Compost Addition Attenuates the Negative Impacts of High Soil Mineral Nitrogen Levels on Rhizosphere Microbial Characteristics and Enhances Cucumber Growth in Monoculture Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Description and Experiment Design
2.2. DNA Extraction and Quantitative PCR
2.3. Nitrogen Mineralization
2.4. Statistical Analysis
3. Results
3.1. Mineral N content
3.2. Microbial Numbers
3.3. Nitrogen Cycling Genes
3.4. Nitrogen Mineralization
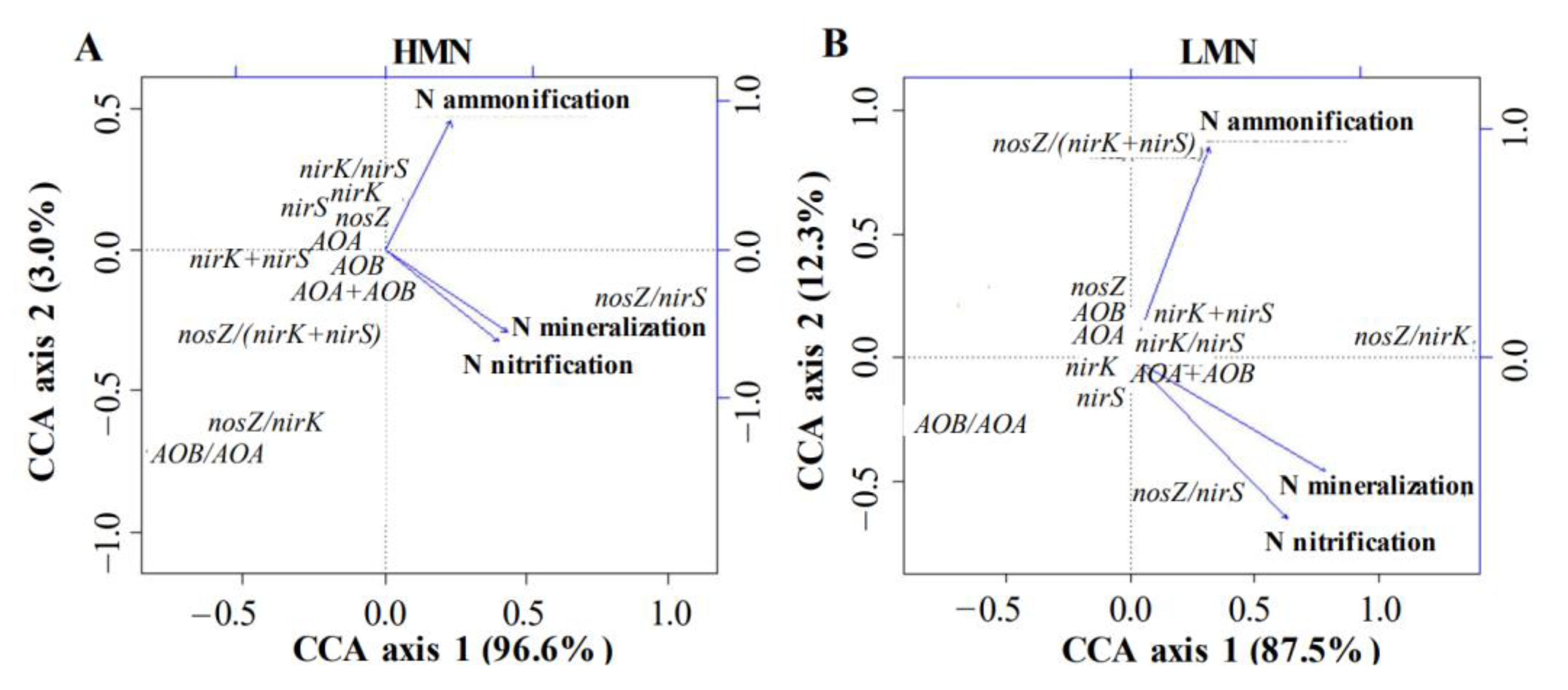
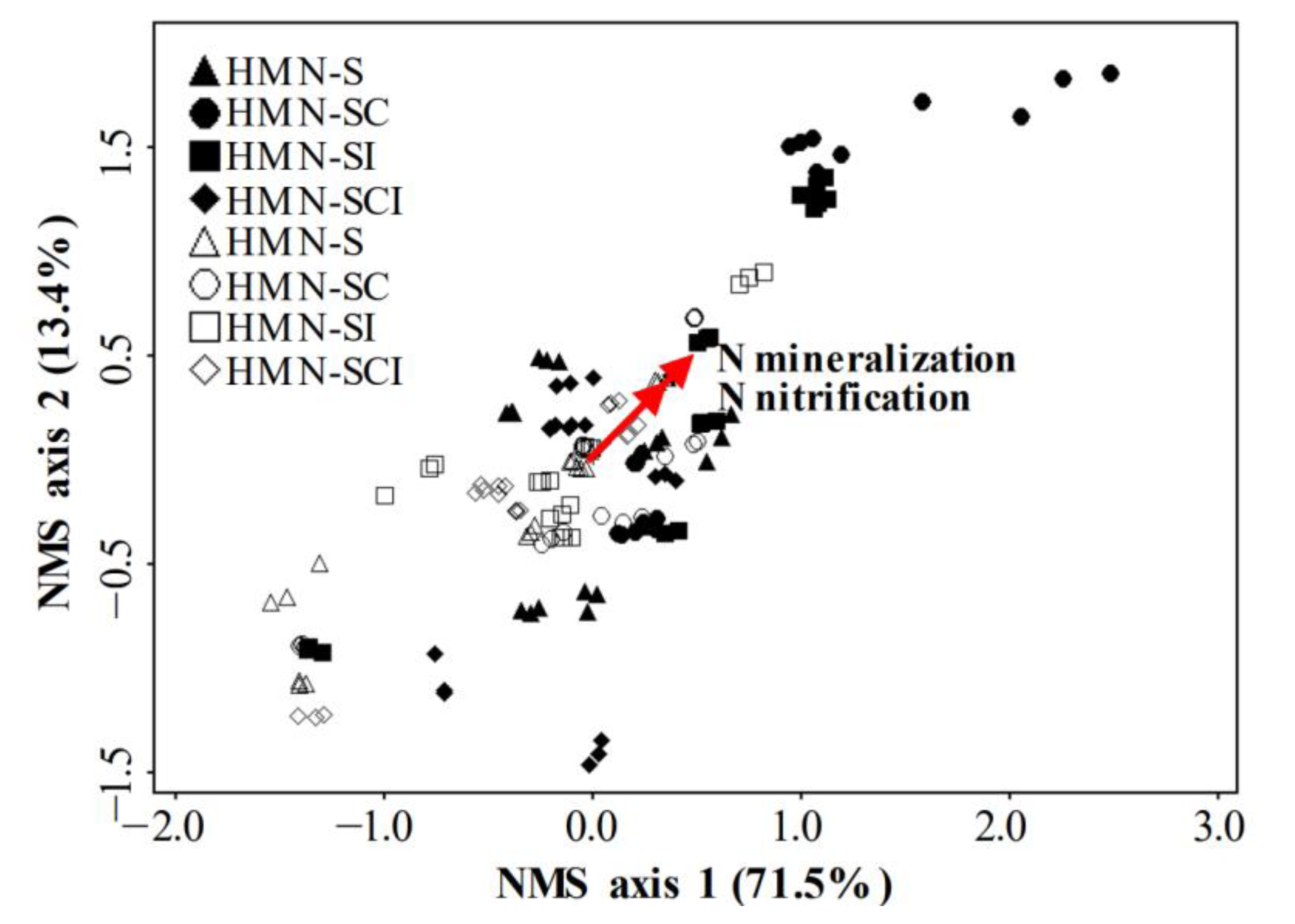
3.5. Relationships between N Cycling Genes and N Mineralization
3.6. Effects of Soil Mineral N Levels and Soil Amendments on N Cycling
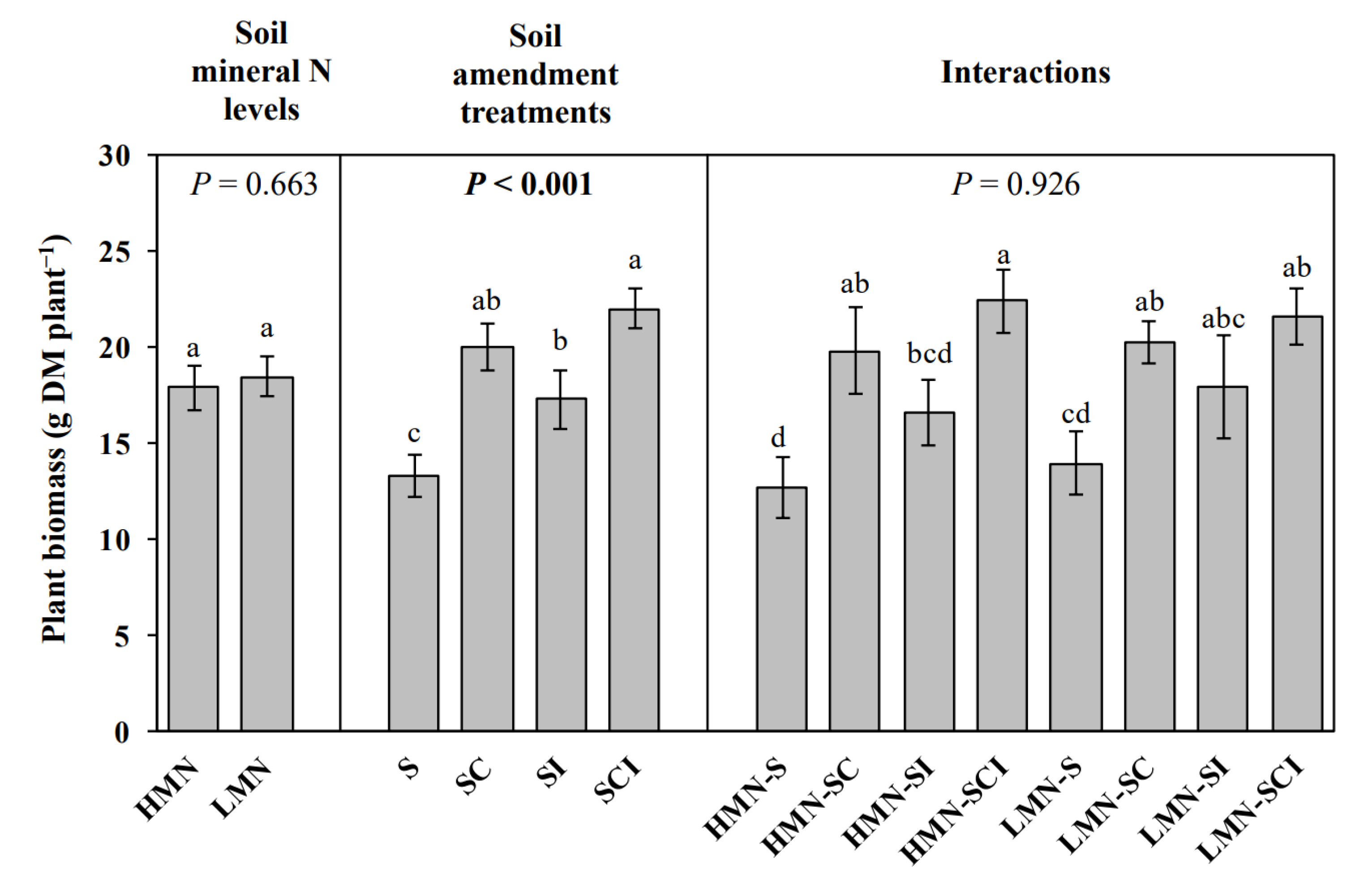
3.7. Plant Biomass
4. Discussion
4.1. Soil Mineral N Level and Microbial Population
4.2. Soil Mineral N Level and N Cycling
4.3. Soil Amendment and N Cycling
4.4. The Responses of Plant Biomass to Mineral N Level, Amendments and N Cycling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rve. 2012, 87, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Ramankutty, N.; Muelle, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nature Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, X.; Christie, P.; Chen, Q.; Jiang, R.; Zhang, F. Influence