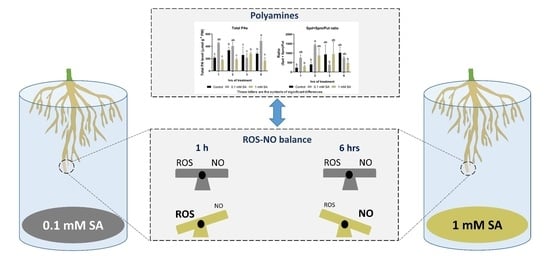
Short-Term Salicylic Acid Treatment Affects Polyamine Metabolism Causing ROS–NO Imbalance in Tomato Roots
Abstract
:1. Introduction
2. Results
2.1. Effects of SA Treatments on Free Polyamines in Tomato Roots
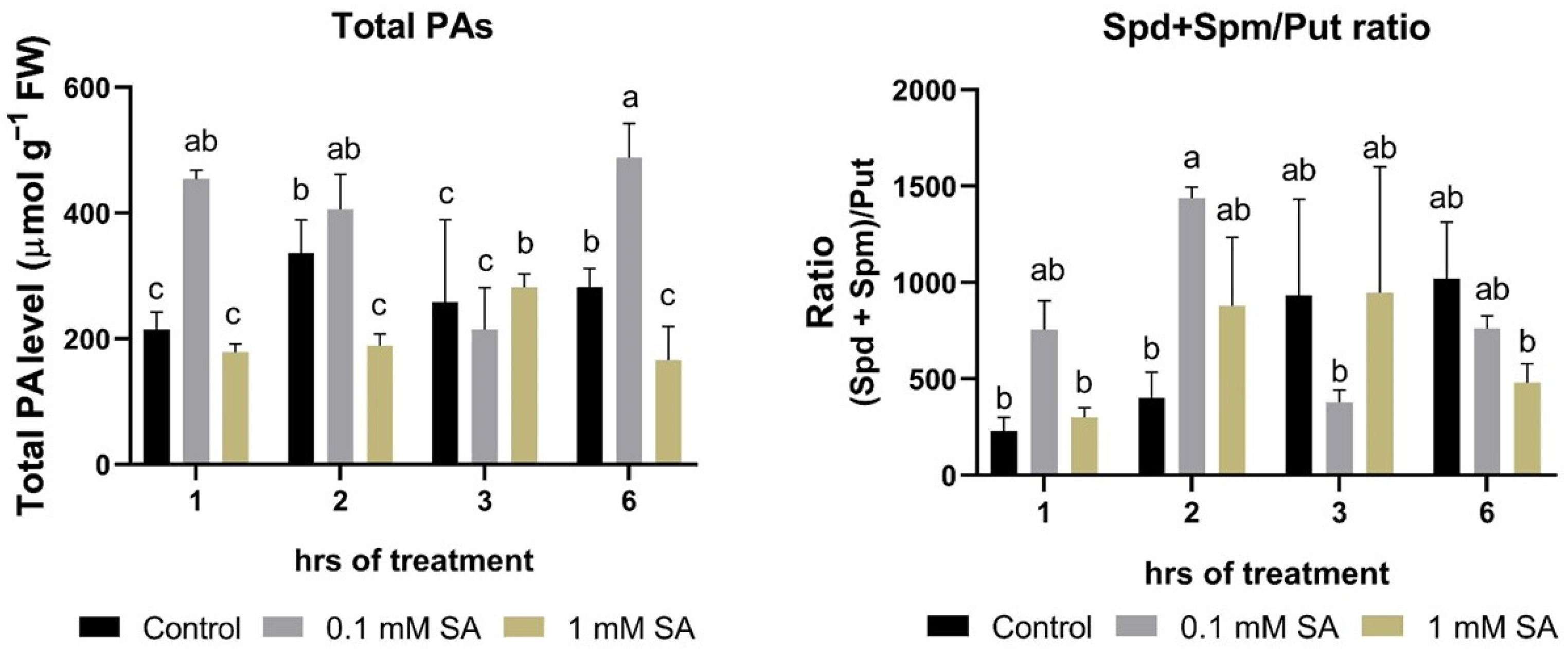
2.2. Effects of SA Treatments on Total Polyamines and Ratio of (Spd + Spm)/Put
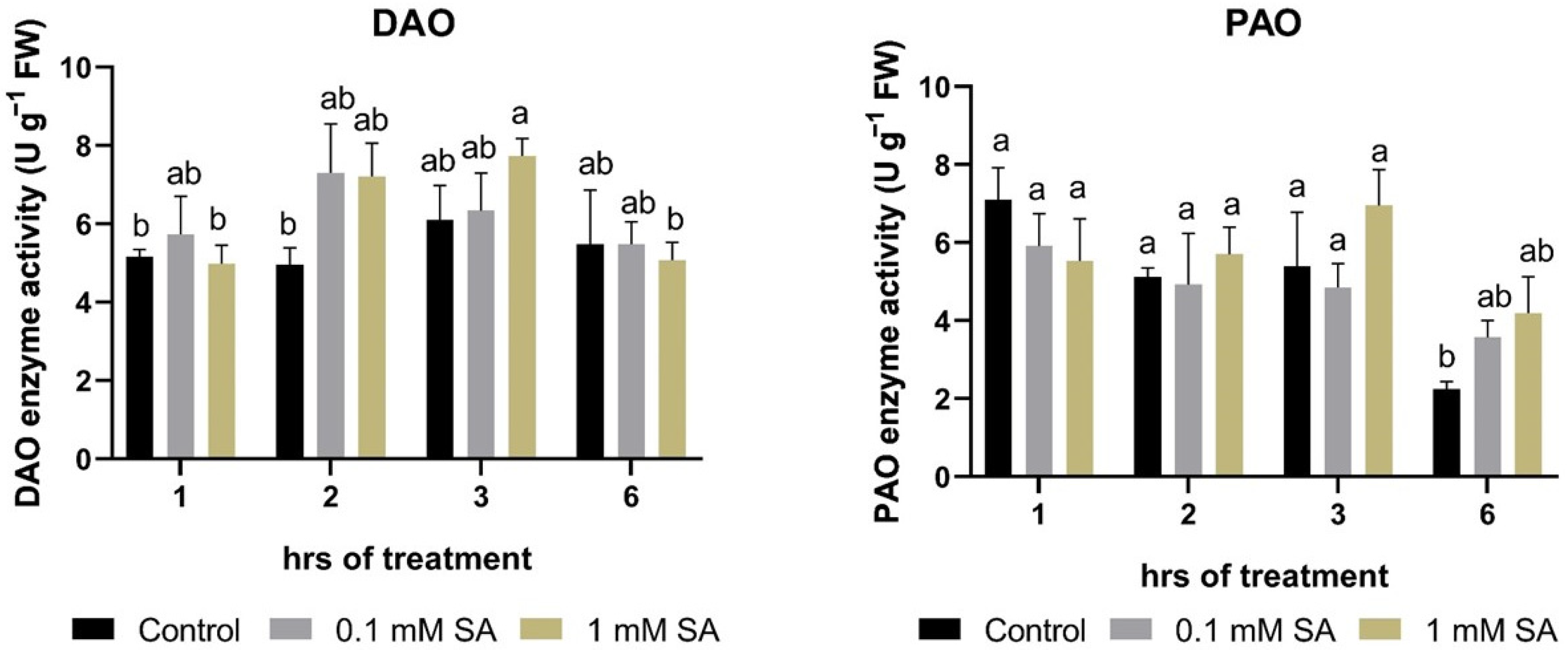
2.3. Effects of SA Treatments on Polyamine Degradation Enzymes
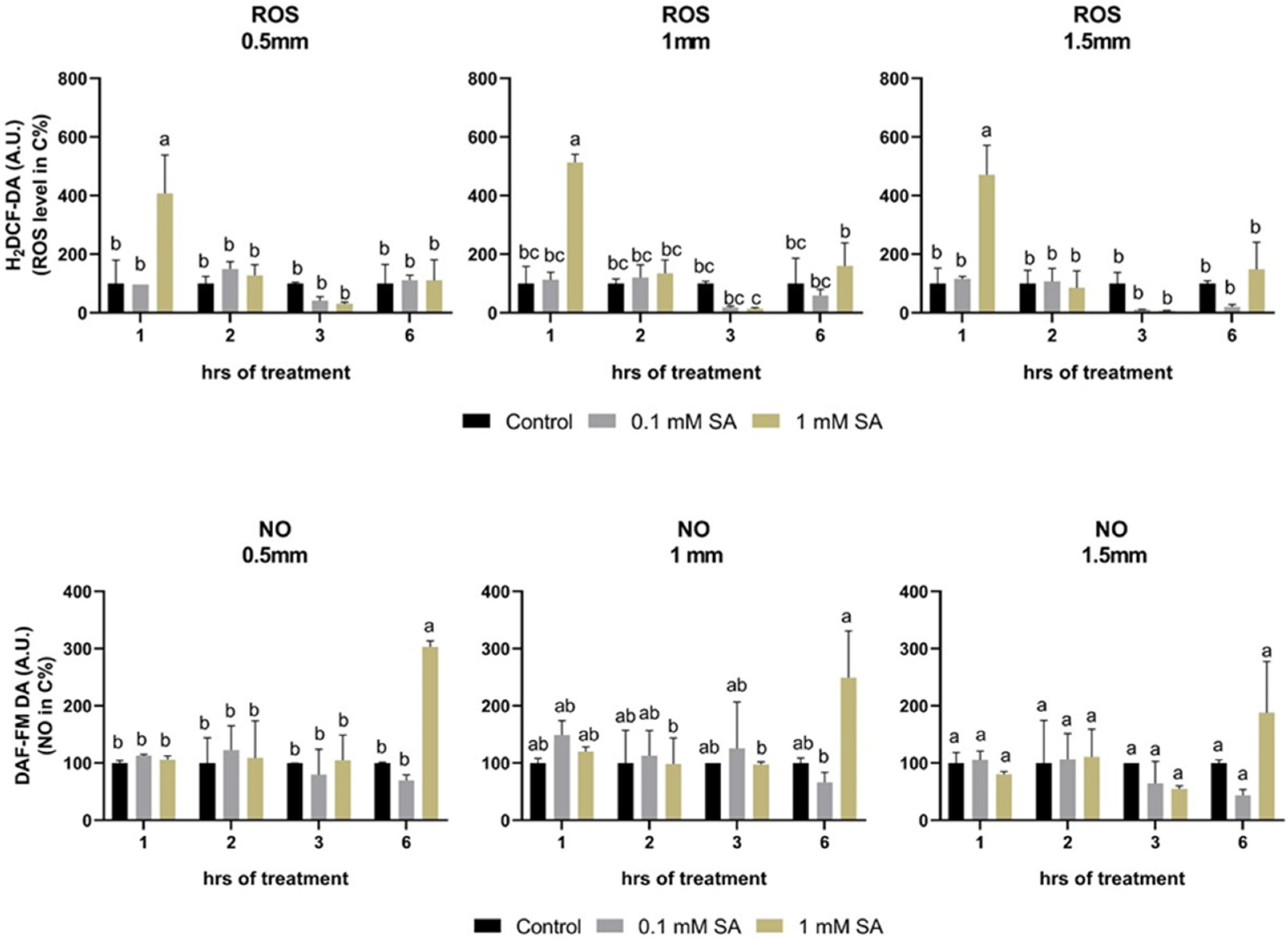
2.4. Effects of SA Treatments on ROS and NO Levels
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Analysis of Free Polyamine Levels by HPLC
4.3. Polyamine Catabolism: Diamine (DAO, EC 1.4.3.6) and Polyamine Oxidase (PAO, EC 1.4.3.4)
4.4. Microscopic Analysis of Reactive Oxygen Species and Nitric Oxide in Tomato Root Tips
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szepesi, Á.; Gémes, K.; Orosz, G.; Pető, A.; Takács, Z.; Vorák, M.; Tari, I. Interaction between salicylic acid and polyamines and their possible roles in tomato hardening processes. Acta Biol. 2011, 55, 165–166. [Google Scholar]
- Szepesi, Á. Chapter 22—Molecular Mechanisms of Polyamines-Induced Abiotic Stress Tolerance in Plants. In Approaches for Enhancing Abiotic Stress Tolerance in Plants, 1st ed.; Hasanuzzaman, M., Nahar, K., Fujita, M., Oku, H., Islam, T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; p. 18. [Google Scholar]
- Rossi, F.R.; Gárriz, A.; Marina, M.; Pieckenstain, F.L. Modulation of polyamine metabolism in Arabidopsis thaliana by salicylic acid. Physiol. Plant 2021, 173, 843–855. [Google Scholar] [CrossRef]
