Magnesium Accumulation in Two Contrasting Varieties of Lycopersicum esculentum L. Fruits: Interaction with Calcium at Tissue Level and Implications on Quality
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
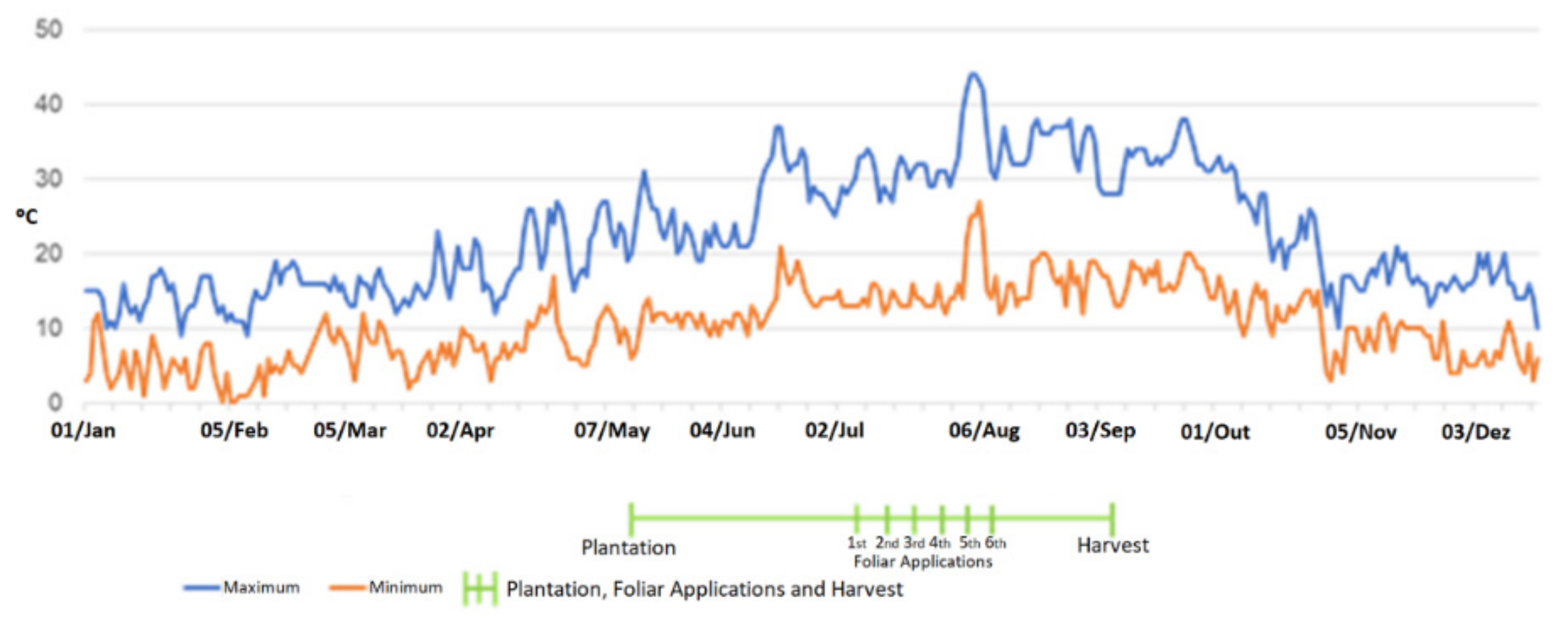
4.1. Agricultural Field and Workflow for Mg Enrichment
4.2. Orthophotomaping
4.3. Soil and Irrigation Water Analysis
4.4. Leaf Gas Exchange Measurements
4.5. Magnesium Content in Leaves and Tissue Localization
4.6. Morphometric, Colorimetric and Total Soluble Solids of Fruits
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role in magnesium fertilizers in agriculture: Plant-soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeen, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceylan, Y.; Kutman, U.B.; Mengutay, M.; Cakmak, I. Magnesium applications to growth medium and foliage the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil 2016, 406, 145–156. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Kashinath, B.L.; Ganesha Murthy, A.N.; Senthivel, T.; Pitchai, G.J.; Sadashiva, A.T. Effect of applied magnesium on yield and quality of tomato in Alfisols of Karnataka. J. Hortic. Sci. 2013, 8, 55–59. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Cakmak, I. Magnesium in crop production, food quality and human health. Plant Soil 2013, 368, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Peters, J.; Berkowitz, G. Surface Charge-Mediated Effects of Mg2+ and K+ Flux across the Chloroplast Envelope Are Associated with regulation of stromal pH and photosynthesis. Plant Physiol. 1991, 97, 580–587. [Google Scholar] [CrossRef] [Green Version]
- Hawkesford, M.J.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Pergamon: Oxford, UK, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Watkins, C.B. Bitter pit in apple fruit. Hortic. Rev. 1989, 11, 289–355. [Google Scholar]
- Nonami, H.; Fukuyama, T.; Yamamoto, M.; Yang, L.; Hashimoto, Y. Blossom-end rot of tomato plants may not be directly caused by calcium deficiency. Acta Hortic. 1995, 396, 107–114. [Google Scholar] [CrossRef]
- Ho, L.C.; White, P.J. A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann. Bot. 2005, 95, 571–581. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Ilyas, M.; Ayub, G.; Hussain, Z.; Ahmad, M.; Bibi, B.; Rashid, A.; Luqman. Response of tomato to different levels of calcium and magnesium concentration. World Appl. Sci. J. 2014, 31, 1560–1564. [Google Scholar]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water Analyses, Ground Water Note 12; US Geological Survey: Washington, DC, USA, 1953.