of root zone nitrogen management and a summer catch crop on cucumber yield and soil mineral nitrogen dynamics in intensive production systems. Plant Soil 2008, 313, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Chalhoub, M.; Garnier, P.; Coquet, Y.; Mary, B.; Lafolie, F.; Houot, S. Increased nitrogen availability in soil after repeated compost applications: Use of the PASTIS model to separate short and long-term effects. Soil Biol. Biochem. 2013, 65, 144–157. [Google Scholar] [CrossRef] [Green Version]
- Hadar, Y. Suppressive compost: When plant pathology met microbial ecology. Phytoparasitica 2011, 39, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Sant, D.; Casanova, E.; Segarra, G.; Avilés, M.; Reis, M.; Trillas, M.I. Effect of Trichoderma asperellum strain T34 on Fusarium wilt and water usage in carnation grown on compost-based growth medium. Biol. Control 2010, 53, 291–296. [Google Scholar] [CrossRef]
- Pardo, L.H.; Templer, P.H.; Goodale, C.L.; Duke, S.; Groffman, P.M.; Adams, M.B. Regional assessment of N saturation using foliar and root δ15 N. Biogeochemistry 2006, 80, 143–171. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, J.; Zhang, X.; Gao, L. Effects of summer catch crop, residue management, soil temperature and water on the succeeding cucumber rhizosphere nitrogen mineralization in intensive production systems. Nut. Cycl. Agroecosyst. 2010, 88, 429–446. [Google Scholar] [CrossRef]
- Wankel, S.D.; Mosier, A.C.; Hansel, C.M.; Paytan, A.; Francis, C.A. Spatial variability in nitrification rates and ammonia-oxidizing microbial communities in the agriculturally impacted Elkhorn Slough estuary, California. Appl. Environ. Microbiol. 2011, 77, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, D.G.; Blazewicz, S.J.; Firestone, M.; Herman, D.J.; Turetsky, M.; Waldrop, M. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 2012, 14, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agri. 1973, 24, 1085–11090. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis. Part 3-Chemical Methods; Sparks, D.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fert. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Government Printing Office, USDA: Washington, DC, USA, 1954; Volume 939.