- Németh, M.; Janda, T.; Horváth, E.; Páldi, E.; Szalai, G. Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci. 2002, 162, 569–574. [Google Scholar] [CrossRef]
- Canales, F.J.; Montilla-Bascón, G.; Rispail, N.; Prats, E. Salicylic acid regulates polyamine biosynthesis during drought responses in oat. Plant Signal. Behav. 2019, 14, e1651183. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y. Regulation of salicylic acid on polyamine synthesize under NaCl stress in leaves of the yali pear. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 3704–3708. [Google Scholar]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Filgueiras, C.C.; Martins, A.D.; Pereira, R.V.; Willett, D.S. The Ecology of Salicylic Acid Signaling: Primary, Secondary and Tertiary Effects with Applications in Agriculture. Int. J. Mol. Sci. 2019, 20, 5851. [Google Scholar] [CrossRef] [Green Version]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef] [Green Version]
- Fraudentali, I.; Ghuge, S.A.; Carucci, A.; Tavladoraki, P.; Angelini, R.; Rodrigues-Pousada, R.A.; Cona, A. Developmental, hormone- and stress-modulated expression profiles of four members of the Arabidopsis copper-amine oxidase gene family. Plant Physiol. Biochem. 2020, 147, 141–160. [Google Scholar] [CrossRef]
- Yariuchi, Y.; Okamoto, T.; Noutoshi, Y.; Takahashi, T. Responses of Polyamine-Metabolic Genes to Polyamines and Plant Stress Hormones in Arabidopsis Seedlings. Cells 2021, 10, 3283. [Google Scholar] [CrossRef]
- Janda, T.; Szalai, G.; Pál, M. Salicylic Acid Signalling in Plants. Int. J. Mol. Sci. 2020, 21, 2655. [Google Scholar] [CrossRef] [Green Version]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Gemperlová, L.; Nováková, M.; Vaňková, R.; Eder, J.; Cvikrová, M. Diurnal changes in polyamine content, arginine and ornithine decarboxylase, and diamine oxidase in tobacco leaves. J. Exp. Bot. 2006, 57, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Paschalidis, K.