- Richard, L.A. Diagnosis and Improvement of Saline and Alkali Soils, Agriculture Handbook N. 60; USDA: Washington, DC, USA, 1954.
- Wilcox, L. Classification and Use of Irrigation Waters, Circular N. 969; USDA: Washington, DC, USA, 1955.
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2012, 368, 5–21. [Google Scholar] [CrossRef] [Green Version]
- DGPC—Direção-Geral de Proteção das Culturas. 2006. Available online: https://www.dgadr.gov.pt/sustentavel/producao-integrada/normas-de-prodi (accessed on 14 March 2020).
- Heinz Company. 2016. Available online: https://www.heinzseed.com/hs_about (accessed on 16 March 2020).
- El-Ramady, H.R.; Alshaal, T.A.; Amer, M.; Domokos-Szabolcsy, É.; Elhawat, N.; Prokisch, J.; Fári, M. Soil quality and plant nutrition. In Sustainable Agriculture Reviews 14: Agroecology and Global Change; Ozier-Lafontaine, H., Lesueur-Jannoyers, M., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 345–447. [Google Scholar] [CrossRef]
- Ayers, R.; Westcot, D. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985. [Google Scholar]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef] [Green Version]
- Sandei, L.; Cocconi, E.; Stingone, C.; Vitelli, R.; Bandini, M.; Sannino, A.; Savini, S.; Zanotti, A.; Zoni, C. Assessment of premium quality factors (nutritional, functional and taste) for five Italian tomato cultivars and relative diced tomatoes products. Acta Hortic. 2019, 1233, 247–254. [Google Scholar] [CrossRef]
- Jarquín-Enríquez, L.; Mercado-Silva, E.M.; Maldonado, J.L.; Lopez-Baltazar, J. Lycopene content and color index of tomatoes are affected by the greenhouse cover. Sci. Hortic. Amsterdam. 2013, 155, 43–48. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An Update on the health effects of tomato lycopene. Annu. Rev. Food Sci. T 2010, 1, 189–210. [Google Scholar] [CrossRef] [Green Version]
- Direção-Geral de Agricultura e Desenvolvimento Rural-Atlas digital de solos de Portugal Continental (1:25000). Available online: https://snisolos.dgadr.gov.pt/ (accessed on 22 March 2022).
- Direcção Geral de Agricultura Desenvolvimento Rural. Carta de Capacidade de Uso do Solo de Portugal—Bases e Normas Adoptadas na Sua Elaboração, 6th ed.; Ministério da Economia, Secretaria de Estado da Agricultura, Serviço de Reconhecimento e de Ordenamento Agrário: Lisboa, Portugal, 1972; pp. 25–26.
