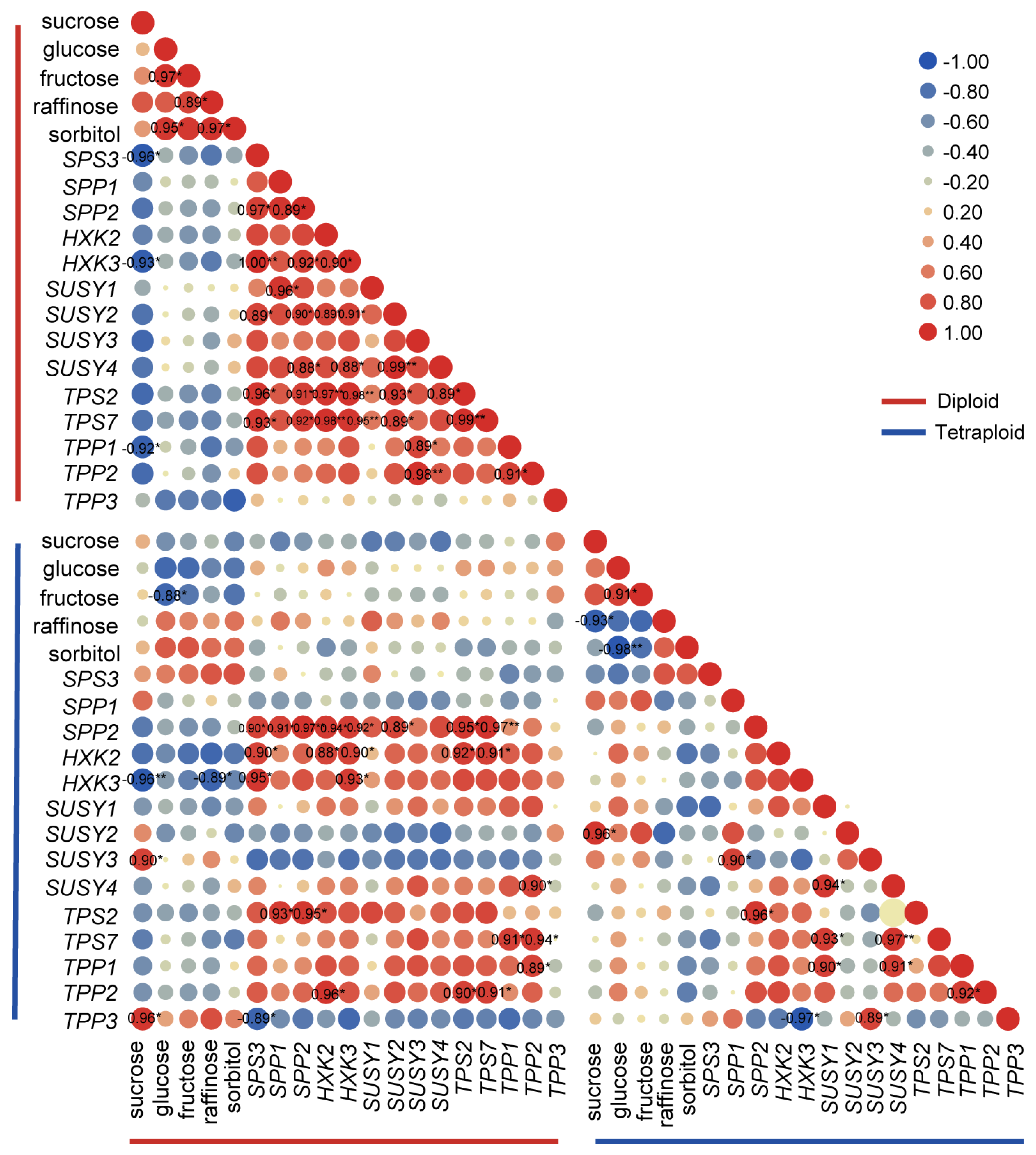
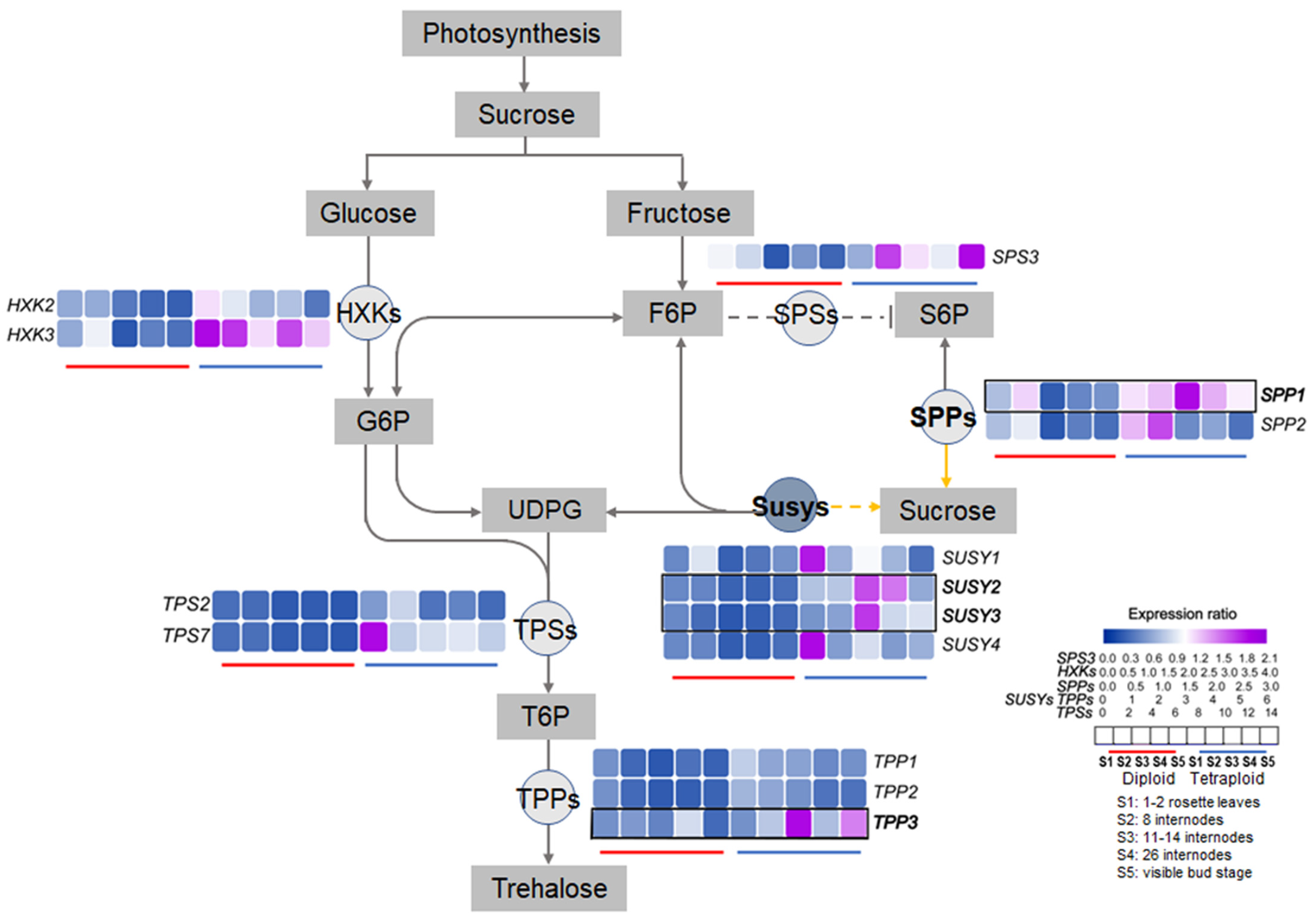
2.4. Expression Patterns of Sucrose-Metabolism-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phenotypic Characteristic and Growth Evaluations
4.3. Leaf Anatomical Structure and Stomatal Characteristics Observations
4.4. Photosynthetic Pigment and Photosynthetic Parameter Measurement
4.5. NSC Content Measurements
4.6. Expression Profile Analyses of Sucrose-Related Genes
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grassotti, A.; Gimelli, F. Bulb and cut flower production in the genus Lilium: Current status and the future. Acta Hortic. 2021, 900, 21–35. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, H.; He, T.; Gao, R.; Guo, G.; Lu, R.; Chen, Z.; Liu, C. Comparative analysis of morphology, photosynthetic physiology, and transcriptome between diploid and tetraploid barley derived from microspore culture. Front. Plant Sci. 2021, 12, 626916. [Google Scholar] [CrossRef] [PubMed]
- Vichiato, M.R.M.; Vichiato, M.; Pasqual, M.; Rodrigues, F.A.; de Castro, D.M. Morphological effects of induced polyploidy in Dendrobium nobile Lindl. (Orchidaceae). Crop Breed. Appl. Biotechnol. 2014, 14, 154–159. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Y.; Xie, Z.; Guo, D.; Chen, C.; Fan, Q.; Deng, X.; Liu, J.H. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol. J. 2019, 17, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Cao, Q.Z.; Jia, G.X. A protocol for fertility restoration of F1 hybrid derived from Lilium x formolongi ‘Raizan 3’ x Oriental hybrid ‘Sorbonne’. Plant Cell Tiss. Organ Cult. 2017, 129, 375–386. [Google Scholar] [CrossRef]
- Tan, F.Q.; Zhang, M.; Xie, K.D.; Fan, Y.J.; Song, X.; Wang, R.; Wu, X.M.; Zhang, H.Y.; Guo, W.W. Polyploidy remodels fruit metabolism by modifying carbon source utilization and metabolic flux in Ponkan mandarin (Citrus reticulata Blanco). Plant Sci. 2019, 289, 110276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, W.Q.; Yang, X.J.; Luo, X.; Jiang, N.; Ma, H.; Zhang, D.B. Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 2010, 22, 672–689. [Google Scholar] [CrossRef]