- Sparks, D.L.; Huang, P.M. Physical chemistry of soil potassium. In Potassium in Agriculture; Munson, R.E., Ed.; ASA, CSSA and SSSA: Madison, WI, USA, 1985; pp. 201–276. [Google Scholar]
- Chung, S.; Kong, H.; Buyer, J.S.; Lakshman, D.K.; Lydon, J.; Kim, S.D.; Roberts, D.P. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Appl. Microbiol. Biotechnol. 2008, 280, 115–123. [Google Scholar] [CrossRef]
- Chen, X.P.; Zhu, Y.G.; Xia, Y.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea: Important players in paddy rhizosphere soil? Environ. Microbiol. 2008, 10, 1978–1987. [Google Scholar] [CrossRef]
- Robertson, G.P.; Wedin, D.; Groffman, P.M.; Blair, J.M.; Holland, E.A.; Nadelhoffer, K.J.; Harris, D. Soil carbon and nitrogen availability. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Bledsoe, C.S., Coleman, D.C., Sollins, P., Eds.; Oxford University Press: New York, NY, USA, 1999; pp. 258–265. [Google Scholar]
- Ter Braak, C.J. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B. Package ‘Vegan’, Community Ecology Package. Version 2.0-8. Available online: http://cran.r-project.org (accessed on 18 October 2013).
- Grandin, U. PC-ORD version 5: A user-friendly toolbox for ecologists. J. Veg. Sci. 2006, 17, 843–844. [Google Scholar] [CrossRef]
- Brockett, B.F.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Huber, D.M.; Watson, R.D. Nitrogen form and plant disease. Annu. Rev. Phytopathol. 1974, 12, 139–165. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C. Plant disease severity in high-input compared to reduced-input and organic farming systems. Plant Dis. 1995, 79, 976–984. [Google Scholar] [CrossRef]
- Garbeva, P.; Van Veen, G.A.; Van Elsas, J.D. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, S.; Porporato, A. Soil carbon and nitrogen mineralization: Theory and models across scales. Soil Biol. Biochem. 2009, 41, 1355–1379. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef] [Green Version]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [Green Version]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Zhang, L.M.; Offre, P.R.; He, J.Z.; Verhamme, D.T.; Nicol, G.W.; Prosser, J.I. Autotrophic ammonia oxidation by soil thaumarchaea. Proc. Natl. Acad. Sci. USA 2010, 107, 17240–17245. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Tourna, M.; Freitag, T.E.; Nicol, G.W.; Prosser, J.I. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ. Microbiol. 2008, 10, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Schleper, C. Ammonia oxidation: Different niches for bacteria and archaea? ISME J. 2010, 4, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Valentine, D.L. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat. Rev. Microbiol. 2007, 5, 316–323. [Google Scholar] [CrossRef]
- Hallin, S.; Jones, C.M.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef]