- Pelica, J.; Barbosa, S.; Reboredo, F.; Lidon, F.; Pessoa, F.; Calvão, T. The paradigm of high concentration of metals of natural or anthropogenic origin in soils—The case of Neves-Corvo mine area (southern Portugal). J. Geochem. Explor. 2018, 186, 12–23. [Google Scholar] [CrossRef]
- Simões, M. Métodos cromatográficos, volumétricos e potenciométricos para análise química quantitativa de água subterrânea e sua aplicação no aquífero cenozóico da Bacia do Baixo Tejo, Portugal. Geociências Rev. Unesp. São Paulo 2008, 27, 161–169. [Google Scholar]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwel, F.; Goulão, L.; et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Glob. Chang. Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, C.C.; Lidon, F.C.; Luís, I.C.; Marques, A.C.; Coelho, A.R.F.; Daccak, D.; Ramalho, J.C.; Silva, M.J.; Rodrigues, A.P.; Guerra, M.; et al. Under calcium spraying nutrients accumulation in the initial stages of fruits development is critical in “Rocha” pears. Emir. J. Food Agr. 2021, 33, 868–883. [Google Scholar] [CrossRef]
- Lidon, F.C.; Oliveira, K.; Galhano, C.; Guerra, M.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitão, A.E.; Almeida, A.S.; et al. Selenium biofortification of rice crops in contrasting genotypes through foliar application with selenite and selenate. Exp. Agr. 2018, 55, 528–542. [Google Scholar] [CrossRef]
- Cardoso, P.; Mateus, T.C.; Velu, G.; Singh, R.P.; Santos, J.P.; Carvalho, M.L.; Lourenço, V.M.; Lidon, F.; Reboredo, F.; Guerra, M. Localization and distribution of Zn and Fe in grains of biofortified bread wheat lines through micro- and triaxial—X-ray fluorescence spectrometry. Spectrochim. Acta B 2018, 141, 70–79. [Google Scholar] [CrossRef]
- Marques, A.C.; Lidon, F.C.; Coelho, A.R.F.; Pessoa, C.C.; Luís, I.C.; Scotti-Campos, P.; Simões, M.; Almeida, A.S.; Legoinha, P.; Pessoa, M.F.; et al. Quantification and tissue localization of selenium in rice (Oryza sativa L., Poaceae) grains: A perspective of agronomic biofortification. Plants 2020, 9, 1670. [Google Scholar] [CrossRef]
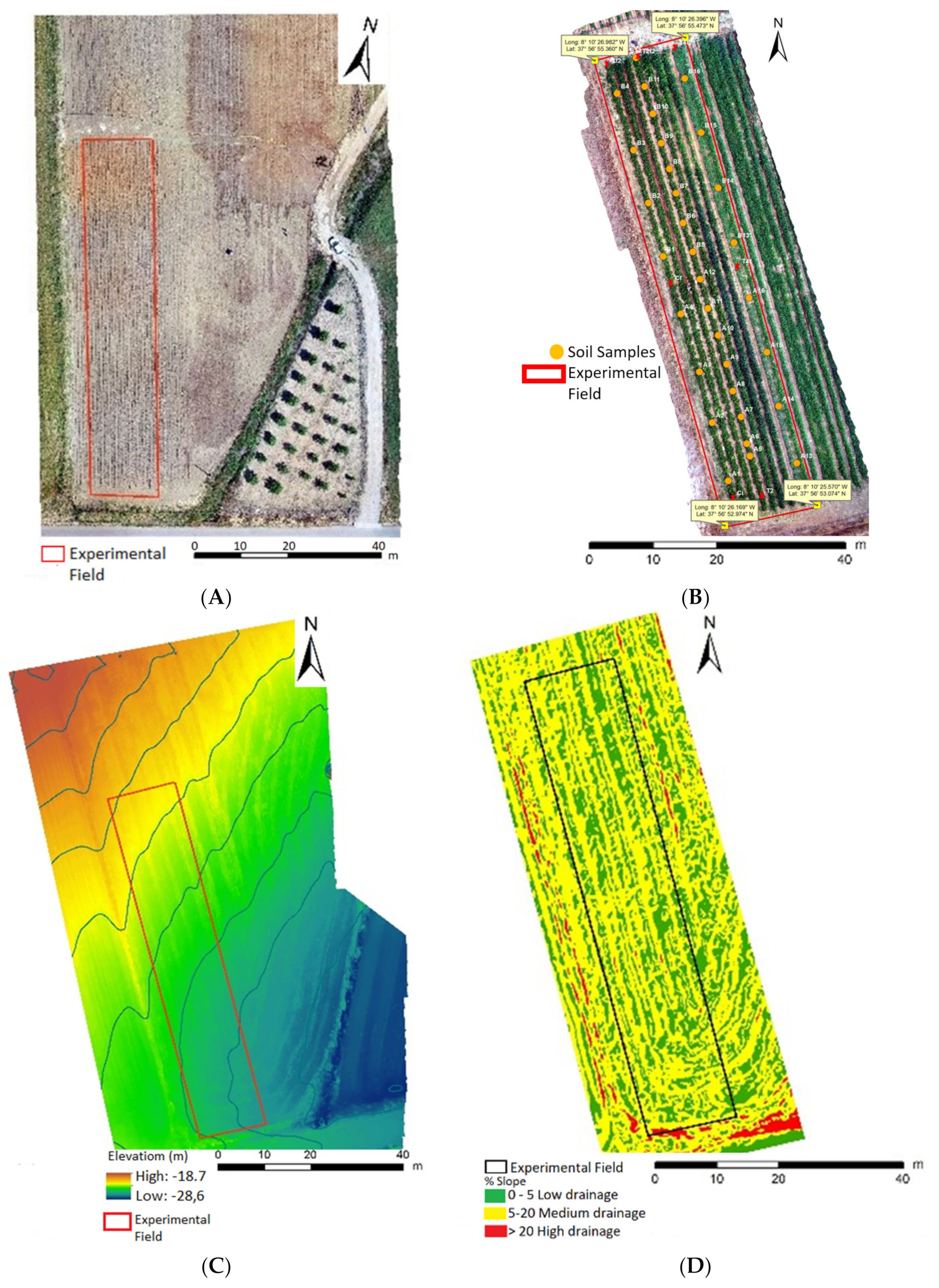
| Physical and Chemical Parameters of Soil Collected from 0–30 cm of Depth | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Electrical Conductivity | Organic Matter | Fe | K | Ca | Mg | P | Mn | S | Zn | As |
| S m −1 | % | % | ppm | ||||||||
| 7.1 | 0.0191 | 2.61 | 1.27 | 0.61 | 0.16 | 0.08 | 0.08 | 301.0 | 49.1 | 17.1 | 5.63 |
