Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat
Abstract
1. Introduction
2. Results
2.1. Effect of SNP or Arginine on the Growth Indices of Wheat Plants Grown under Salinity Stress
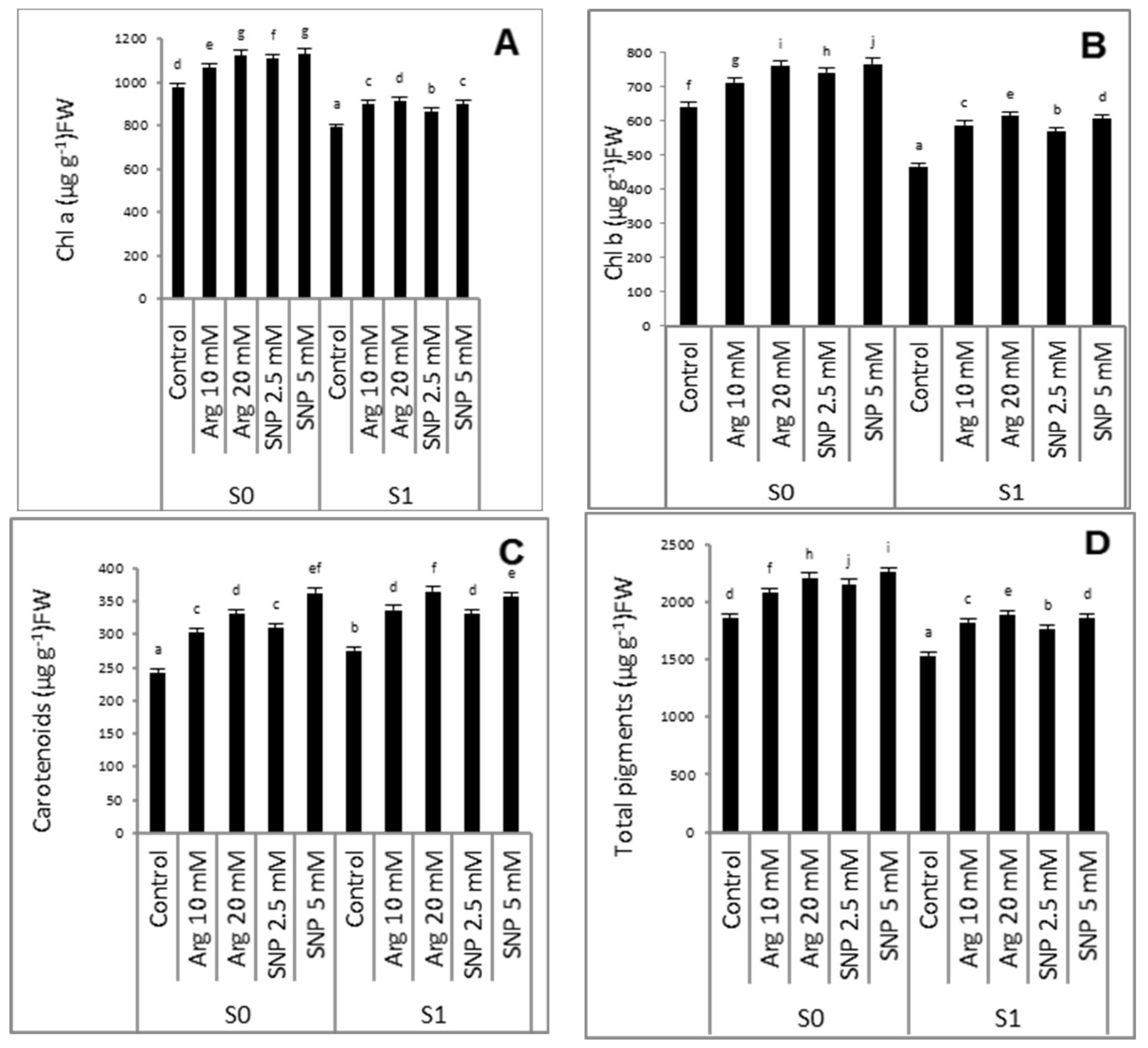
2.2. Effect of SNP or Arginine on the Photosynthetic Pigments of Wheat Plants Grown under Salinity Stress
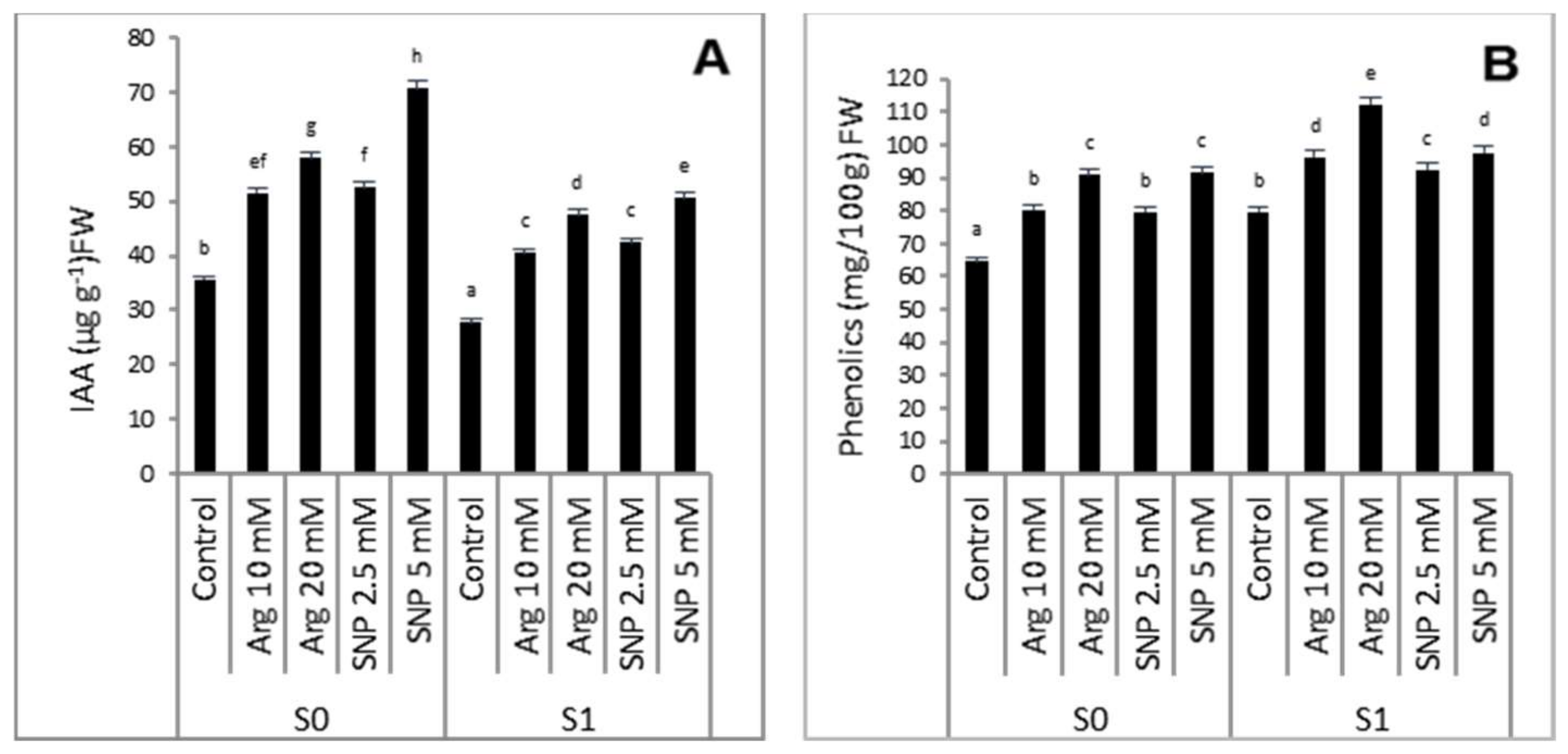
2.3. Effect of SNP or Arginine on the Endogenous Phytohormone IAA and Phenolic Contents of Wheat Plants Grown under Salinity Stress
2.4. Effect of SNP or Arginine on the Compatible Solutes of Wheat Plants Grown under Salinity Stress
2.5. Effect of SNP or Arginine on H2O2 and Lipid Peroxidation of Wheat Plants Grown under Salinity Stress
2.6. Effect of SNP or Arginine on Antioxidant Enzymes as Well as Nitrate Reductase Activity of Wheat Plants Grown under Salinity Stress
2.7. Effect of SNP or Arginine on the Yield and Yield Component Traits of Wheat Plants Grown under Salinity Stress
2.8. Effect of SNP or Arginine on Amino Acid Profile of Wheat Grains Grown under Salinity Stress
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Growth Characteristics and Yield Components Traits
4.3. Biochemical Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sofy, M.; Mohamed, H.; Dawood, M.; Abu-Elsaoud, A.; Soliman, M. Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch. Agron. Soil Sci. 2021, 21, 1–22. [Google Scholar] [CrossRef]
- Dawood, M.F.; Zaid, A.; Abdel Latef, A.A.H. Salicylic acid spraying-induced resilience strategies against the damaging impacts of drought and/or salinity stress in two varieties of Vicia faba L. Seedlings. J. Plant Growth Regul. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Inafuku, M.; Nahar, K.; Fujita, M.; Oku, H. Nitric Oxide Regulates Plant Growth; Physiology; Antioxidant Defense; and Ion Homeostasis to Confer Salt Tolerance in the Mangrove Species, Kandelia obovata. Antioxidants 2021, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Sohag, A.M.; Tahjib-Ul-Arif, M.; Latef, A.A.H.A. Hydrogen sulfide priming can enhance the tolerance of artichoke seedlings to individual and combined saline-alkaline and aniline stresses. Plant Physiol. Biochem. 2021, 159, 347–362. [Google Scholar] [CrossRef]
- Mahmoud, M.M.R. Ameliorative effect of salicylic acid on growth, minerals and nitrogenous compounds of Vicia faba L. plants under salt stress. Egypt. J. Bot. 2017, 57, 11–29. [Google Scholar] [CrossRef]
- Sadak, M.S. Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull. Natl. Res. Cent. 2019, 43, 53. [Google Scholar] [CrossRef]
- Cai, G.; Sobieszczuk-Nowicka, E.; Aloisi, I.; Fattorini, L.; Serafini-Fracassini, D.; del Duca, S. Polyamines are common players in different facets of plant programmed cell death. Amino Acids 2015, 47, 27–44. [Google Scholar] [CrossRef]
- Nayyar, H. Calcium as environmental sensor in plants. Curr. Sci. 2003, 84, 893–902. [Google Scholar]
- Rasheed, R.; Ashraf, M.A.; Ali, S.; Iqbal, M.; Zafar, S. Plant metabolism adjustment in exogenously applied NO under stress. In Nitric Oxide in Plant Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 261–296. [Google Scholar]
