1. Introduction
Cowpea beans (
Vigna unguiculata (L.) Walp.), also known as rope or Macassar beans, are a significant source of protein in the north and northeastern regions of Brazil [
1]. Family and small farms are the leading producers, using mostly native or traditional seeds [
2]. These farmers have little technological apparatus and use family labor and seeds from selection made by the farmer himself, with well-defined and recognized phenotypic characteristics that characterize them as native or traditional seeds. Traditional or local varieties are highly adapted to the places where they are conserved and managed, and they are part of family autonomy, constituting the main factor in people’s food security.
Farms in the northeastern region, especially those in Mossoró/Açu, RN, Brazil, require a substantial amount of water, which has driven the use of saline water up to 4.5 dS m
−1, e.g., groundwater from the Calcário Jandaíra aquifer [
3]. Cowpea beans are moderately tolerant to salinity, up to 3.3 dS m
−1 in irrigation water and 4.8 dS m
−1 in the soil [
4]. However, it is necessary to use saline water due to the scarcity of good quality water and the increase in water consumption to meet population growth and irrigated agriculture.
Water with a high sodium adsorption ratio (SAR) can modify the physicochemical conditions of the soil, and excess soluble salts result in lower osmotic potential, water deficit, stomatal closure, limited CO
2 assimilation/water usage, and alterations to the photochemical process [
5,
6]. Osmotic restrictions combined with ionic restrictions and nutritional imbalance limit gas exchange and biomass accumulation and production [
5,
6,
7,
8]. The decrease in productivity of cowpea under saline stress occurs due to the decrease in the water potential, survival rate, plant’s initial vigor, growth, and photosynthetic activity and excessive accumulation of Cl and Na ions and reactive oxygen species (ROS) [
5,
6,
7,
8,
9,
10,
11]. Therefore, studies that evaluate gas exchange and photosynthetic efficiency are important for the identification of salinity-tolerant plants.
Identifying and selecting salinity-tolerant cowpea bean varieties can facilitate the use of saline water without affecting cowpea production. While there are early growth stage studies that designate salinity-tolerant cowpea bean genotypes [
2,
12,
13], studies investigating the complete production cycle are lacking. In this study, we hypothesized that the genetic diversity of cowpea beans grown in the semi-arid region would create salinity-tolerant cowpea bean varieties that may be cultivated in farms with poor water quality. Thus, the goals of this study were to assess the effect of irrigation with brackish water on the growth, gas exchange, and production of 15 traditional varieties of cowpea and determine the most saltwater-tolerant varieties.
3. Discussion
The soil salinity limit for cowpea culture is 4.8 dS m
−1 [
4]. Irrigation water with 4.5 dS m
−1 increased by 1.4 to 2.15 times the electrical conductivity of the soil saturation extract considering the soil salinity limit. Therefore, every cowpea variety was under possible salt stress. This behavior was similar to that observed in irrigated areas with lower leaching fractions where irrigation-water salinity influenced the soil salinity at the end of the cycle [
7,
14].
Soil from pots with the highest SDM levels presented the highest ECse values (Canário and Roxão). This behavior relates to the diversity of salinity tolerance mechanisms [
15], e.g., water consumption restrictions, selective salt absorption, and salt exclusion at the roots. Soil from pots with the lowest SDM levels exhibited the lowest ECse values (Costela de Vaca, Lisão, Canapu Miúdo, and Paulistinha) because of vacuolar ion compartmentation and non-selective ion absorption in the most susceptible varieties [
16,
17].
Irrigation water with 4.5 dS m
−1 reduced the photosynthetic rate (
AN) in the Boquinha, Roxão, Feijão Branco, Canapu Miúdo, Coruja, and Paulistinha varieties. The
AN reductions were related to stomatal factors, i.e., the reduction of stomatal conductance limited the influx of CO
2 and consequently the internal concentration of CO
2 (
Ci) in the substomatal cavity, reducing water absorption and transpiration [
6,
17,
18]. The reduction of stomatal conductance occurred with increased soil salt concentrations and led to a decrease in osmotic and water potentials, resulting in toxicity of specific ions—Na
+ and Cl
- [
5,
8,
9]. Plants experience issues absorbing water from the soil under salt stress and tend to reduce water loss by closing stomates and reducing transpiration [
6,
18].