- Patrick, J.W.; Botha, F.C.; Birch, R.G. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar] [CrossRef]
- Gutiérrez-Miceli, F.A.; Rodríguez-Mendiola, M.A.; Ochoa-Alejo, N.; Méndez-Salas, R.; Dendooven, L.; Arias-Castro, C. Relationship between sucrose accumulation and activities of sucrose-phosphatase, sucrose synthase, neutral invertase and soluble acid invertase in micropropagated sugarcane plants. Acta Physiol. Plant. 2002, 24, 441–446. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Xu, S.M.; Brill, E.; Llewellyn, D.J.; Furbank, R.T.; Ruan, Y.L. Overexpression of a potato sucrose synthase gene in cotton accelerates leaf expansion, reduces seed abortion, and enhances fiber production. Mol. Plant 2012, 5, 430–441. [Google Scholar] [CrossRef]
- Chourey, P.S.; Taliercio, E.W.; Carlson, S.J.; Ruan, Y.L. Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol. Gen. Genet. 1998, 259, 88–96. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Z.; Gao, C.; Sun, M.; Li, S.; Min, R.; Wu, J.; Li, D.; Wang, X.; Wei, Y.; et al. Change in sucrose cleavage pattern and rapid starch accumulation govern lily shoot-to-bulblet transition in vitro. Front Plant Sci. 2021, 11, 564713. [Google Scholar] [CrossRef]
- Vargas, W.A.; Salerno, G.L. The Cinderella story of sucrose hydrolysis: Alkaline/neutral invertases, from cyanobacteria to unforeseen roles in plant cytosol and organelles. Plant Sci. 2010, 178, 1–8. [Google Scholar] [CrossRef]
- Leloir, L.F.; Cardini, C.E. The biosynthesis of sucrose phosphate. J. Biol. Chem. 1955, 214, 157–165. [Google Scholar] [CrossRef]
- Micallef, B.J.; Haskins, K.A.; Vanderveer, P.J.; Roh, K.S.; Shewmaker, C.K.; Sharkey, T.D. Altered photosynthesis, flowering, and fruiting in transgenic tomato plants that have an in-creased capacity for sucrose synthesis. Planta 1995, 196, 327–334. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Eastmond, P.J.; van Dijken, A.J.; Spielman, M.; Kerr, A.; Tissier, A.F.; Dickinson, H.G.; Jones, J.D.; Smeekens, S.C.; Graham, I.A. Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J. 2002, 29, 225–235. [Google Scholar] [CrossRef]