- Knapp, C.W.; Dodds, W.K.; Wilson, K.C.; O’Brien, J.M.; Graham, D.W. Spatial heterogeneity of denitrification genes in a highly homogenous urban stream. Environ. Sci. Technol. 2009, 43, 4273–4279. [Google Scholar] [CrossRef]
- Philippot, L.; Cuhel, J.; Saby, N.P.A.; Cheneby, D.; Chrona-kova, A.; Bru, D.; Arrouays, D.; Martin-Laurent, F.; Simek, M. Mapping field-scale spatial patterns of size and activity of the denitrifier community. Environ. Microbiol. 2009, 11, 1518–1526. [Google Scholar] [CrossRef]
- Abell, G.C.J.; Revill, A.T.; Smith, C.; Bissett, A.P.; Volkman, J.K.; Robert, S.S. Archaeal ammonia oxidizers and nirS-type denitrifiers dominate sediment nitrifying and denitrifying populations in a subtropical macrotidal estuary. ISME J. 2010, 4, 286–300. [Google Scholar] [CrossRef]

| Mineral N Level | Soil Codes a | Cropping Years | Organic Matter | Total Nitrogen | Ammonium Nitrogen | Nitrate Nitrogen | Mineral Nitrogen | Available Phosphorus | Available Potassium | pH | Electrical Conductivity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mS cm−1) | ||||
| LMN a | Soil-1 | 20 years | 25.6 ± 0.8 | 1.57 ± 0.09 | 6.63 ± 0.28 | 50.8 ± 0.8 | 57.4 ± 3.4 | 144 ± 5 | 599 ± 27 | 7.09 ± 0.48 | 0.50 ± 0.01 |
| Soil-2 | 16 years | 9.10 ± 0.1 | 0.55 ± 0.02 | 12.4 ± 0.42 | 60.5 ± 3.6 | 72.9 ± 4.6 | 148 ± 5 | 450 ± 19 | 7.16 ± 0.07 | 0.39 ± 0.03 | |
| Soil-5 | 22 years | 29.3 ± 1.1 | 2.66 ± 0.17 | 11.2 ± 0.17 | 33.6 ± 1.3 | 44.8 ± 2.4 | 104 ± 6 | 314 ± 9 | 7.52 ± 0.37 | 0.50 ± 0.01 | |
| Soil-7 | 7 years | 13.7 ± 1.0 | 0.84 ± 0.03 | 7.22 ± 0.04 | 42.0 ± 1.7 | 49.2 ± 1.7 | 79.1 ± 2.9 | 31.0 ± 2 | 7.46 ± 0.05 | 0.40 ± 0.02 | |
| Soil-8 | 1 year | 15.3 ± 1.1 | 0.93 ± 0.01 | 7.32 ± 0.40 | 64.9 ± 4.0 | 72.2 ± 2.8 | 167 ± 1 | 48.3 ± 0.1 | 7.44 ± 0.35 | 0.57 ± 0.02 | |
| Soil-12 | 3 years | 19.2 ± 1.0 | 1.18 ± 0.03 | 8.02 ± 0.35 | 70.3 ± 4.5 | 78.3 ± 0.3 | 176 ± 11 | 132 ± 3 | 6.75 ± 0.47 | 0.84 ± 0.03 | |
| HMN b | Soil-3 | 10 years | 19.0 ± 0.3 | 1.16 ± 0.02 | 16.8 ± 0.4 | 285 ± 6 | 303 ± 16 | 113 ± 3 | 500 ± 6 | 7.15 ± 0.23 | 1.30 ± 0.09 |
| Soil-4 | 4 years | 25.6 ± 0.9 | 1.89 ± 0.03 | 8.40 ± 0.38 | 96.1 ± 1.1 | 104 ± 7 | 66.1 ± 4 | 374 ± 21 | 7.31 ± 0.35 | 0.61 ± 0.02 | |
| Soil-6 | 15 years | 11.8 ± 0.1 | 0.76 ± 0.03 | 10.4 ± 0.5 | 143 ± 11 | 153 ± 9 | 131 ± 2 | 238 ± 12 | 7.51 ± 0.14 | 0.76 ± 0.01 | |
| Soil-9 | 18 years | 20.8 ± 1.2 | 1.27 ± 0.02 | 12.8 ± 0.2 | 241 ± 14 | 254 ± 16 | 341 ± 7 | 293 ± 20 | 6.28 ± 0.07 | 1.75 ± 0.03 | |
| Soil-10 | 12 years | 27.8 ± 0.8 | 2.90 ± 0.17 | 27.1 ± 0.8 | 657 ± 21 | 684 ± 27 | 358 ± 5 | 687 ± 30 | 5.87 ± 0.15 | 5.52 ± 0.43 | |
| Soil-11 | 5 years | 20.3 ± 0.2 | 1.21 ± 0.57 | 14.9 ± 0.6 | 162 ± 6 | 177 ± 14 | 137 ± 2 | 230 ± 2 | 6.41 ± 0.43 | 1.17 ± 0.07 | |
| Means c | LMN | 18.7 ± 3.1 | 1.29 ± 0.31 | 8.80 ± 0.98 | 53.7 ± 5.8 | 62.5 ± 5.7 | 136 ± 15 | 262 ± 94 | 7.24 ± 0.12 | 0.53 ± 0.07 | |