The reduced A
N in the Boquinha, Feijão Branco, Canapu Miúdo, Coruja, and Paulistinha varieties was also related to non-stomatal factors. There were reductions in the
A/Ci, indicating decreased ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) enzymic activity under stress conditions, e.g., the lack of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) from the electron transport chain of photosystem II [
6,
19].
The Ceará, Canário, Pingo de Ouro, Canapu Branco, Baeta, and Sempre Verde varieties increased A
N under salt stress. The increased production of photo-assimilates under salt stress improves the energetic input and allows the plant to use mechanisms to tolerate energy expenditure, such as vacuolar ion compartmentation, the exclusion of specific ions, and attempting to attain ionic homeostasis [
16,
17].
The increased
AN in the Ceará, Canário, Pingo de Ouro, Canapu Branco, Baeta, and Sempre Verde varieties coincided with increases in
gs, CO
2 influx, transpiration, and the consequent water absorption. Increased water loss because of transpiration results in reduced water potential at the roots, overcoming the osmotic stress and aiding in water absorption [
9,
10,
11]. The
AN of the Pingo de Ouro, Canapu Branco, Baeta, and Sempre Verde varieties coincided with increased
AN/Ci and
WUEi, improved water usage, and increased Rubisco activity. However, the increased
AN in the Canapu Branco variety also coincided with a decrease in gs and E; in this case, the higher salt concentration, improved water usage (
WUEi), and increased Rubisco activity (
A/Ci) resulted in water consumption restrictions.
The
AN of the Costela de Vaca, Lisão, and Ovo de Peru varieties was not affected by salinity, but the
WUEi increased because of lower transpiration. Soil salinity impairs water absorption by cowpea bean plants; therefore, lower transpiration is a strategy to reduce water losses [
6,
18].
Chlorophyll fluorescence did not differ among varieties, indicating similar photochemical activities. Increases of
Fv and
ETR in cowpea bean varieties under salt stress compared with control demonstrated an increased ability to transfer energy from the excited electrons of chlorophyll molecules to assemble NADPH and ATP and had reduced ferredoxin (Fdr). This increased energy transfer was vital for preventing the decrease in photosynthesis or improving photosynthesis under salt stress once the quantum efficiency of photosystem II (
Y(II)) in cowpea bean varieties decreased under salt stress, indicating a decrease in the fraction of energy absorption by chlorophyll in PSII [
20]. However, some varieties susceptible to salt stress, e.g., Boquinha, Roxão, Feijão Branco, Canapu Miúdo, Coruja, and Paulistinha, exhibited decreased
AN and
A/Ci, despite this mechanism for increasing the photochemical energy transfer.
Cowpea bean plants increased their photoprotective capability under salt stress because of a higher quantum yield of regulated photochemical quenching (
Y(NPQ)) through thermal energy dissipation by the xanthophyll cycle [
21]. This photoprotective mechanism is efficient in cowpea bean plants once the
Fv/Fm values are greater than 0.75, indicating a lack of degradation of the photosynthetic apparatus [
17]. Compared with the control, the
Fo values were lower under salt stress, corroborating the absence of damage in the PSII reaction centers [
20].
Despite improvements in
AN,
WUE, and
A/Ci, the growth, biomass accumulation, and production of cowpea beans decreased under salt stress, especially the primary branch length in the Costela de Vaca, Pingo de Ouro, Roxão, Canapu Branco, Canapu Miúdo, and Baeta varieties, characterized by their prostrate and semi-prostrate size and indeterminate growth habit. The reduced growth resulted from lower energetic stability because of decreased
Y(II) under salt stress. The authors in [
22] reported that reduced biomass in cowpea bean plants under salt stress relates to an energy bypass because of the metabolic cost incurred during acclimation.
In plants, salt stress results in morphologic and anatomic modifications with strategies for adapting to adverse conditions, e.g., fewer leaves and shorter branches, reflecting decreased transpiration to improve water absorption [
14,
23].