- Griffiths, C.A.; Paul, M.J.; Foyer, C.H. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochim. Biophys. Acta. 2016, 1857, 1715–1725. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Feng, Y.; Tu, M.; Wittich, P.E.; Bate, N.J.; Messing, J. Transcriptome and metabolome reveal distinct carbon allocation patterns during internode sugar accumulation in different sorghum genotypes. Plant Biotechnol. J. 2019, 17, 472–487. [Google Scholar] [CrossRef]
- Nagle, W. Cell growth and nuclear DNA increase by endoreduplication and differential DNA replication. In Cell Growth; Nicolini, C., Ed.; Plenum Press: New York, NY, USA, 1982; pp. 619–651. [Google Scholar]
- Cionini, P.G.; Cavallini, A.; Baroncelli, S.; Lercari, B.; D’Amato, F. Diploidy and chromosome endoreduplication in the development of epidermal cell lines in the first foliage leaf of durum wheat (Triticum durum Desf.). Protoplasma 1983, 118, 36–43. [Google Scholar] [CrossRef]
- Dudits, D.; Török, K.; Cseri, A.; Paul, K.; Nagy, A.V.; Nagy, B.; Sass, L.; Ferenc, G.; Vankova, R.; Dobrev, P.; et al. Response of organ structure and physiology to autotetraploidization in early development of energy willow Salix viminalis. Plant Physiol. 2016, 170, 1504–1523. [Google Scholar] [CrossRef]
- Silvertown, J.W.; Doust, J.L. Introduction to Plant Population Biology; Blackwell Scientific Publications: Oxford, UK, 1993. [Google Scholar]
- Chen, T.; Sheng, Y.; Hao, Z.; Long, X.; Fu, F.; Liu, Y.; Tang, Z.; Ali, A.; Peng, Y.; Liu, Y.; et al. Transcriptome and proteome analysis suggest enhanced photosynthesis in tetraploid Liriodendron sino-americanum. Tree Physiol. 2021, 41, 1953–1971. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, X.; Gao, X.; Wang, L.; Jia, G. Effects of ploidy level on the cellular, photochemical and photosynthetic characteristics in Lilium FO hybrids. Plant Physiol. Biochem. 2018, 133, 50–56. [Google Scholar] [CrossRef]