| Physical and Chemical Parameters of Irrigation Water | |||||||||||
| pH | Electrical Conductivity | Ca2+ | K+ | Mg2+ | Na+ | Cl− | HCO3− | SO42− | NO3− | PO43− | |
| S m −1 | mg L−1 (meq L−1) | ||||||||||
| 7.1 | 0.0886 | 58.9 (2.9) | 5.9 (0.1) | 34.7 (2.8) | 48.6 (2.1) | 77.1 (2.2) | 225.0 (3.6) | 78.0 (1.6) | 0.2 (0.004) | <1.5 (<0.04) | |
| Area of Agricultural Parcel Under Effect of High or Low Water Accumulation | |||||||||||
| Slope Classes (%) | Drainage Type | Partial Area (m2) | Partial Area (%) | ||||||||
| 1− [0–5%] | Low | 391.7 | 35.6 | ||||||||
| 2− [5–20%] | Medium | 705.5 | 64.0 | ||||||||
| 3− >20% | High | 4.5 | 0.41 | ||||||||
| Treatments | Date of Measured Parameters, 24 July 2018 | |||||||
|---|---|---|---|---|---|---|---|---|
| H1534 | H9205 | |||||||
| Pn (µmol CO2 m−2 s−1) | ||||||||
| Ctr | 15.26 | ± | 1.44 | a | 18.51 | ± | 1.56 | a |
| 0.25% | 13.08 | ± | 1.45 | a | 14.60 | ± | 0.67 | ab |
| 1% | 15.16 | ± | 1.50 | a | 11.60 | ± | 1.37 | b |
| 4% | 13.72 | ± | 1.25 | a | 10.05 | ± | 1.62 | b |
| gs (mmol H2O m−2 s−1) | ||||||||
| Ctr | 264.6 | ± | 21.9 | a | 300.5 | ± | 28.3 | a |
| 0.25% | 298.6 | ± | 9.2 | a | 309.5 | ± | 36.7 | a |
| 1% | 241.7 | ± | 19.7 | a | 191.1 | ± | 23.1 | b |
| 4% | 241.5 | ± | 42.4 | a | 169.7 | ± | 35.1 | b |
| Ci (ppm) | ||||||||
| Ctr | 199.7 | ± | 5.9 | ab | 186.4 | ± | 8.2 | a |
| 0.25% | 211.2 | ± | 7.4 | a | 183.1 | ± | 6.4 | a |
| 1% | 196.8 | ± | 2.0 | ab | 184.8 | ± | 3.6 | a |
| 4% | 158.4 | ± | 24.0 | b | 193.6 | ± | 2.8 | a |
| E (mmol H2O m−2 s−1) | ||||||||
| Ctr | 4.20 | ± | 0.24 | a | 4.63 | ± | 0.23 | a |
| 0.25% | 4.25 | ± | 0.18 | a | 3.97 | ± | 0.14 | ab |
| 1% | 4.00 | ± | 0.13 | a | 3.57 | ± | 0.18 | b |
| 4% | 3.76 | ± | 0.21 | a | 3.36 | ± | 0.16 | b |
| iWUE (mmol CO2 mol−1 H2O) | ||||||||
| Ctr | 3.58 | ± | 0.19 | a | 3.94 | ± | 0.21 | a |
| 0.25% | 3.00 | ± | 0.24 | a | 3.78 | ± | 0.28 | a |
| 1% | 3.76 | ± | 0.34 | a | 3.23 | ± | 0.29 | a |
| 4% | 3.85 | ± | 0.51 | a | 3.90 | ± | 0.31 | a |
| Treatments | Mg Contents in Leaves (% ± SE) | Mg Contents in Whole Fruits (ppm ± SE) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variety H1534 | Variety H9205 | Variety H1534 | Variety H9205 | |||||
| Crt | 0.31 a | ±0.05 | 0.39 a | ±0.00 | 404 b | ±9 | 412 a | ±18 |
| 0.25% | 0.37 a | ±0.08 | 0.33 a | ±0.00 | 435 ab | ±0 | 412 a | ±12 |