- Jaspers, P.; Kangasjärvi, J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant. 2010, 138, 405–413. [Google Scholar] [CrossRef]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Liu, J.H.; Nada, K.; Honda, C.; Kitashiba, H.; Wen, X.P. Polyamine biosynthesis of apple callus under salt stress. Importance of the arginine decarboxylase pathway in stress responses. J. Exp. Bot. 2006, 57, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. J. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Fan, H.F.; Guo, S.R.; Li, J.; Du, C.X.; Huang, B.J. Effects of exogenous nitric oxide on Cucumis sativus seedlings growth and osmoatic adjustment substances contents under NaCl stress. J. Chin. Ecol. 2007, 26, 2045–2050. [Google Scholar]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil Sci. Plant Nutr. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Liu, W.; Jing, Q.; Cao, W. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ. Exp. Bot. 2009, 67, 222–227. [Google Scholar] [CrossRef]
- Singh, B.K.; Singh, S.P.; Shekhawat, K.; Rathore, S.S.; Pandey, A.; Kumar, S.; Singh, K.D.; Choudhry, S.B.; Kumar, S.; Singh, D. Comparative analysis for understanding salinity tolerance mechanism in Indian mustard (Brassica juncea L.). Acta Physiol. Plant. 2019, 41, 104. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Chen, Y.; Gao, J.; Sun, Y.; Sun, M.; Chen, J. Exogenous application of gibberellic acid and ascorbic acid improved tolerance of okra seedlings to NaCl stress. Acta Physiol. Plant. 2019, 41, 93. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effect of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. J. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants; Kumar, V., Ed.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 85–136. [Google Scholar]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Nejadalimoradi, H.A.V.V.A.; Nasibi, F.A.T.E.M.E.H.; Kalantari, K.M.; Zanganeh, R.O.Y.A. Effect of seed priming with L-arginine and sodium nitroprusside on some physiological parameters and antioxidant enzymes of sunflower plants exposed to salt stress. Agric. Commun. 2014, 2, 23–30. [Google Scholar]
- Ramadan, A.A.; Abd Ebtihal, E.M.; Sadak, M.S. Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull. Natl. Res. Cent. 2019, 43, 118. [Google Scholar] [CrossRef]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 2000, 210, 215–221. [Google Scholar] [CrossRef]
- Leshem, Y.Y.; Wills, R.B.H.; Ku, V.V.V. Evidence for the function of the free radical gas-nitric oxide (NO) as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol. Biochem. 1998, 36, 825–833. [Google Scholar] [CrossRef]
- Takahashi, S.; Yamasaki, H. Reversible inhibition of photophosphorylation in chloroplasts by nitric oxide. FEBS Lett. 2002, 512, 145–148. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 1999, 208, 337–344. [Google Scholar] [CrossRef]
- Crawford, N.M.; Guo, F.Q. New insights into nitric oxide metabolism and regulatory functions. Trends Plant Sci. 2005, 10, 195–200. [Google Scholar] [CrossRef]
- Leshem, Y.; Kuiper, P. Is there a GAS (general adaptation syndrome) response to various types of environmental stress. Biol. Plant. 1996, 38, 1–18. [Google Scholar] [CrossRef]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Muday, G.K.; Lomax, T.L.; Rayle, D.L. Characterization of the growth and auxin physiology of roots of the tomato mutant; diageotropica. Planta 1995, 195, 548–553. [Google Scholar] [CrossRef]
- Beligni, M.V.; Fath, A.; Bethke, P.C.; Lamattina, L.; Jones, R.L. Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 2002, 129, 1642–1650. [Google Scholar] [CrossRef]