- Mooney, H.A.; Johnson, W. Comparative physiological ecology of an arctic and alpine population of Thalictrum alpinum L. Ecology 1965, 46, 721–727. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Xu, Y.; Zhang, S.; Wang, J.; Hu, B.; Hou, X.; Li, Y.; Liu, T. Enhanced relative electron transport rate contributes to increased photosynthetic capacity in autotetraploid Pak Choi. Plant Cell Physiol. 2020, 61, 761–774. [Google Scholar] [CrossRef]
- Dai, F.; Wang, Z.; Luo, G.; Tang, C. Phenotypic and transcriptomic analyses of autotetraploid and diploid Mulberry (Morus alba L.). Int. J. Mol. Sci. 2015, 16, 22938–22956. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhang, Q.; Cao, Q.Z.; Gao, X.; Jia, G.X. An efficient method for inducing multiple genotypes of allotetraploids Lilium rosthornii Diels. Plant Cell Tiss. Organ Cult. 2020, 141, 499–510. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, Y.L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef]
- Hartig, K.; Beck, E. Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol 2006, 8, 389–396. [Google Scholar] [CrossRef]
- Lingle, S.E. Sugar metabolism during growth and development in sugarcane internodes. Crop Sci. 1999, 39, 480–486. [Google Scholar] [CrossRef]
- Grof, C.P.L.; Albertson, P.L.; Bursle, J.; Perroux, J.M.; Bonnett, G.D.; Manners, J.M. Sucrose-phosphate synthase, a biochemical marker of high sucrose accumulation in sugarcane. Crop Sci. 2007, 47, 1530–1539. [Google Scholar] [CrossRef]
- Schluepmann, H.; Paul, M. Trehalose metabolites in Arabidopsis-elusive, active and central. Arab. Book 2009, 7, e0122. [Google Scholar] [CrossRef]
- van Dijken, A.J.H.; Schluepmann, H.; Smeekens, S.C.M. Arabidopsis trehalose-6-phosphate synthase 1 is essential for normal vegetative growth and transition to flowering. Plant Physiol. 2004, 135, 969–977. [Google Scholar] [CrossRef]