| 1% | 0.39 a | ±0.09 | 0.34 a | ±0.01 | 437 ab | ±10 | 399 a | ±3 |
| 4% | 0.39 a | ±0.03 | 0.35 a | ±0.03 | 478 a | ±12 | 394 a | ±7 |
| Location of the 2 Transverse Sections in the Longitudinal Section of Tomato Fruit | Ratio of Mg/Ca in the Transverse Section of the Fruit | |
|---|---|---|
| Mg/Ca | Zones | |
| H1534 | ||
 |  |  |
| H9205 | ||
 |  |  |
| Fruit Segments in the Transverse Section | Macroscopic Visualization of Fruit Segments in the Longitudinal Sections | Fruit Segments in the Transverse Section | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H1534 | ||||||||||
| 0% | 4% | |||||||||
 |  |  | ||||||||
| Average values of Mg contents ± SE of each section | Average values of Mg contents ± SE of each section | |||||||||
| 0.74 ± 0.04 | 0.00 ± 0.00 | 0.45 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.10 ± 0.00 | 2.73 ± 0.14 | 2.78 ± 0.14 | 0.00 ± 0.00 | 1.00 ± 0.05 | |
| H9205 | ||||||||||
| 0% | 4% | |||||||||
 |  |  | ||||||||
| Average values of Mg contents ± SE of each section | Average values of Mg contents ± SE of each section | |||||||||
| 0.00 ± 0.00 | 0.00 ± 0.00 | 1.33 ± 0.07 | 0.00 ± 0.00 | 4.76 ± 0.24 | 2.24 ± 0.11 | 4.63 ± 0.23 | 5.97 ± 0.30 | 13.1 ± 0.66 | 9.72 ± 0.49 | |
| Treatments | Height (mm) | Diameter (mm) | Dry Weight (%) | Density (kg m−3) | °Brix | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Tomato Variety H1534 | ||||||||||
| Crt | 54.3 a | ±0.9 | 48.7 a | ±1.7 | 6.5 a | ±0.3 | 1151 a | ±19 | 5.0 a | ±0.0 |
| 0.25% | 51.3 a | ±0.9 | 52.0 a | ±0.8 | 7.0 a | ±0.3 | 1178 a | ±43 | 5.9 a | ±0.1 |
| 1% | 50.0 a | ±2.1 | 48.3 a | ±2.5 | 8.0 a | ±0.4 | 1106 ab | ±6 | 5.1 a | ±0.1 |
| 4% | 50.0 a | ±1.5 | 50.3 a | ±1.2 | 7.3 a | ±0.3 | 1038 b | ±12 | 5.3 a | ±0.2 |
| Tomato Variety H9205 | ||||||||||
| Crt | 55.3 a | ±3.3 | 48.7 a | ±1.3 | 7.5 a | ±0.2 | 1052 a | ±22 | 6.2 a | ±0.1 |
| 0.25% | 54.0 a | ±3.5 | 48.3 a | ±1.3 | 8.2 a | ±0.0 | 1042 a | ±3 | 5.3 a | ±0.3 |
| 1% | 55.0 a | ±1.0 | 45.7 a | ±1.8 | 8.0 a | ±0.2 | 999 a | ±69 | 5.7 a | ±0.3 |
| 4% | 55.7 a | ±2.2 | 47.0 a | ±0.6 | 7.7 a | ±0.3 | 1088 a | ±25 | 5.4 a | ±0.2 |
| Treatments | Wavelength (nm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 450 | 500 | 550 | 570 | 600 | 650 | |||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Tomato Variety H1534 | ||||||||||||
| Crt | 567 a | ±4 | 452 a | ±5 | 706 a | ±6 | 398 a | ±7 | 659 a | ±6 | 1433 a | ±9 |