- Nasibi, F.; Yaghoobi, M.; Kalantari, K. Effect of exogenous arginine on alleviation of oxidative damage in tomato plant under water stress. J. Plant Interact. 2011, 6, 291–296. [Google Scholar] [CrossRef]
- Tan, J.; Zhao, H.; Hong, J.; Han, Y.; Li, H.; Zhao, W. Effects of exogenous nitric oxide on photosynthesis, antioxidant capacity and proline accumulation in wheat seedlings subjected to osmotic stress. World J. Agric. Sci. 2008, 4, 307–313. [Google Scholar]
- Mishra, P.; Bhoomika, K.; Dubey, R.S. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 2013, 250, 3–19. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant responding mechanisms to salt stress, physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Esmail, N.Y.; Hashem, H.A.; Hassanein, A.A. Effect of treatment with different concentrations of sodium nitroprusside on survival, germination, growth, photosynthetic pigments and endogenous nitric oxide content of Lupines termis L. plants. Acta Sci. Agric. 2018, 2, 48–52. [Google Scholar]
- Abd Elhamid, E.M.; Sadak, M.S.; Tawfik, M.M. Glutathione treatment alleviate salinity adverse effects on growth, some biochemical aspects, yield quantity and nutritional value of chickpea plant. Sci. Fed J. Glob. Warm. 2018, 2, 1–11. [Google Scholar]
- Silva, E.N.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Vie’gas, R.A.; Silveira, J.A. Salt stress induced damages on the photosynthesis of physic nut young plants. Sci. Agric. 2011, 68, 62–68. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res. Int. 2014, 8, 757219. [Google Scholar] [CrossRef]
- Naeem, M.S.; Warusawitharana, H.; Liu, H.; Liu, D.; Ahmad, R.; Waraich, E.A.; Xu, L.; Zhou, W. 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol. Biochem. 2012, 57, 84–92. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photo inhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Lei, Y.; Yin, C.; Ren, J.; Li, C. Effect of osmotic stress and sodium nitroprusside pretreatment on proline metabolism of wheat seedlings. Biol. Plant. 2007, 51, 386–390. [Google Scholar] [CrossRef]
- Kausar, F.; Shahbaz, M.; Ashraf, M. Protective role of foliar-applied nitric oxide in Triticum aestivum under saline stress. Turk. J. Bot. 2013, 37, 1155–1165. [Google Scholar] [CrossRef]
- Graziano, M.; Beligni, M.V.; Lamattina, L. Nitric oxide improves internal iron availability in plants. Plant Physiol. 2002, 130, 1852–1859. [Google Scholar] [CrossRef]
- Jasim, A.H.; Timmen, W.M.A.; Abid, A.S. Effect of salt stress on plant growth and free endogenous hormones of primed radish (Raphanus Sativus L.) seeds with salicylic acid. Int. J. Chem. Tech. Res. 2016, 9, 339–346. [Google Scholar]
- Bano, A.; Yasmeen, S. Role of phytohormones under induced drought stress in wheat. Pak. J. Bot. 2010, 42, 2579–2587. [Google Scholar]
- Mostafa, H.A.M.; Hassanein, R.A.; Khalil, S.I.; El-Khawas, S.A.; El-Bassiouny, H.M.S.; El-Monem, A.A. Effect of arginine or putrescine on growth, yield and yield components of late sowing Wheat. J. Appl. Sci. Res. 2010, 6, 177–183. [Google Scholar]