- Wahl, V.; Ponnu, J.; Schlereth, A.; Arrivault, S.; Langenecker, T.; Franke, A.; Feil, R.; Lunn, J.E.; Stitt, M.; Schmid, M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 2013, 339, 704–707. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, M.; Zhao, Y.Q.; Hu, H.; Huang, Y.X.; Jia, G.X. Identification of trehalose-6-phosphate synthase (TPS)-coding genes involved in flowering induction of Lilium× formolongi. Plant Physiol. Biochem. 2022, 171, 84–94. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhang, M.F.; Zhang, M.; Jia, G.X. Analysis of global gene expression profiles during the flowering initiation process of Lilium × formolongi. Plant Mol. Biol. 2017, 94, 361–379. [Google Scholar] [CrossRef]
- Wang, L.J.; Cao, Q.Z.; Zhang, X.Q.; Jia, G.X. Effects of polyploidization on photosynthetic characteristics in three Lilium species. Sci. Hortic. 2021, 284, 27. [Google Scholar] [CrossRef]
- Zhou, S.J. Intergenomic Recombination and Introgression Breeding in Longiflorum x Asiatic lilies. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Holland, 2007. [Google Scholar]
- Aversano, R.; Scarano, M.; Aronne, G.; Caruso, I.; D’Amelia, V.; Micco, V.D.; Fasano, C.; Termolino, P.; Carputo, D. Genotype-specific changes associated to early synthesis of autoallotetraploids in wild potato species. Euphytica 2014, 202, 1–10. [Google Scholar]
- Ozturk, A.; Umit, S.; Gürgör, P.N.; Korkmaz, A. The effect of different nursery conditions on some of the leaf and stomata characteristics in Chestnuts. J. Appl. Bot. Food Qual. 2014, 87, 190–195. [Google Scholar]
- Wasmund, N.; Topp, I.; Schories, D. Optimising the storage and extraction of chlorophyll samples. Oceanologia 2006, 48, 125–144. [Google Scholar]
- Lichtenthaler, H.; Wellbum, A.R. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Yan, J.; Shi, S.; Wang, H.; Liu, R.; Li, N.; Chen, Y.; Wang, S. Neutral monosaccharide composition analysis of plant-derived oligo- and polysaccharides by high performance liquid chromatography. Carbohydr Polym. 2016, 136, 1273–1280. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Q.; Zhao, Y.Q.; Yang, J.; He, H.B.; Jia, G.X. The lre-miR159a-LrGAMYB pathway mediates resistance to grey mould infection in Lilium regale. Mol. Plant Pathol. 2020, 21, 749–760. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, C.; Zhang, M.F.; Jia, G.X. Evaluation of putative reference genes for quantitative real-time PCR normalization in Lilium regale during development and under stress. PeerJ. 2016, 4, e1837. [Google Scholar] [CrossRef]