Roxão was the only variety without SDM modifications caused by salinity and was unique in the susceptible group with an investment in biomass at the expense of grain production. The authors in [
7,
8,
24,
25] and other authors observed reduced growth and biomass accumulation in cowpea beans under salt stress. These reductions are part of the acclimation process of cowpea bean plants that have the potential to tolerate salt stress as they seek to secure production and perpetuate the species. In this sense, varieties such as Ovo de Peru, Pingo de Ouro, Sempre Verde, Costela de Vaca, and Ceará presented reduced growth and biomass accumulation but exhibited the highest production compared with the control.
Grain production was reduced (
p < 0.05) in cowpea bean varieties under salt stress, except for the Ceará, Costela de Vaca, Pingo de Ouro, Ovo de Peru, and Sempre Verde varieties. Reduced water absorption associated with specific ion toxicity and physiological effects of salinity resulted in reduced growth and production [
6,
26]. The stress from salt accumulation in plants resulted in fewer reproductive branches and higher abortion rates [
14,
27]. All varieties produced fewer pods under salt stress; however, the Ceará, Pingo de Ouro, and Sempre Verde had more seeds per pod, compensating for the final grain production.
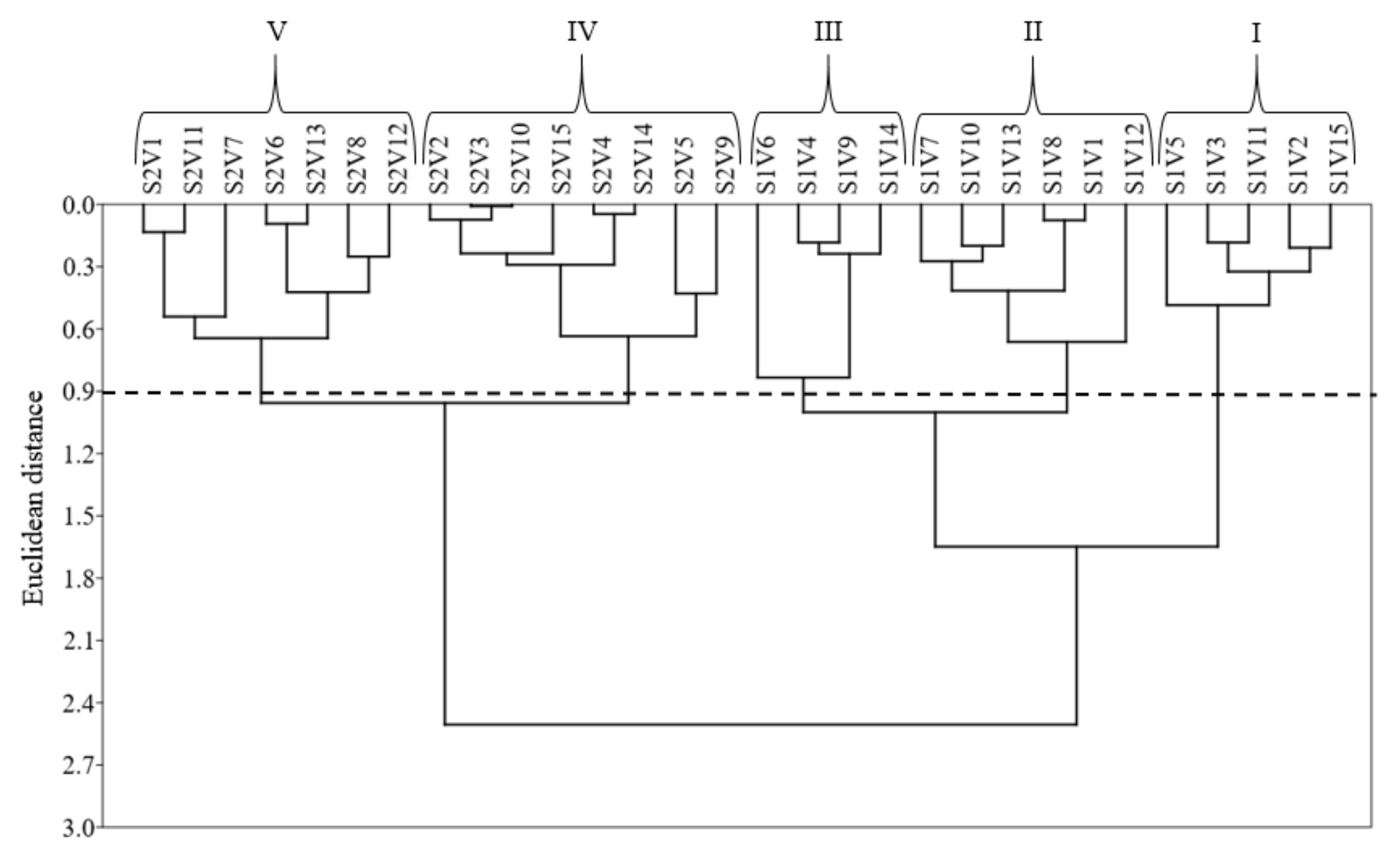
Cluster analysis revealed heterogenicity among plants under saline water irrigation with the Ceará, Costela de Vaca, Lisão, Canário, Canapu Branco, Canapu Miúdo, Paulistinha, and Sempre Verde varieties presenting similar SDM and production compared with the control, indicating tolerance to high irrigation-water salinity. The tolerance of Lisão, Canário, Canapu Branco, Canapu Miúdo, and Paulistinha occurred due to SDM. The Ceará, Costela de Vaca, and Semper Verde varieties are more tolerant to saline stress for SDM and grain production. We recommend the Ceará, Costa de Vaca, Pingo de Ouro, Ovo de Peru, and Semper Verde varieties for grain production under saline stress conditions. Those results differed from studies with conventional cowpea bean varieties under salt stress, e.g., [
28] (EPACE 10); [
29] (MNC04-762F-9, MNC04-762F-3, MNC04-762F-21, MNC04-769F-62, and MNC04-765F-153); [
30] (IPA-206 and BRS Guariba); [
27] (BRS Pajeu); [
24] (BRS Imponente, MNC04-795F-168, and MNC04-795F-161); [