- Dawood, M.F.A.; Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Abdel Latef, A.A.H.; Ragaey, M.M. Mechanistic insight of allantoin in protecting tomato plants against ultraviolet C stress. Plants 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Saeidi-Sar, S.; Afshari, H.; Yaghoobi, S.R. Effects of ascorbic acid and gibberellin a on alleviation of salt stress in common bean (Phaseolus vulgaris L.) seedlings. Acta Physiol. Plant. 2013, 35, 667–677. [Google Scholar] [CrossRef]
- Abeed, A.H.; Dawood, M.F. Comparative impact of different iso-osmotic solutions on osmotic adjustment in Gossypium barbadense. Glob. Nest J. 2020, 22, 75–84. [Google Scholar]
- Polash, M.A.S.; Sakil, M.A.; Tahjib-Ul-Arif, M.; Hossain, M.A. Effect of salinity on osmolytes and relative water content of selected rice genotypes. Trop. Plant Res. 2018, 5, 227–232. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Acosta, M.; Hernandez, J.A. Effect of biostimulants on plant responses to salt stress. In Plant Tolerance to Environmental Stress; Hasanuzzaman, M., Fujita, M., Oku, H., Tofazzal Islam, M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 363–380. [Google Scholar]
- Hosseini, S.M.; Hasanloo, T.; Mohammadi, S. Physiological characteristics, antioxidant enzyme activities, and gene expression in 2 spring canola (Brassica napus L.) cultivars under drought stress conditions. Turk. J. Agric. For. 2014, 39, 413–420. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 10537. [Google Scholar] [CrossRef]
- Zamani, M.; Hakimi, M.H.; Arany, M.; Kiani, B.; Rashtian, A. The effects of salicylic Acid (SA) and sodium nitroprusside (SNP) on physical and growth characteristics of Pinus eldarica. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 31–35. [Google Scholar]
- Singh, N.B.; Yadav, K.; Amist, N. Positive effects of nitric oxide on Solanum lycopersicum. J. Plant Interact. 2016, 9, 10–18. [Google Scholar] [CrossRef]
- Gan, L.; Wu, X.; Zhong, Y. Exogenously applied nitric oxide enhances the drought tolerance in hulless barley. Plant Prod. Sci. 2015, 91, 52–56. [Google Scholar] [CrossRef]
- Zhang, X.; Azhar, G.; Rogers, S.C.; Foster, S.R.; Luo, S.; Wei, J.Y. Overexpression of p49/STRAP alters cellular cytoskeletal structure and gross anatomy in mice. BMC Cell Biol. 2014, 15, 32. [Google Scholar] [CrossRef][Green Version]
- Grun, S.; Lindermayr, C.; Sell, S.; Durner, J. Nitric oxide and gene regulation in plants. J. Exp. Bot. 2006, 57, 507–516. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O. Exogenous applied nitric oxide confers tolerance to salinity-induced oxidative stress in two maize (Zea mays L.) cultivars differing in salinity tolerance. Turk. J. Agric. For. 2015, 39, 909–919. [Google Scholar] [CrossRef]
- Wendehenne, D.; Pugin, A.; Klessig, D.; Durner, J. Nitric oxide, comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef]
- Sallam, A.; Amro, A.; Ammar, E.; Dawood, M.F.A.; Kumamaru, T.; Baenziger, P.S. Genetic diversity and genetic variation in morpho-physiological traits to improve heat tolerance in spring barley. Mol. Biol. Rep. 2018, 45, 2441–2453. [Google Scholar] [CrossRef]
- Anjum, F.; Yaseen, M.; Rasul, E.; Wahid, A.; Anjum, S. Water stress in barley (Hordeum vulgare L.). II. Effect on chemical composition and chlorophyll contents. Pak. J. Agric. Sci. 2003, 40, 45–49. [Google Scholar]
- Khan, H.A.; Siddique, K.H.M.; Colmer, T.D. Vegetative and reproductive growth of salt-stressed chickpea are carbon-limited, sucrose infusion at the reproductive stage improves salt tolerance. J. Exp. Bot. 2016, 68, 2001–2011. [Google Scholar] [CrossRef]
