Amino Acids in Rice Grains and Their Regulation by Polyamines and Phytohormones
Abstract
:1. Introduction
2. Content, Compositions, Anabolism of Amino Acids in Rice Grains
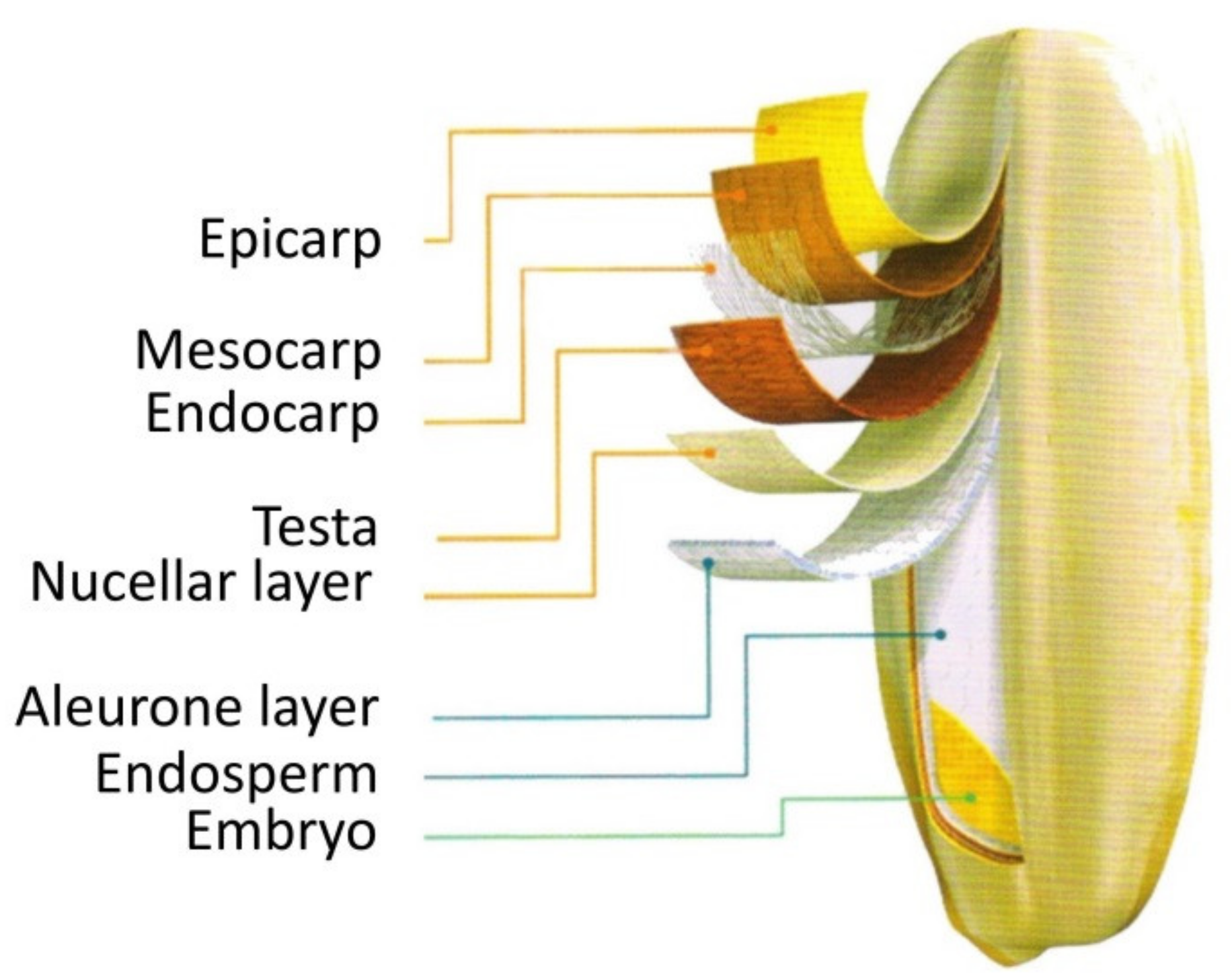
2.1. Content, Compositions, and Distributions of Amino Acids in the Ripe Grain
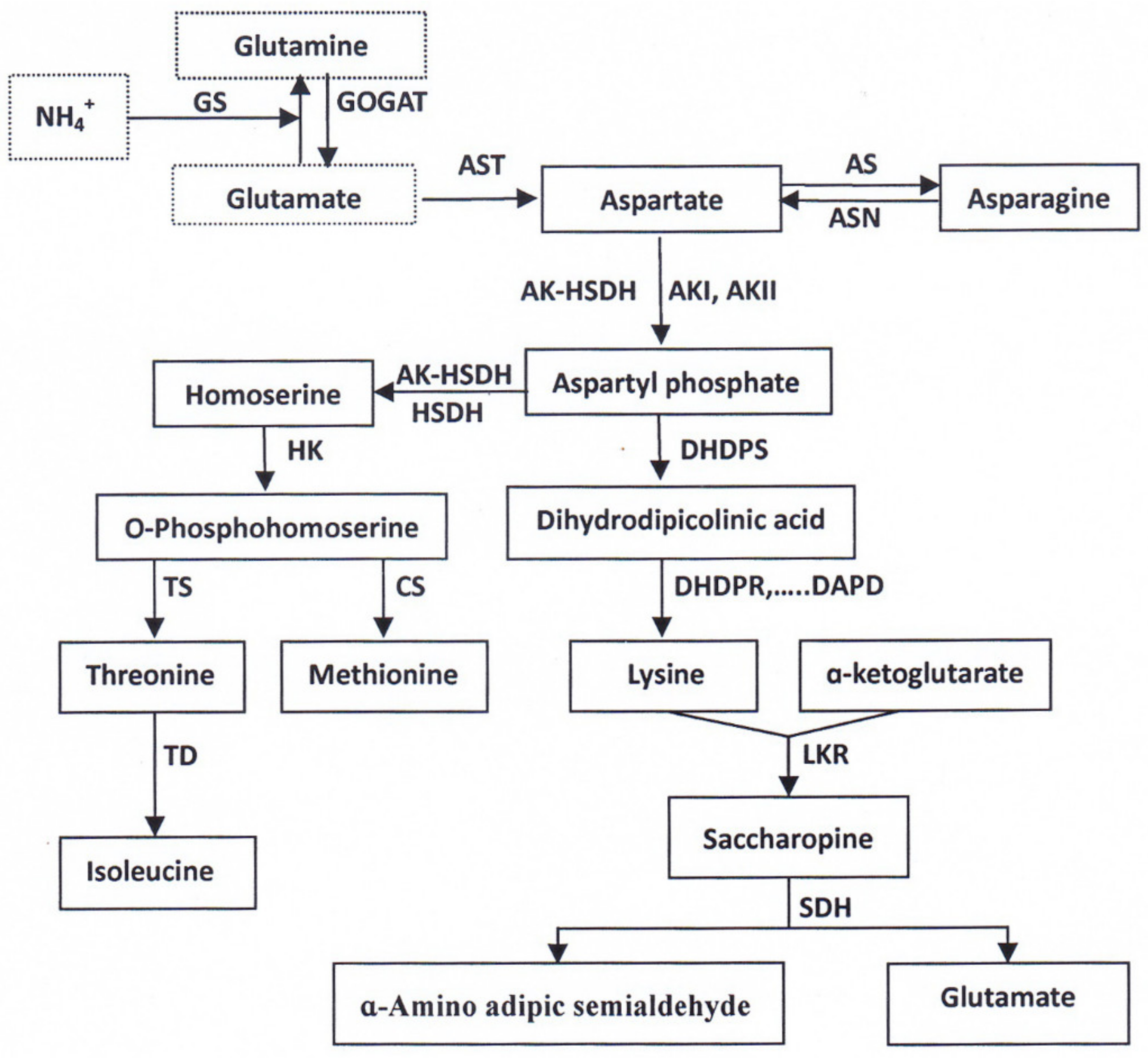
2.2. Biosynthesis and Metabolism of Amino Acids in the Filling Grain
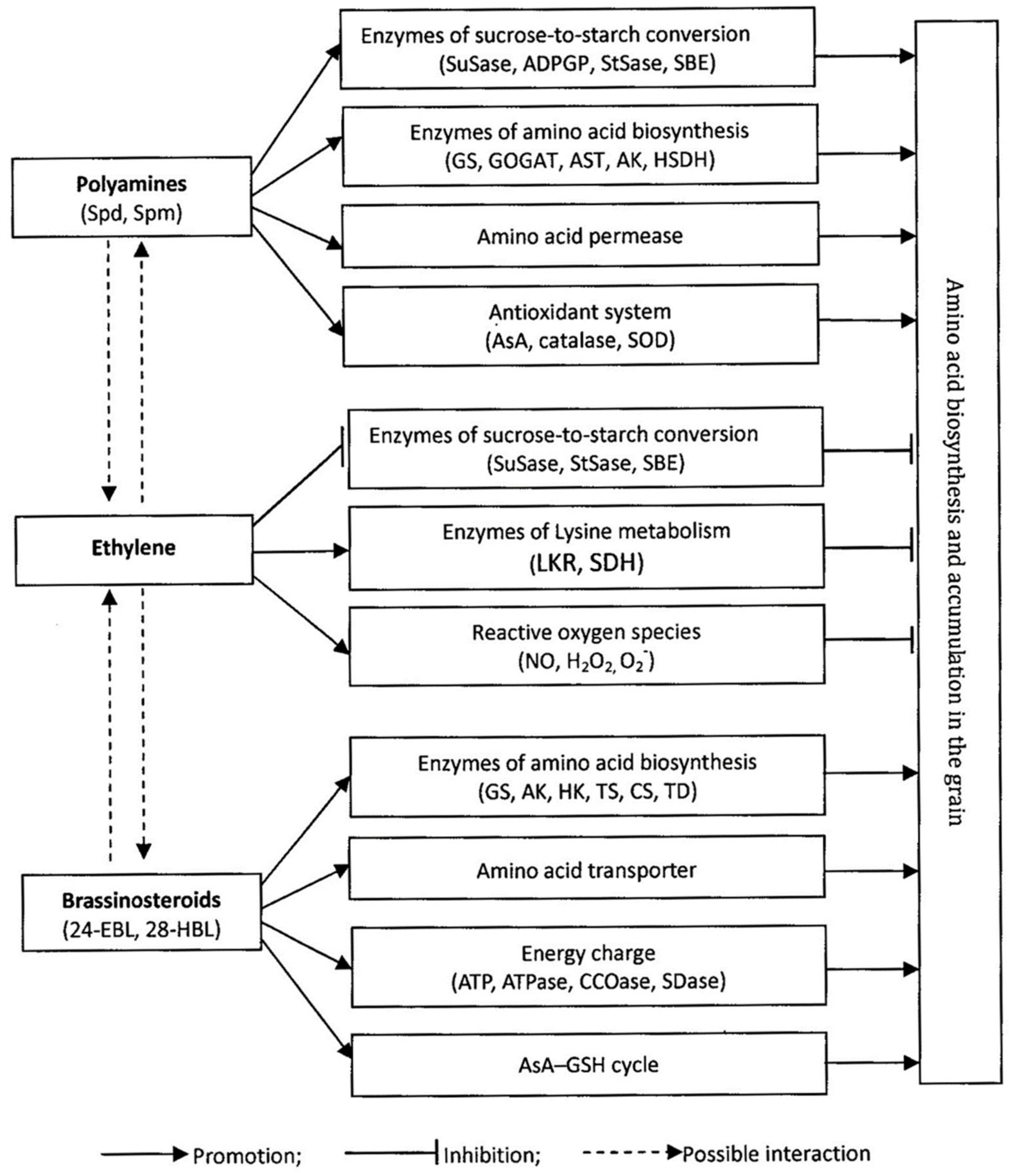
3. Roles of PAs and Phytohormones in Regulating Anabolism of Amino Acids in Rice
3.1. Roles of PAs and Ethylene
3.2. Roles of BRs and Other Phytohormones
4. Proposal for Further Research
4.1. Temporal and Spatial Distributions of Amino Acids in the Filling Grain
4.2. Cross-Talk of PAs and Phytohormones in Mediating the Anabolism of Amino Acids
4.3. Regulation Techniques for Promoting Biosynthesis of Amino Acids in the Grain
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.Q.; Zhao, D.S.; Zhang, C.Q.; Sun, S.S.M.; Liu, Q.Q. Lysine biofortification of crops to promote sustained human health in the 21st century. J. Exp. Bot. 2022, 73, 1258–1267. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Y.F.; Huang, Y.M.; Zhao, X.M.; Dong, Z.B.; Jin, W.W.; Huang, W. Amino acid permease 6 regulates grain protein content in maize. Crop J. 2022; in press. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Nawaz, A.; Farooq, M. Rice production systems and grain quality. J. Cereal Sci. 2022, 105, 103463. [Google Scholar] [CrossRef]
- Albarracin, M.; Dyner, L.; Giacomino, M.S.; Weisstaub, A.; Zuleta, A.; Drago, S.R. Modification of nutritional properties of whole rice flours (Oryza sativa L.) by soaking, germination, and extrusion. J. Food Biochem. 2019, 43, e12854. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Kurpad, A.; Tano-Debrah, K.; Otoo, G.E.; Aaron, G.A.; Toride, Y.; Ghosh, S. Role of protein and amino acids in infant and young child nutrition: Protein and amino acid needs and relationship with child growth. J. Nutr. Sci. Vitaminol. 2015, 61 (Suppl.), S192–S194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Shivakumar, N.; Kashyap, S.; Kishore, S.; Varkey, A.; Devi, S.; Preston, T.; Jahoor, F.; Sheshshayee, M.S.; Kurpad, A.V. Protein-quality evaluation of complementary foods in Indian children. Am. J. Clin. Nutr. 2019, 109, 1319–1327. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Chen, L.; Wang, W.L.; Xu, Y.J.; Zhang, W.Y.; Zhang, H.; Liu, L.J.; Wang, Z.Q.; Gu, J.F.; Yang, J.C. Effects of application of rapeseed cake as organic fertilizer on rice quality at high yield level. J. Sci. Food Agric. 2022, 102, 1832–1841. [Google Scholar] [CrossRef]
- Wong, H.W.; Liu, Q.; Sun, S.S.M. Biofortification of rice with lysine using endogenous histones. Plant Mol. Biol. 2015, 87, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Ajadi, A.A.; Cisse, A.; Ahmad, S.; Wang, Y.F.; Shu, Y.Z.; Li, S.F.; Liu, X.X.; Bello, B.K.; Sani, M.T.; Tong, X.H.; et al. Protein phosphorylation and phosphoproteome: An overview of rice. Rice Sci. 2020, 27, 184–200. [Google Scholar] [CrossRef]
- Krishnan, H.B.; White, J.A. Morphometric analysis of rice seed protein bodies. Plant Physiol. 1995, 109, 1491–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.M.; Zhang, X.C.; Wang, Z.X.; Jiang, Y.T.; Liu, Z.H.; Alexander, D.; Li, G.H.; Wang, S.H.; Ding, Y.F. Metabolomic analysis of pathways related to rice grain chalkiness by a notched-belly mutant with high occurrence of white-belly grains. BMC Plant Biol. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.Q.; Yu, W.H.; Wu, H.Y.; Zhang, C.Q.; Sun, S.S.M.; Liu, Q.Q. Lysine biofortification in rice by modulating feedback inhibition of aspartate kinase and dihydrodipicolinate synthase. Plant Biotechnol. J. 2021, 19, 490–501. [Google Scholar] [CrossRef]