| 0.25% | 570 a | ±5 | 454 a | ±5 | 705 a | ±3 | 399 a | ±5 | 660 a | ±12 | 1412 a | ±14 |
| 1% | 564 a | ±2 | 447 a | ±2 | 701 a | ±3 | 398 a | ±6 | 674 a | ±13 | 1425 a | ±30 |
| 4% | 560 a | ±5 | 444 a | ±6 | 696 a | ±5 | 395 a | ±7 | 675 a | ±9 | 1447 a | ±6 |
| Tomato Variety H9205 | ||||||||||||
| Crt | 574 a | ±7 | 460 a | ±5 | 717 a | ±8, | 415 a | ±15 | 704 a | ±36 | 1451 a | ±30 |
| 0.25% | 577 a | ±8 | 466 a | ±11 | 734 a | ±16 | 419 a | ±15 | 699 a | ±23 | 1462 a | ±10 |
| 1% | 567 a | ±6 | 457 a | ±7 | 719 a | ±17 | 411 a | ±16 | 703 a | ±30 | 1483 a | ±20 |
| 4% | 573 a | ±2 | 460 a | ±3 | 721 a | ±6 | 419 a | ±5 | 714 a | ±13 | 1491 a | ±14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessoa, C.C.; Lidon, F.C.; Coelho, A.R.F.; Marques, A.C.; Daccak, D.; Luís, I.C.; Caleiro, J.C.; Kullberg, J.C.; Legoinha, P.; Brito, M.G.; et al. Magnesium Accumulation in Two Contrasting Varieties of Lycopersicum esculentum L. Fruits: Interaction with Calcium at Tissue Level and Implications on Quality. Plants 2022, 11, 1854. https://doi.org/10.3390/plants11141854
Pessoa CC, Lidon FC, Coelho ARF, Marques AC, Daccak D, Luís IC, Caleiro JC, Kullberg JC, Legoinha P, Brito MG, et al. Magnesium Accumulation in Two Contrasting Varieties of Lycopersicum esculentum L. Fruits: Interaction with Calcium at Tissue Level and Implications on Quality. Plants. 2022; 11(14):1854. https://doi.org/10.3390/plants11141854
Chicago/Turabian StylePessoa, Cláudia Campos, Fernando C. Lidon, Ana Rita F. Coelho, Ana Coelho Marques, Diana Daccak, Inês Carmo Luís, João Cravidão Caleiro, José Carlos Kullberg, Paulo Legoinha, Maria Graça Brito, and et al. 2022. "Magnesium Accumulation in Two Contrasting Varieties of Lycopersicum esculentum L. Fruits: Interaction with Calcium at Tissue Level and Implications on Quality" Plants 11, no. 14: 1854. https://doi.org/10.3390/plants11141854
APA StylePessoa, C. C., Lidon, F. C., Coelho, A. R. F., Marques, A. C., Daccak, D., Luís, I. C., Caleiro, J. C., Kullberg, J. C., Legoinha, P., Brito, M. G., Ramalho, J. C., Silva, M. J., Rodrigues, A. P., Guerra, M., Leitão, R. G., Campos, P. S., Pais, I. P., Semedo, J. N., Silva, M. M., ... Simões, M. (2022). Magnesium Accumulation in Two Contrasting Varieties of Lycopersicum esculentum L. Fruits: Interaction with Calcium at Tissue Level and Implications on Quality. Plants, 11(14), 1854. https://doi.org/10.3390/plants11141854



