31] (CE 790 and CE 104); [
32] (BRS Pajeú); and [
33] (BRS Itaim), who reported that these conventional varieties were susceptible to high irrigation-water salinity (5.0, 4.8, 5.0, 4.5, 6.4, 5.0, 6.0, 4.5, and 6.0 dS m
−1, respectively). These findings support the hypothesis that traditional varieties are more tolerant of salt stress than conventional varieties; however, further field studies are necessary. The Boquinha, Pingo de Ouro, Roxão, Feijão Branco, Ovo de Peru, Baeta, and Coruja varieties presented significantly different values compared to the control, indicating high susceptibility to irrigation-water salinity (4.5 dS m
−1).
In summary, the reduced photosynthetic rates in cowpea bean varieties are mainly caused by reductions in stomatal conductance resulting from salt stress. Salt stress increases the energy transferability of photosystem II in cowpea bean varieties, increasing the CO2 assimilation rate and the instantaneous carboxylation efficiency in varieties more tolerant to salt stress. Salt stress decreases 26% of the production of tolerant varieties to salt stress and 54% of susceptible varieties. The Ceará, Costela de Vaca, Pingo de Ouro, Ovo de Peru, and Sempre Verde varieties exhibited the best physiological and production performance under salt stress; therefore, these varieties are tolerant to salt stress. The Lisão, Canário, Canapu Branco, Canapu Miúdo, Paulistinha, Boquinha, Roxão, Feijão Branco, Baeta, and Coruja present the worst physiological and production performances under salt stress; therefore, those varieties are susceptible to salt stress.
4. Material and Methods
4.1. Location, Experimental Design, and Plant Material
The experiment was conducted in a greenhouse at the Federal Rural University of the Semi-Arid Region—UFERSA, East campus, Mossoró/RN, Brazil, from May to August 2019. The municipality is located at the geographical coordinates of 5°12′ S and 37°19′ W, with an average altitude of 18 m. According to Köppen’s classification, the climate of the region is BSwh’, and maximum and minimum temperatures of 44.2 and 20.4 °C and maximum and minimum relative humidity (RH) of 86 and 22%, respectively, were recorded during the experimental period. The average temperature and average daily relative humidity throughout the experiment were 33.8 °C and 49% RH, respectively.