| HMN | 20.9 ± 2.3 | 1.53 ± 0.30 | 15.1 ± 2.7 | 264 ± 83 | 279 ± 86 | 191 ± 51 | 387 ± 73 | 6.76 ± 0.27 | 1.85 ± 0.75 | ||
| p-Value | 0.583 | 0.591 | 0.054 | 0.031 | 0.031 | 0.330 | 0.320 | 0.133 | 0.111 |
| DNA Extract | Microbial Properties a (Gene Copies (g−1 Dry Compost)) | |||||||
|---|---|---|---|---|---|---|---|---|
| (μg DNA g−1 Dry Compost) | Bacteria | Fungi | Archaea | AOB amoA | AOA amoA | nirK | nirS | nosZ |
| 16.26 ± 3.14 | 7.05 × 1012 ± 0.52 × 1012 | 1.25 × 1012 ± 0.02 × 1012 | 2.76 × 1010 ± 0.06 × 1010 | 6.20 × 108 ± 0.12 × 108 | 1.01 × 107 ± 0.07 × 107 | 2.54 × 108 ± 0.11 × 108 | 7.75 × 109 ± 0.29 × 109 | 7.87 × 109 ± 0.09 × 109 |
| Organic carbon | Total nitrogen | Ammonium nitrogen | Nitrate nitrogen | Mineral nitrogen | Available phosphorus | Available potassium | pH | Electrical conductivity |
| (g kg−1) | (g kg−1) | (g kg−1) | (g kg−1) | (g kg−1) | (g kg−1) | (g kg−1) | (mS cm−1) | |
| 297.9 ± 6.3 | 17.11 ± 0.46 | 0.17 ± 0.01 | 1.90 ± 0.09 | 2.07 ± 0.14 | 3.58 ± 0.23 | 16.35 ± 1.31 | 6.25 ± 0.21 | 4.05 ± 0.03 |
| Target | Primer Name | Primer Sequence 5’ to 3’ | Fragment Length | Thermal Profile | Number of Cycles | Reference |
|---|---|---|---|---|---|---|
| Bacteria | Eub338 | ACTCCTACGGGAGGCAGCAG | 181 bp | 95 °C-5 min | 1 | Fierer et al. (2005) Petersen et al. (2012) |
| Eub518 | ATTACCGCGGCTGCTGG | 95 °C-60 s/53 °C-30 s/ 72 °C-60 s | 40 | |||
| Fungi | 5.8 s | CGCTGCGTTCTTCATCG | 300 bp | 95 °C-5 min | 1 | Fierer et al. (2005) |
| ITS1f | TCCGTAGGTGAACCTGCGG | 95 °C-60 s/53 °C-30 s/ 72 °C-60 s | 40 | |||
| Archaea | Arch344 | TTCGCGCCTGSTGCRCCCCG | 572 bp | 95 °C-3 min | 1 | Mori et al. (2003) Petersen et al. (2012) |
| Arch915 | GTGCTCCCCCGCCAATTCCT | 95 °C-30 s/65 °C-30 s/ 72 °C-45 s | 40 | |||
| AOB amoA | amoA1F | GGGGTTTCTACTGGTGGT | 491 bp | 94 °C-5 min | 1 | Rotthauwe et al. (1997) Petersen et al. (2012) |
| amoA2R | CCCCTCKGSAAAGCCTTCTTC | 94 °C-30 s/55 °C-45 s/ 72 °C-60 s | 40 | |||
| AOA amoA | Arch-amoAF | STAATGGTCTGGCTTAGACG | 635 bp | 94 °C-5 min | 1 | Francis et al. (2005) Petersen et al. (2012) |
| Arch-amoAR | GCGGCCATCCATCTGTATGT | 94 °C-30 s/53 °C-45 s/ 72 °C-60 s | 40 | |||
| nirK | NirK876 NirK1040 | ATYGGCGGVAYGGCGA GCCTCGATCAGRTTRTGGTT | 165 bp | 95 °C-5 min | 1 | Henry et al. (2004) Petersen et al. (2012) |
| 95 °C-15 s/63–58 °C-30 s/ 72 °C-30 s | 6 (touchdown) | |||||
| 95 °C-15 s/58 °C-30 s/72 °C-30 s | 40 | |||||
| niS | Cd3F | GT(C/G)AACGT(C/G)AAGGA(A/G) AC(C/G)GG | 407 bp | 95 °C-5 min 95 °C-15 s/63–58 °C-30 s/ 72 °C-30 s 95 °C-15 s/58 °C-30 s/72 °C-30 s | 1 6 (touchdown) 40 | Michotey et al. (2000) Throbäck et al. (2004) Petersen et al. (2012) |
| R3cd | GA(C/G)TTCGG(A/G)TG(C/G)GTCT TGA | |||||
| nosZ | NosZ2F NosZ2R | CGCRACGGCAASAAGGTSMSSGT CAKRTGCAKSGCRTGGCAGAA | 267 bp | 95 °C-5 min | 1 | Henry et al. (2006) Petersen et al. (2012) |
| 95 °C-30 s/65–60 °C-30 s/ 72 °C-30 s | 6 (touchdown) | |||||