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine Catabolism in Plants: A Universal Process with Diverse Functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef] [Green Version]
- Gémes, K.; Poór, P.; Horváth, E.; Kolbert, Z.; Szopkó, D.; Szepesi, Á.; Tari, I. Cross-talk between salicylic acid and NaCl-generated reactive oxygen species and nitric oxide in tomato during acclimation to high salinity. Physiol. Plant 2011, 142, 179–192. [Google Scholar] [CrossRef]
- Moschou, P.; Dellis, I.; Paschalidis, K.; Roubelakis-Angelakis, K.A. Transgenic tobacco plants over-expressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol. Plant 2008, 133, 140–156. [Google Scholar] [CrossRef]
- Fraudentali, I.; Rodrigues-Pousada, R.A.; Angelini, R.; Ghuge, S.A.; Cona, A. Plant Copper Amine Oxidases: Key Players in Hormone Signaling Leading to Stress-Induced Phenotypic Plasticity. Int. J. Mol. Sci. 2021, 22, 5136. [Google Scholar] [CrossRef]
- Jiménez-Bremont, J.F.; Chávez-Martínez, A.I.; Ortega-Amaro, M.A.; Guerrero-González, M.L.; Jasso-Robles, F.I.; Maruri-López, I.; Liu, J.H.; Gill, S.S.; Rodríguez-Kessler, M. Translational and post-translational regulation of polyamine metabolic enzymes in plants. J. Biotechnol. 2022, 344, 1–10. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Scalschi, L.; Vicedo, B.; Marcos-Barbero, E.L.; Morcuende, R.; Camañes, G. Putrescine: A Key Metabolite Involved in Plant Development, Tolerance and Resistance Responses to Stress. Int. J. Mol. Sci. 2022, 23, 2971. [Google Scholar] [CrossRef]