- Ali, Q.; Daud, M.K.; Haider, M.Z.; Ali, S.; Rizwan, M.; Aslam, N.; Noman, A.; Iqbal, N.; Shahzad, F.; Deeba, F.; et al. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 2017, 119, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Moursi, Y.S.; Thabet, S.G.; Amro, A.; Dawood, M.F.A.; Baenziger, P.S.; Sallam, A. Detailed Genetic Analysis for Identifying QTLs Associated with Drought Tolerance at Seed Germination and Seedling Stages in Barley. Plants 2021, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, A.J.; Pawelzik, E. Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under NaCl salinity. Food Chem. 2008, 111, 642–647. [Google Scholar] [CrossRef]
- López-Gómez, M.; Palma, F.; Lluch, C. Strategies of salt tolerance in the rhizobia-legume symbiosis. In Beneficial Plant-Microbial Interactions, Ecology and Applications; CRC Press: Boca Raton, FL, USA, 2013; pp. 99–121. [Google Scholar]
- Rokebul Anower, M.; Peel, M.; Mott, I.; Wu, Y. Physiological processes associated with salinity tolerance in an alfalfa half-sib family. J. Agron. Crop Sci. 2017, 203, 506–518. [Google Scholar] [CrossRef]
- Rao, K.M.; Raghavendra, A.S.; Reddy, K.J. Physiology and Molecular Biology of Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Bertrand, A.; Bipfubusa, M.; Dhont, C.; Chalifour, F.P.; Drouin, P.; Beauchamp, C.J. Rhizobial strains exert a major effect on the amino acid composition of alfalfa nodules under NaCl stress. Plant Physiol. Biochem. 2016, 108, 344–352. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Improving amino acid composition of soybean under salt stress by salicylic acid and jasmonic acid. J. Appl. Bot. Food Qual. 2016, 89, 243–248. [Google Scholar]
- Stroganov, B.P. Physiological Basis of the Salt Tolerance Of Plants (under Different Types of Soil Salinization); Izd. Akad. Nauk. USSR: Moscow, Russia, 1962. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids, measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- Larsen, P.; Harbo, A.; Klungron, S.; Ashein, T.A. On the biosynthesis of some indole compounds in Acetobacter xylinum. Physiol. Plant 1962, 15, 552–565. [Google Scholar] [CrossRef]
- Danil, A.D.; George, C.M. Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. J. Am. Soc. Hortic. Sci. 1972, 17, 621–624. [Google Scholar]
- Kalsoom, U.; Bennett, I.J.; Boyce, M.C. A Review of Extraction and Analysis, Methods for Studying Osmoregulants in Plants. J. Chromatogr. Sep. Tech. 2016, 7, 1–11. [Google Scholar]
- Sorrequieta, A.; Ferraro, S.; Boggio, S.B.; Valle, E.M. Free amino acid production during tomato fruit ripening, a focus on L-glutamate. Amino Acids 2009, 38, 1523–1532. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldan, R.P.; Teare, L.D. Rapid determination of free proline under water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhausser, S.M.A. method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Yu, C.W.; Murph, T.M.; Lin, C.H. Hydrogen peroxide-induces chilling tolerance in mung bean mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Hodges, D.M.; J De Long, M.; Forney, C.; Prange, P.K. Improving the thiobarbaturic acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Chen, J.X.; Wang, X.F. Plant Physiology Experimental Guide; Higher Education Press: Beijing, China, 2006; pp. 24–25; 55–56. [Google Scholar]