| 95 °C-15 s/60 °C-15 s/ 72 °C-30 s | 40 | |||||
| Bacillus | Bsub5F | AAGTCGAGCGGACAGATGG | 595 bp | 95 °C-5 min | 1 | Wattiau et al. (2001) |
| subtilis | Bsub3R | CCAGTTTCCAATGACCCTCCCC | 95 °C-60 s/65 °C-30 s/ 72 °C-60 s | 40 | ||
| F. oxysporum f. sp. cucumerinum | FocF3 FocR7 | AAACGAGCCCGCTATTTGAG TATTTCCTCCACATTGCCATG | 244 bp | 95 °C-5 min 95 °C-15 s/60 °C-45 s/ 72 °C-60 s | 1 40 | Lievens et al. (2007) |
| Treatments a | Total Microbial Biomass b | Selected Microorganisms (Genes g−1 Soil) | ||||
|---|---|---|---|---|---|---|
| (×103 DNA ng g−1 Soil) | Bacteria (×109) | Fungi (×109) | Archaea (×108) | Bacillus subtilis (×108) | Fusarium oxysporum f. sp. Cucumerinum (×105) | |
| Soil mineral N levels: | ||||||
| HMN | 9.57 b c | 10.61 b | 7.29 | 6.91 | 1.61 | 15.01 a |
| LMN | 11.01 a | 14.12 a | 7.81 | 9.53 | 1.93 | 1.53 b |
| p-Value d | 0.000 | 0.000 | 0.450 | 0.096 | 0.091 | 0.038 |
| Soil amendment treatments: | ||||||
| S | 8.16 c | 10.23 b | 5.15 b | 10.01 | 1.91 ab | 26.52 a |
| SC | 12.02 a | 14.36 a | 10.01 a | 6.10 | 1.43 b | 2.03 b |
| SI | 9.18 b | 10.21 b | 6.09 b | 9.41 | 2.12 a | 1.89 b |
| SCI | 11.71 a | 14.50 a | 8.93 a | 7.23 | 1.51 b | 2.59 b |
| p-Value | 0.000 | 0.000 | 0.000 | 0.235 | 0.019 | 0.024 |
| Interactions: | ||||||
| HMN-S | 7.77 e | 8.40 c | 5.85 cd | 8.40 ab | 1.41 bc | 51.90 a |
| HMN-SC | 11.53 b | 12.04 bc | 9.03 ab | 4.30 b | 1.00 c | 2.57 b |
| HMN-SI | 8.99 cde | 9.85 bc | 7.65 bc | 9.11 ab | 2.42 a | 2.79 b |
| HMN-SCI | 10.10 c | 12.23 b | 6.63 bcd | 5.82 ab | 1.49 bc | 2.66 b |
| LMN-S | 8.55 de | 12.02 b | 4.46 d | 11.71 a | 2.45 a | 1.11 b |
| LMN-SC | 12.60 ab | 16.60 a | 11.02 a | 8.03 ab | 1.87 ab | 1.49 b |
| LMN-SI | 9.36 cd | 10.62 bc | 4.54 d | 9.70 ab | 1.85 ab | 0.99 b |
| LMN-SCI | 13.31 a | 16.91 a | 11.21 a | 8.52 ab | 1.44 bc | 2.53 b |
| p-Value | 0.007 | 0.398 | 0.001 | 0.893 | 0.008 | 0.019 |
| Treatments a | Selected N Cycling Genes b (Genes g−1 Soil) | AOB/AOA | ||||
|---|---|---|---|---|---|---|
| AOB amoA (×106) | AOA amoA (×106) | nirK (×107) | nirS (×107) | nosZ (×107) | ||
| Soil mineral N levels: | ||||||
| HMN | 4.52 | 2.24 b c | 3.80 | 2.34 b | 6.16 | 1.92 a |
| LMN | 5.00 | 3.32 a | 3.79 | 4.67 a | 6.81 | 1.46 b |
| p-Value d | 0.475 | 0.000 | 0.980 | 0.016 | 0.236 | 0.029 |
| Soil amendment treatments: | ||||||
| S | 4.90 | 2.61 | 3.03 b | 4.13 | 5.95 b | 1.79 a |
| SC | 4.45 | 2.92 | 4.34 a | 2.73 | 7.79 a | 1.45 b |
| SI | 5.77 | 2.68 | 3.51 b | 3.78 | 5.56 b | 2.13 a |
| SCI | 3.92 | 2.90 | 4.29 a | 3.40 | 6.63 ab | 1.39 b |
| p-Value | 0.256 | 0.653 | 0.000 | 0.757 | 0.023 | 0.048 |
| Interactions: | ||||||
| HMN-S | 4.50 ab | 2.02 d | 3.42 c | 1.51 bc | 6.14 abc | 2.01 a |
| HMN-SC | 3.70 b | 2.38 cd | 4.50 ab | 0.88 c | 7.32 abc | 1.47 b |
| HMN-SI | 6.55 a | 2.10 d | 4.05 bc | 2.26 bc | 5.84 bc | 2.76 a |
| HMN-SCI | 3.32 b | 2.45 bcd | 3.22 cd | 4.71 ab | 5.34 c | 1.43 b |