- Recalde, L.; Gómez Mansur, N.M.; Cabrera, A.V.; Matayoshi, C.L.; Gallego, S.M.; Groppa, M.D.; Benavides, M.P. Unravelling ties in the nitrogen network: Polyamines and nitric oxide emerging as essential players in signalling roadway. Ann. Appl. Biol. 2021, 178, 192–208. [Google Scholar] [CrossRef]
- Szepesi, A.; Csiszár, J.; Gémes, K.; Horváth, E.; Horváth, F.; Simon, M.L.; Tari, I. Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L. J. Plant Physiol. 2009, 166, 914–925. [Google Scholar] [CrossRef]
- Szepesi, Á.; Bakacsy, L.; Kovács, H.; Szilágyi, Á.; Köhler, Z.M. Inhibiting Copper Amine Oxidase Using L-Aminoguanidine Induces Cultivar and Age-Dependent Alterations of Polyamine Catabolism in Tomato Seedlings. Agriculture 2022, 12, 274. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szepesi, Á.; Poór, P.; Bakacsy, L. Short-Term Salicylic Acid Treatment Affects Polyamine Metabolism Causing ROS–NO Imbalance in Tomato Roots. Plants 2022, 11, 1670. https://doi.org/10.3390/plants11131670
Szepesi Á, Poór P, Bakacsy L. Short-Term Salicylic Acid Treatment Affects Polyamine Metabolism Causing ROS–NO Imbalance in Tomato Roots. Plants. 2022; 11(13):1670. https://doi.org/10.3390/plants11131670
Chicago/Turabian StyleSzepesi, Ágnes, Péter Poór, and László Bakacsy. 2022. "Short-Term Salicylic Acid Treatment Affects Polyamine Metabolism Causing ROS–NO Imbalance in Tomato Roots" Plants 11, no. 13: 1670. https://doi.org/10.3390/plants11131670
APA StyleSzepesi, Á., Poór, P., & Bakacsy, L. (2022). Short-Term Salicylic Acid Treatment Affects Polyamine Metabolism Causing ROS–NO Imbalance in Tomato Roots. Plants, 11(13), 1670. https://doi.org/10.3390/plants11131670