- Yabuki, Y.; Ohashi, M.; Imagawa, F.; Ishiyama, K.; Beier, M.P.; Konishi, N.; Umetsu-Ohashi, T.; Hayakawa, T.; Yamaya, T.; Kojima, S. A temporal and spatial contribution of asparaginase to asparagine catabolism during development of rice grains. Rice 2017, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.B.; Wang, Z.D.; Wang, C.R.; Li, H.; Huang, D.Q.; Zhou, D.G.; Zhao, L.; Pan, Y.Y.; Gong, R.; Zhou, S.C. Comparisons of metabolic profiles for carbohydrates, amino acids, lipids, fragrance and flavones during grain development in indica rice cultivars. Rice Sci. 2022, 29, 155–165. [Google Scholar]
- Fath, A.; Bethke, P.C.; Belligni, M.V.; Spiegel, Y.N.; Jones, R.L. Signalling in the cereal aleurone: Hormones, reactive oxygen and cell death. New Phytol. 2001, 151, 99–107. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, J.K.; Huang, K.; Su, Y.; Tong, J.H.; Huang, Z.G.; Huang, C.; Wei, M.L.; Lin, W.H.; Xiao, L.T. Dynamic formation and transcriptional regulation mediated by phytohormones during chalkiness formation in rice. BMC Plant Biol. 2021, 21, 308. [Google Scholar] [CrossRef]
- Zhang, X.F.; Tong, J.H.; Bai, A.N.; Liu, C.M.; Xiao, L.T.; Xue, H.W. Phytohormone dynamics in developing endosperm influence rice grain shape and quality. J. Integr. Plant Biol. 2020, 62, 1625–1637. [Google Scholar] [CrossRef]
- Gu, J.F.; Li, Z.K.; Mao, Y.Q.; Struik, P.C.; Zhang, H.; Liu, L.J.; Wang, Z.Q.; Yang, J.C. Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci. 2018, 274, 320–331. [Google Scholar] [CrossRef]
- Kamara, J.S.; Konishi, S.; Sasanuma, T.; Abe, T. Variation in free amino acid profile among some rice (Oryza sativa L.) cultivars. Breed. Sci. 2010, 60, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Chu, H.D.; Le, N.Q. Improving nutritional quality of plant proteins through genetic engineering. Curr. Genom. 2016, 17, 220–229. [Google Scholar]