| LMN-S | 5.30 ab | 3.20 abc | 2.65 d | 6.76 a | 5.76 c | 1.57 b |
| LMN-SC | 5.20 ab | 3.46 a | 4.19 bc | 4.56 abc | 8.27 a | 1.44 b |
| LMN-SI | 5.00 ab | 3.27 ab | 2.96 d | 5.30 ab | 5.29 c | 1.49 b |
| LMN-SCI | 4.51 ab | 3.35 a | 5.36 a | 2.08 bc | 7.91 ab | 1.34 b |
| p-Value | 0.369 | 0.966 | 0.000 | 0.025 | 0.151 | 0.041 |
| N Cycling Genes a | HMN b | LMN | ||||
|---|---|---|---|---|---|---|
| Net N Ammonification | Net N Nitrification | Net N Mineralization | Net N Ammonification | Net N Nitrification | Net N Mineralization | |
| AOB amoA | −0.395 ***c | −0.137 | −0.180 | −0.213 | −0.144 | −0.221 |
| AOA amoA | −0.500 *** | −0.005 | −0.057 | 0.119 | 0.053 | 0.093 |
| AOB + AOA | −0.446 *** | −0.119 | −0.167 | −0.130 | −0.097 | −0.145 |
| nirK | −0.057 | 0.131 | 0.126 | 0.276 * | −0.047 | 0.030 |
| nirS | −0.175 | −0.019 | −0.038 | −0.259 * | 0.143 | 0.081 |
| nirK + nirS | −0.208 | 0.018 | −0.203 | −0.222 | −0.162 | 0.113 |
| nosZ | −0.405 *** | −0.113 | −0.157 | 0.384 *** | 0.006 | 0.120 |
| AOB/AOA | −0.270 * | −0.151 | −0.181 | −0.267 * | −0.248 * | −0.351 ** |
| nosZ/nirK | −0.408 *** | −0.149 | −0.194 | 0.076 | 0.142 | 0.179 |
| nosZ/nirS | 0.202 | 0.479 *** | 0.506 *** | −0.089 | 0.193 | 0.187 |
| nosZ/(nirK + nirS) | −0.275 * | −0.079 | −0.108 | 0.375 *** | −0.097 | 0.004 |
| nirK/nirS | 0.315 ** | 0.448 *** | 0.487 *** | −0.172 | 0.231 | 0.203 |
| Total Microbial Biomass | Bacteria | Fungi | Archaea | Fusarium oxysporum f. sp. cucumerinum | Net N Mineralization | |
|---|---|---|---|---|---|---|
| r | 0.515 | 0.423 | 0.060 | −0.385 | −0.390 | 0.599 |
| p-Value | <0.001 a | 0.003 | 0.685 | 0.007 | 0.006 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Gao, Y.; Tian, Y.; Li, J. Compost Addition Attenuates the Negative Impacts of High Soil Mineral Nitrogen Levels on Rhizosphere Microbial Characteristics and Enhances Cucumber Growth in Monoculture Systems. Plants 2022, 11, 1621. https://doi.org/10.3390/plants11131621
Cao Y, Gao Y, Tian Y, Li J. Compost Addition Attenuates the Negative Impacts of High Soil Mineral Nitrogen Levels on Rhizosphere Microbial Characteristics and Enhances Cucumber Growth in Monoculture Systems. Plants. 2022; 11(13):1621. https://doi.org/10.3390/plants11131621
Chicago/Turabian StyleCao, Yune, Yanming Gao, Yongqiang Tian, and Jianshe Li. 2022. "Compost Addition Attenuates the Negative Impacts of High Soil Mineral Nitrogen Levels on Rhizosphere Microbial Characteristics and Enhances Cucumber Growth in Monoculture Systems" Plants 11, no. 13: 1621. https://doi.org/10.3390/plants11131621
APA StyleCao, Y., Gao, Y., Tian, Y., & Li, J. (2022). Compost Addition Attenuates the Negative Impacts of High Soil Mineral Nitrogen Levels on Rhizosphere Microbial Characteristics and Enhances Cucumber Growth in Monoculture Systems. Plants, 11(13), 1621. https://doi.org/10.3390/plants11131621