The experimental design used was randomized blocks, with treatments arranged in a 15 × 2 factorial scheme, consisting of the combination of fifteen cowpea varieties (V1 (Boquinha), V2 (Ceará), V3 (Costela de Vaca), V4 (Lisão), V5 (Canário), V6 (Pingo de Ouro), V7 (Roxão), V8 (Feijão Branco), V9 (Canapu Branco), V10 (Canapu Miúdo), V11 (Ovo de Peru), V12 (Baeta), V13 (Coruja), V14 (Paulistinha), and V15 (Sempre Verde)) with two levels of salinity of irrigation water (0.5 dS m−1 and 4.5 dS m−1), with five replicates.
The seeds used were acquired from collections from Traditional Seed Guardians belonging to rural communities located in municipalities of the western region of the Rio Grande do Norte state. The seeds came from the 2018 season and were stored in PET bottles, which were sealed to avoid any change in the degree of moisture and stored in dry, well-ventilated warehouses without the use of preservatives. The varieties used in this study were chosen based on a preliminary study conducted on the germination and initial growth stages of cowpea [
2].
4.2. Experiment Setup and Fertilization Management
Sowing was performed using 9 seeds, with the first thinning performed at 4 days after germination, leaving 3 plants per pot, and the second thinning 15 days later, leaving only one plant per pot.
Each experimental unit consisted of a plastic pot with a capacity of 12.0 L, with 1.0 L filled with crushed stone at the bottom, 1.0 L free at the top, and 10.0 L filled with soil classified as
Latossolo Vermelho Amarelo distrófico (Oxisol), sandy loam texture [
34], whose physical and chemical characteristics are presented in
Table 8.
Soil acidity was corrected with calcium hydroxide (Ca(OH)
2), with 54% calcium. The soil was corrected to increase base saturation to 90%. After 15 days, the soil was fertilized according to the recommendations of [
35] for pots in protected cultivation, applying 300 mg of P
2O
5−, 150 mg of K
2O, and 100 mg of N per dm
3 of soil through fertigation, using urea (45% of N), potassium chloride (KCl = 60% of K
2O), and monoammonium phosphate (MAP = 12% of N and 50% of P
2O
5−). Fertilization with micronutrients was performed by foliar application in pre-flowering and 15 days after flowering, with the foliar fertilizer Liqui-Plex Fruit
® in the proportion of the 3 mL L
−1 of the solution, following the manufacturer’s recommendation (
Table 9).
4.3. Saline Waters and Irrigation and Drainage Management
In the preparation of irrigation waters, local-supply water (ECw = 0.50 dS m
−1) was used for the lowest level of salinity. For the highest level of salinity (ECw = 4.50 dS m
−1), local-supply water was mixed with reject brine from brackish water desalination (ECw = 9.50 dS m
−1). The desalination reject brine was obtained at the Jurema Settlement, located beside the RN-013 highway, km 4 (
Table 10). Local supply water and brine tailings were stored in water tanks with a volume of 2000 L. We monitored the electrical conductivity during mixing with a portable conductivity meter.
Irrigation management was based on the drainage lysimeter method to leave the soil with moisture close to the maximum retention capacity, and irrigations were performed once a day, applying a leaching fraction (LF) of 15% every seven days along with the applied depth. The volume applied (Va) per container was obtained by the difference between the previous depth applied (La) minus the mean drainage (D), divided by the number of containers (n), as indicated in Equation (1):
The irrigation system comprised a self-venting Metalcorte/Eberle circulation motor pump, driven by a single-phase motor, 210 V voltage, 60 Hz frequency, installed in a reservoir with a capacity of 50 L and 16 mm diameter hoses with pressure-compensating drippers with a flow rate of 1.3 L h−1.