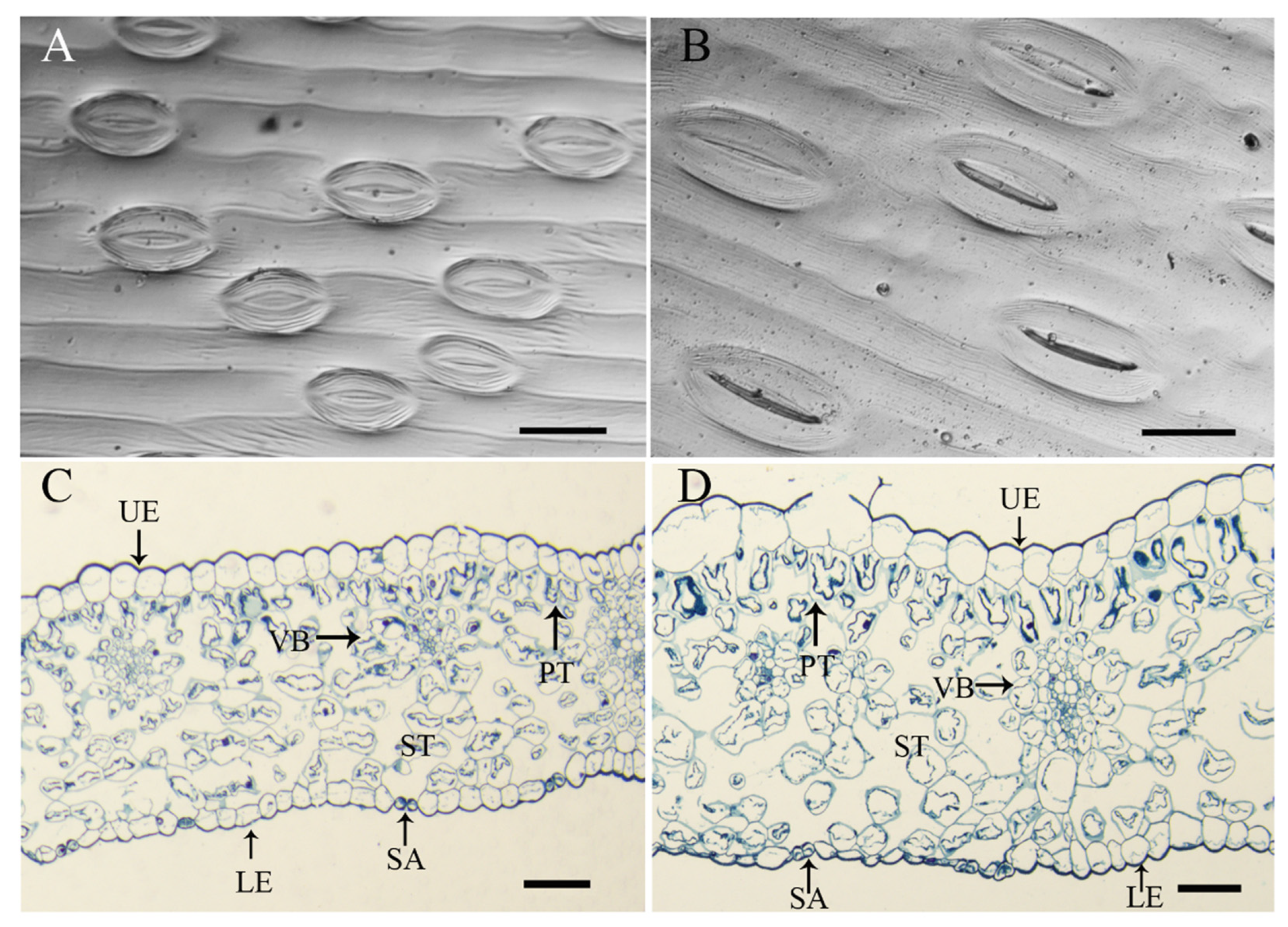
| Ploidy | Stomatal Length (μm) | Stomatal Width (μm) | Stomatal Length-Width Ratio | Stomatal Density (/mm2) |
|---|---|---|---|---|
| Allodiploid | 82.16 ± 5.87b | 28.10 ± 1.35b | 2.92a | 77.96b |
| Allotetraploid | 129.83 ± 6.12a | 43.85 ± 1.35a | 2.96a | 23.86a |
| Difference (%) | 58.02 | 56.04 | 1.37 | 69.39 |
| Ploidy Level | Leaf Thickness (µm) | Upper Epidermis (µm) | Lower Epidermis (µm) | Palisade Tissue (µm) | Spongy Tissue (µm) | Vascular Bundle (µm) |
|---|---|---|---|---|---|---|
| Allodiploid | 403.34 ± 100.96b | 57.16 ± 17.06fg | 40.56 ± 15.98i | 57.77 ± 10.59d | 31.40 ± 13.92gh | 29.85 ± 6.81hi |
| Allotetraploid | 601.30 ± 32.27a | 64.33 ± 15.84f | 47.12 ± 12.73gh | 72.68 ± 15.39c | 54.71 ± 17.89e | 37.53 ± 11.04fg |
| Difference (%) | 49.08 | 12.54 | 16.17 | 25.81 | 74.24 | 25.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Hu, H.; Jiang, Y.; Wang, L.; Kong, X.; Huang, Y.; Jia, G. Effects of Polyploidization on Morphology, Photosynthetic Parameters and Sucrose Metabolism in Lily. Plants 2022, 11, 2112. https://doi.org/10.3390/plants11162112
Zhang Q, Hu H, Jiang Y, Wang L, Kong X, Huang Y, Jia G. Effects of Polyploidization on Morphology, Photosynthetic Parameters and Sucrose Metabolism in Lily. Plants. 2022; 11(16):2112. https://doi.org/10.3390/plants11162112
Chicago/Turabian StyleZhang, Qian, Hao Hu, Yuzhou Jiang, Lianjuan Wang, Xiangfeng Kong, Yixuan Huang, and Guixia Jia. 2022. "Effects of Polyploidization on Morphology, Photosynthetic Parameters and Sucrose Metabolism in Lily" Plants 11, no. 16: 2112. https://doi.org/10.3390/plants11162112
APA StyleZhang, Q., Hu, H., Jiang, Y., Wang, L., Kong, X., Huang, Y., & Jia, G. (2022). Effects of Polyploidization on Morphology, Photosynthetic Parameters and Sucrose Metabolism in Lily. Plants, 11(16), 2112. https://doi.org/10.3390/plants11162112