- Kong, F.X.; Hu, W.; Chao, W.L.; Sang, W.L.; Wang, L.S. Physiological responses of Mexicana to oxidative stress of SO2. Environ. Exp. Bot. 1999, 42, 201–209. [Google Scholar] [CrossRef]
- Kumar, K.B.; Khan, P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Foyer, C.H.; Valadier, M.H.; Migge, A.; Becker, T.H. Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiol. 1998, 117, 283–292. [Google Scholar] [CrossRef]
- Jaworski, E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef]
- Gehrke, C.W.; Wall, L.L.; Absheer, J.S.; Kaiser, F.E.; Zumwolt, R.W. Sample preparation for chromatography of amino acids, acid hydrolysis of proteins. J. Assoc. Off. Anal. Chem. 1985, 68, 811–816. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons: Hoboken, NJ, USA, 1984; p. 460. [Google Scholar]

| Salinity | Treatments | Shoot Length (cm) | Leaves No/tiller | Flag Leaf Area (cm2) | Tillers Fresh Weight (g) | Tillers Dry Weight (g) | Root Length (cm) | Root Fresh WT (g) | Root Dry WT (g) |
|---|---|---|---|---|---|---|---|---|---|
| S0 | Control | 56.3 c ± 1.45 | 4.7 bc ± 0.33 | 30.5 b ± 0.14 | 5.6 c ± 0.12 | 1.8 b ± 0.05 | 13.7 b ± 0.33 | 1.8 b ± 0.05 | 0.92 b ± 0.02 |
| Arg 10 mM | 63.3 d ± 0.88 | 5.3 bc ± 0.33 | 36.9 de ± 0.96 | 6.6 e ± 0.15 | 2.1 cd ± 0.06 | 16.3 de ± 0.33 | 2.1 cd ± 0.06 | 1.10 d ± 0.02 | |
| Arg 20 mM | 70.3 e ± 1.20 | 5.7 bc ± 0.33 | 42.6 f ± 0.79 | 8.1 f ± 0.20 | 2.7 e ± 0.03 | 17.3 e ± 0.88 | 2.7 e ± 0.03 | 1.28 e ± 0.00 | |
| SNP 2.5 mM | 63.0 d ± 0.57 | 5.7 bc ± 0.33 | 35.4 d ± 0.26 | 6.1 d ± 0.09 | 2.3 d ± 0.04 | 16.7 de ± 0.33 | 2.3 d ± 0.04 | 1.12 d ± 0.031 | |
| SNP 5.0 mM | 70.0 e ± 1.00 | 6.0 c ± 0.00 | 42.2 f ± 0.41 | 8.3 f ± 0.11 | 2.8 e ± 0.05 | 20.0 f ± 0.57 | 2.8 e ± 0.05 | 1.26 e ± 0.01 | |
| S1 | Control | 43.7 a ± 0.67 | 4.3 a ± 0.33 | 24.2 a ± 0.38 | 4.1 a ± 0.05 | 1.1 a ± 0.06 | 11.7 a ± 0.33 | 1.1 a ± 0.06 | 0.84 a ± 0.02 |
| Arg 10 mM | 51.7 b ± 0.33 | 4.7 ab ± 0.33 | 31.8 bc ± 0.38 | 5.2 b ± 0.04 | 1.7 b ± 0.023 | 13.3 b ± 0.33 | 1.7 b ± 0.023 | 0.95 bc ± 0.018 | |
| Arg 20 mM | 59.0 c ± 1.00 | 5.0 ab ± 0.00 | 37.5 e ± 0.93 | 5.8 c ± 0.08 | 2.0 c ± 0.052 | 15.3 cd ± 0.66 | 2.0 c ± 0.052 | 1.03 c ± 0.03 | |
| SNP 2.5 mM | 51.0 b ± 1.15 | 5.3 bc ± 0.33 | 32.9 c ± 0.27 | 4.9 b ± 0.10 | 1.8 b ± 0.06 | 14.0 bc ± 0.57 | 1.8 b ± 0.06 | 0.97 bc ± 0.03 | |
| SNP 5.0 mM | 58.7 c ± 0.33 | 5.3 bc ± 0.33 | 38.5 e ±0.66 | 5.8 c ± 0.0 | 2.1 cd ± 0.04 | 16.3 de ± 0.33 | 2.1 cd ± 0.04 | 1.12 d ± 0.03 |
| Salinity | Treatments | Free Amino Acids | Proline | TSS |
|---|---|---|---|---|
| S0 | Control | 137.15 a ± 0.86 | 23.83 a ± 0.10 | 21.55 a ± 0.11 |
| Arg 10 mM | 152.84 c ± 0.28 | 36.25 c ± 0.23 | 23.44 b ± 0.05 | |
| Arg 20 mM | 160.15 d ± 1.44 | 49.22 e ± 0.2 4 | 27.30 d ± 0.19 | |
| SNP 2.5 mM | 147.92 b ± 0.92 | 33.93 b ± 0.13 | 25.66 c ± 0.008 | |
| SNP 5.0 mM | 154.83 c ± 1.43 | 42.43 d ± 0.62 | 28.90 e ± 0.14 | |
| S1 | Control | 152.59 c ± 0.14 | 37.25 c ± 0.34 | 25.56 c ± 0.049 |
| Arg 10 mM | 168.59 f ± 0.60 | 51.13 f ± 0.10 | 31.18 f ± 0.19 | |
| Arg 20 mM | 176.15 g ± 1.44 | 54.25 g ± 0.34 | 36.31 h ± 0.36 | |
| SNP 2.5 mM | 163.93 e ± 0.33 | 54.75 g ± 0.63 | 33.75 g ± 0.05 | |
| SNP 5.0 mM | 171.08 f ± 0.82 | 68.08 h ± 0.32 | 37.30 i ± 0.37 |
| Salinity | Treatments | MDA | H2O2 | CAT | SOD | POX | NR |
|---|---|---|---|---|---|---|---|