The electrical conductivity of the saturation extract (ECse) was estimated according to the methodology suggested by [
4] for medium-textured soils. For this, at 80 days after sowing, an additional leaching fraction (15%) was applied, the drained volume was collected, and the electrical conductivity of the drainage water (ECd) was measured using a benchtop conductivity meter, with the data expressed in dS m
−1 adjusted to the temperature of 25 °C. The data were applied in Equation (2):
4.4. Analysis of Gas Exchange and Chlorophyll a Fluorescence
Physiological analyses were performed during the flowering stage of the plants, at 58 days after sowing. Gas exchange was analyzed in the period from 6 to 9 a.m., with evaluations on fully expanded leaves located in the upper third of each plant, using a portable infrared gas analyzer (IRGA), LCPro
+ Portable Photosynthesis System
® (ADC BioScientific Limited, Hertfordshire, UK) with temperature control at 25 °C, irradiation of 1200 μmol photons m
−2 s
−1, and airflow of 200 mL min
−1. The quantified variables were CO
2 assimilation rate (
AN) (µmol (CO
2) m
−2 s
−1), transpiration (
E) (mmol (H
2O) m
−2 s
−1), stomatal conductance (
gs) (mol (H
2O) m
−2 s
−1), and internal CO
2 concentration (
Ci) (mol m
−2 s
−1). These data were then used to estimate the instantaneous water use efficiency (
WUEi) (
AN/
E) [(µmol (CO
2) m
−2 s
−1) (mmol (H
2O) m
−2 s
−1)
−1] and instantaneous carboxylation efficiency (
CEi) (
AN/Ci) [(µmol (CO
2) m
−2 s
−1) (mol (CO
2) m
−2 s
−1)
−1] [
6].
Immediately after gas exchange measurements, chlorophyll
a fluorescence was evaluated using the OS5p pulse-modulated fluorometer from Opti science; the Fv/Fm protocol was used for evaluations under dark conditions. Under these conditions, the following fluorescence induction variables were estimated: initial fluorescence (
Fo) (µmol (photons) m
−2 s
−1), maximum fluorescence (
Fm) (µmol (photons) m
−2 s
−1), variable fluorescence (
Fv = Fm-Fo) (µmol (photons) m
−2 s
−1), and the maximum quantum efficiency of PSII (
Fv/Fm) [
6].
The pulse-modulated fluorometer was also used to perform evaluations under light conditions, through the yield protocol. Readings were taken by applying the actinic light source with a multi-flash saturating pulse, coupled to a photosynthetically active radiation determination clip (PAR-Clip) to estimate the following variables: initial fluorescence before the saturation pulse (
F’), maximum fluorescence after adaptation to saturating light (
Fm’), electron transport rate (
ETR) (µmol (photons) m
−2 s
−1), and quantum efficiency of photosystem II (
Y(II)). With these data, the following parameters were determined: minimum fluorescence of the illuminated plant tissue (Fo’) [
36], photochemical quenching coefficient by the lake model (
qL) [
37], quantum yield of regulated photochemical quenching (
Y(NPQ)) [
37], and the quantum yield of non-regulated photochemical quenching (
Y(NO)) [
37].
4.5. Growth Analysis and Biomass Accumulation
At 58 DAP, the following parameters were determined: main branch length (MBL), using a measuring tape and measured from the plant collar to the last leaf insertion; stem diameter (SD), measured at 1.0 cm from the plant collar using a digital caliper; and the number of leaves (NL). After harvesting the pods of all varieties 80 days after sowing, the aerial part of the plants was collected and dried in an oven with forced air circulation, at a temperature of 65 °C, until reaching constant weight, to quantify the values of shoot dry mass (SDM).
4.6. Production Quantification
The pods were harvested as each traditional variety reached the phenological stage R9 (maturity stage), when the fruits were dry with the color and brightness that are characteristic of the genotype. The pods were transported to the laboratory, where the number of pods per plant (NPP), the number of seeds per pod (NSPo), the number of seeds per plant (NSPl), and production per plant (PP) (g) were counted.
4.7. Statistical Analysis
The data were subjected to analysis of variance and F-test. In cases of significant effect, the Scott–Knott test (
p < 0.05) was performed for the variety factor, and Student’s
t-test (
p < 0.05) was performed for the salinity factor, using SISVAR
® statistical analysis software [
38]. The data of shoot dry mass and grain production per plant were used to classify salinity tolerance; for this, the data were subjected to standardization, leaving mean zero (
= 0) and variance one (S
2 = 1). Subsequently, cluster analysis was performed by hierarchical method, Ward’s minimum variance, using the Euclidean distance as a measure of dissimilarity. PAST 3 free software was used for univariate and multivariate statistical analyses.