| S0 | Control | 7.66 d ± 0.00 | 5.24 c ± 0.05 | 55.83 a ± 0.28 | 38.18 a ± 0.28 | 11.75 a ± 0.057 | 320.76 d ± 0.25 |
| Arg 10 mM | 6.58 c ± 0.05 | 4.23 b ± 0.04 | 58.16 bc ± 0.85 | 43.95 b ± 0.17 | 12.97 bc ± 0.31 | 328.50 fg ± 0.66 | |
| Arg 20 mM | 6.34 bc ± 0.07 | 3.73 a ± 0.22 | 59.15 cd ± 0.28 | 46.25 c ± 0.23 | 13.15 bc ± 0.28 | 331.52 g ± 0.57 | |
| SNP 2.5 mM | 6.08 ab ± 0.03 | 4.29 b ± 0.13 | 57.40 b ± 0.14 | 45.60 c ± 0.01 | 12.53 ab ± 0.06 | 321.52 de ± 0.00 | |
| SNP 5.0 mM | 5.93 a ± 0.04 | 3.53 a ± 0.06 | 59.65 d ± 0.00 | 47.30 d ± 0.20 | 13.70 c ± 0.02 | 329.95 g ± 0.17 | |
| S1 | Control | 13.45 h ± 0.11 | 9.23 e ± 0.06 | 63.69 e ± 0.02 | 51.38 e ± 0.02 | 20.93 d ± 0.33 | 296.83 a ± 0.29 |
| Arg 10 mM | 11.6 g ± 0.14 | 6.53 d ± 0.06 | 70.45 f ± 0.46 | 60.58 f ± 0.53 | 32.23 e ± 0.41 | 306.38 b ± 3.54 | |
| Arg 20 mM | 8.40 e ± 0.14 | 5.38 c ± 0.03 | 77.25 h ± 0.80 | 72.15 h ± 0.28 | 40.58 g ± 0.53 | 318.08 d ± 1.40 | |
| SNP 2.5 mM | 10.44 f ± 0.11 | 6.28 d ± 0.07 | 73.58 g ± 0.037 | 62.88 g ± 0.21 | 34.08 f ± 0.25 | 314.08 c ± 0.90 | |
| SNP 5.0 mM | 7.65 d ± 0.17 | 5.07 c ± 0.14 | 81.27 i ± 0.14 | 75.00 i ± 0.20 | 46.25 h ± 0.23 | 325.15 ef ± 0.28 |
| Salinity | Treatments | Shoot Length (cm) | Spike Length (cm) | Spike Weight (g) | Shoot Weight (cm) | Grain Weight (g) | 1000 Kernel Weight (g) | Total Carbohydrate (%) |
|---|---|---|---|---|---|---|---|---|
| S0 | Control | 63 bc ± 0.33 | 9.33 b ± 0.33 | 2.63 c ± 0.14 | 2.18 b ± 0.04 | 1.80 bc ± 0.06 | 35.38 c ± 0.37 | 45.83 d ± 0.11 |
| Arg 10 mM | 70 d ± 1.00 | 11.66 c ± 0.33 | 3.50 e ± 0.05 | 2.59 c ± 0.01 | 2.22 d ± 0.035 | 38.50 d ± 0.19 | 46.90 f ± 0.028 | |
| Arg 20 mM | 79 e ± 0.88 | 13.33 d ± 0.33 | 3.88 f ± 0.07 | 2.64 c ± 0.03 | 2.43 e ± 0.025 | 42.97 e ± 0.79 | 48.80 g ± 0.08 | |
| SNP 2.5 mM | 76 e ± 0.66 | 14.00 d ± 0.57 | 3.20 d ± 0.04 | 2.45 c ± 0.01 | 2.28 de ± 0.06 | 39.36 d ± 0.20 | 46.98 f ± 0.02 | |
| SNP 5.0 mM | 76 e ± 2.72 | 14.33 d ± 0.33 | 4.05 f ± 0.10 | 2.90 d ± 0.14 | 2.61 f ± 0.14 | 42.57 e ± 0.49 | 48.88 g ± 0.13 | |
| S1 | Control | 52 a ± 1.00 | 8.00 a ± 0.57 | 1.97 a ± 0.07 | 1.90 a ± 0.03 | 1.16 a ± 0.03 | 29.85 a ± 0.62 | 43.45 a ± 0.11 |
| Arg 10 mM | 63 bc ± 0.88 | 9.66 b ± 0.33 | 2.33 b ± 0.04 | 2.19 b ± 0.02 | 1.75 b ± 0.03 | 32.30 b ± 0.39 | 44.90 b ± 0.13 | |
| Arg 20 mM | 66 cd ± 1.20 | 11.66 c ± 0.33 | 2.65 c ± 0.05 | 2.45 c ± 0.11 | 1.93 c ± 0.02 | 34.87 c ± 0.21 | 45.90 d ± 0.13 | |
| SNP 2.5 mM | 62 b ± 0.66 | 9.66 b ± 0.33 | 2.19 b ± 0.05 | 2.25 b ± 0.01 | 1.25 a ± 0.01 | 33.32 b ± 0.24 | 45.18 c ± 0.03 | |
| SNP 5.0 mM | 68 d ± 0.66 | 11.66 c ± 0.33 | 2.35 b ± 0.005 | 2.52 c ± 0.03 | 1.69 b ± 0.029 | 34.65 c ± 0.27 | 46.23 e ± 0.06 |
| Treatments | S0 | S1 | ||||
|---|---|---|---|---|---|---|
| Control | Arg 20 mM | SNP 5.0 mM | Control | Arg 20 mM | SNP 5.0 mM | |
| Cysteine | 14.73 a ± 0.18 | 19.25 c ± 0.23 | 19.68 c ± 0.18 | 17.52 b ± 0.20 | 20.85 d ± 0.24 | 24.65 e ± 0.23 |
| Methionine | 1.49 b ± 0.09 | 1.35 ab ± 0.06 | 1.75 c ± 0.07 | 1.81 c ± 0.086 | 1.23 a ± 0.46 | 1.35 ab ± 0.04 |
| Total sulfur AA | 16.22 a ± 0.11 | 20.60 c ± 0.14 | 21.43 d ± 0.12 | 19.33 b ± 0.17 | 22.08 e ± 0.23 | 26.00 f ± 0.19 |
| Phenylalanine | 4.39 a ± 0.017 | 6.68 c ± 0.075 | 6.27 b ± 0.75 | 7.65 d ± 0.086 | 8.95 f ± 0.11 | 8.35 e ± 0.09 |
| Tyrosine | 6.79 a ± 0.08 | 8.75 c ± 0.04 | 8.95 c ± 0.07 | 7.75 b ± 0.05 | 9.45 d ± 0.07 | 9.68 e ± 0.03 |
| Histidine | 9.52 a ± 0.05 | 13.78 c ± 0.07 | 14.65 d ± 0.11 | 9.65 a ± 0.08 | 11.68 b ± 0.06 | 11.65 b ± 0.03 |
| Tryptophan | 2.32 b ± 0.01 | 2.51 c ± 0.03 | 1.65 a ± 0.01 | 3.12 d ± 0.06 | 3.32 e ± 0.04 | 4.34 f ± 0.02 |
| Total aromatic AA | 23.02 a ± 0.17 | 31.72 c ± 0.23 | 31.52 c ± 0.26 | 28.17 b ± 0.20 | 33.40 d ± 0.26 | 34.02 d ± 0.36 |
| Threonine | 8.27 a ± 0.07 | 13.49 d ± 0.11 | 13.68 d ± 0.11 | 9.65 b ± 0.08 | 9.68 b ± 0.07 | 11.52 c ± 0.17 |
| Valine | 9.79 a ± 0.09 | 12.62 c ± 0.12 | 13.84 d ± 0.06 | 9.64 a ± 0.02 | 11.68 b ± 0.08 | 13.85 d ± 0.24 |
| Leucine | 8.46 a ± 0.02 | 16.75 d ± 0.08 | 17.65 e ± 0.07 | 8.68 a ± 0.02 | 9.68 b ± 0.13 | 9.95 c ± 0.02 |
| Isoleucine | 31.74 c ± 0.19 | 37.95 d ± 0.23 | 38.64 e ± 0.12 | 23.68 a ± 0.13 | 29.68 b ± 0.05 | 31.52 c ± 0.07 |
| Lysine | 32.34 c ± 0.12 | 45.25 e ± 0.13 | 46.95 f ± 0.17 | 27.75 a ± 0.05 | 31.52 b ± 0.11 | 33.52 d ± 0.11 |
| Essential AA | 106.00 b ± 0.42 | 147.87 e ± 0.28 | 153.43 f ± 0.31 | 98.51 a ± 0.21 | 114.10 c ± 0.34 | 121.71 d ± 0.17 |
| Aspartic acid | 13.18 c ± 0.17 | 11.85 a ± 0.12 | 14.65 d ± 0.06 | 12.65 b ± 0.08 | 15.85 e ± 0.13 | 16.38 f ± 0.08 |
| Serine | 4.63 b ± 0.06 | 8.13 d ± 0.12 | 7.68 c ± 0.15 | 3.68 a ± 0.19 | 4.84 b ± 0.08 | 4.69 b ± 0.17 |
| Glutamic acid | 37.87 c ± 0.29 | 43.49 e ± 0.17 | 43.65 e ± 0.35 | 32.68 a ± 0.11 | 35.68 b ± 0.12 | 39.68 d ± 0.28 |
| Proline | 40.26 a ± 0.25 | 51.63 c ± 0.29 | 45.65 b ± 0.36 | 59.68 d ± 0.17 | 85.65 e ± 0.28 | 96.35 f ± 0.52 |
| Glycine | 36.52 a ± 0.14 | 46.59 c ± 0.23 | 47.65 d ± 0.28 | 45.62 b ± 0.23 | 59.68 e ± 0.34 | 59.68 e ± 0.38 |
| Alanine | 14.52 d ± 0.17 | 13.85 c ± 0.07 | 15.84 e ± 0.28 | 8.65 a ± 0.11 | 11.68 b ± 0.18 | 11.68 b ± 0.28 |
| NH4 | 13.72 e ± 0.29 | 8.24 b ± 0.07 | 6.65 a ± 0.08 | 13.85 e ± 0.08 | 11.65 c ± 0.20 | 12.65 d ± 0.11 |
| Arginine | 28.35 b ± 0.17 | 32.52 d ± 0.14 | 35.68 e ± 0.07 | 23.68 a ± 0.12 | 32.68 d ± 0.28 | 29.85 c ± 0.06 |
| Non-essential AA | 184.54 a ± 0.70 | 214.29 c ± 1.1 | 212.05 c ± 0.75 | 205.20 b ± 0.63 | 258.65 d ± 1.2 | 279.78 e ± 1.4 |
| Total amino acids | 318.89 a ± 1.2 | 394.68 c ± 0.86 | 401.16 d ± 1.6 | 327.39 b ± 0.66 | 405.43 e ± 1.1 | 431.34 f ± 1.5 |
| Ess/Non Ess | 0.574 b ± 0.02 | 0.690 c ± 0.02 | 0.724 c ± 0.01 | 0.480 a ± 0.01 | 0.441 a ± 0.01 | 0.435 a ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragaey, M.M.; Sadak, M.S.; Dawood, M.F.A.; Mousa, N.H.S.; Hanafy, R.S.; Latef, A.A.H.A. Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat. Plants 2022, 11, 1786. https://doi.org/10.3390/plants11141786
Ragaey MM, Sadak MS, Dawood MFA, Mousa NHS, Hanafy RS, Latef AAHA. Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat. Plants. 2022; 11(14):1786. https://doi.org/10.3390/plants11141786
Chicago/Turabian StyleRagaey, Marwa M., Mervat Shamoon Sadak, Mona F. A. Dawood, Nermin H. S. Mousa, Rania Samy Hanafy, and Arafat Abdel Hamed Abdel Latef. 2022. "Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat" Plants 11, no. 14: 1786. https://doi.org/10.3390/plants11141786
APA StyleRagaey, M. M., Sadak, M. S., Dawood, M. F. A., Mousa, N. H. S., Hanafy, R. S., & Latef, A. A. H. A. (2022). Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat. Plants, 11(14), 1786. https://doi.org/10.3390/plants11141786