- Gaby, A.R. Natural remedies for Herpes simplex. Altern. Med. Rev. 2006, 11, 93–101. [Google Scholar] [PubMed]
- Sacksteder, K.A.; Biery, B.J.; Morrell, J.C.; Goodman, B.K.; Geisbrecht, B.V.; Cox, R.P.; Gould, S.J.; Geraghty, M.T. Identification of the alpha-aminoadipic semialdehyde synthase gene, which is defective in familial hyperlysinemia. Am. J. Hum. Genet. 2000, 66, 1736–1743. [Google Scholar] [CrossRef] [Green Version]
- Poirier, L.A. The effects of diet, genetics and chemicals on toxicity and aberrant DNA methylation: An introduction. J. Nutr. 2002, 132, 2336s–2339s. [Google Scholar] [CrossRef]
- Fukagawa, N.K.; Galbraith, R.A. Advancing age and other factors influencing the balance between amino acid requirements and toxicity. J. Nutr. 2004, 134, 1569s–1574s. [Google Scholar] [CrossRef]
- Fukagawa, N.K. Sparing of methionine requirements: Evaluation of human data takes sulfur amino acids beyond protein. J. Nutr. 2006, 136, 1676s–1681s. [Google Scholar] [CrossRef] [Green Version]
- Karau, A.; Grayson, I. Amino acids in human and animal nutrition. Adv. Biochem. Eng. Biotechnol. 2014, 143, 189–228. [Google Scholar]
- Long, X.H.; Liu, Q.Q.; Chan, M.L.; Wang, Q.; Sun, S.S.M. Metabolic engineering and profiling of rice with increased lysine. Plant Biotect. J. 2013, 11, 490–501. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhang, C.Q.; Chan, M.L.; Zhao, D.S.; Chen, J.Z.; Wang, Q.; Li, Q.F.; Yu, H.X.; Gu, M.H.; Sun, S.S.M. Biofortification of rice with the essential amino acid lysine: Molecular characterization, nutritional evaluation, and field performance. J. Exp. Bot. 2016, 67, 4285–4296. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, D.; Rizzi, V.; Gaziola, S.A.; Medici, L.O.; Vincze, E.; Kozak, M.; Lea, P.J.; Azevedo, R.A. Lysine metabolism in antisense C-hordein barley grains. Plant Physiol. Biochem. 2015, 87, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Juliano, B.O. Rice: Chemistry and Technology; American Association of Cereal Chemists: Saint Paul, MN, USA, 1985; pp. 56–174. [Google Scholar]
- WHO. Energy and Protein Requirements; WHO Technology Report Series, 522; World Health Organization: Geneva, Switzerland, 1973. [Google Scholar]
- Kaur, P.; Pal, P.; Virdi, A.S.; Singh, N.; Mahajan, G. Protein and starch characteristics of milled rice from different cultivars affected by transplantation date. J. Food Sci. Technol. 2016, 53, 3186–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, S.; Ebenezer, G.A.I.; Dayanandan, P. Histochemical localization of storage components in caryopsis of rice (Oryza sativa L.). Curr. Sci. 2001, 80, 567–571. [Google Scholar]
- Kumamaru, T.; Ogawa, M.; Satoh, H.; Okita, T.W. Protein body biogenesis in cereal endosperms. Plant Cell Monogr. 2007, 8, 141–158. [Google Scholar]
- Gan, L.; Huang, B.S.; Song, Z.J.; Zhang, Y.C.; Zhang, Y.J.; Chen, S.; Tong, L.Q.; Wei, Z.S.; Yu, L.X.; Luo, X.B.; et al. Unique glutelin expression patterns and seed endosperm structure facilitate glutelin accumulation in polyploid rice seed. Rice 2021, 14, 61. [Google Scholar]
- Santos, K.F.D.N.; Silveira, R.D.D.; Martin-Didonet, C.C.G. Storage protein profile and amino acid content in wild rice Oryza glumaepatula. Pesqui. Agropecu. Bras. 2013, 48, 66–72. [Google Scholar] [CrossRef]
- Hoshikawa, K. The Growing Rice Plant, an Anatomical Monograph; Nobunkyo: Tokyo, Japan, 1989; pp. 290–297. [Google Scholar]
- Krishnan, S.; Dayanandan, P. Structural and histochemical studies on grain-filling in the caryopsis of rice. J. Biosci. 2003, 28, 455–469. [Google Scholar] [CrossRef]
- Ishimaru, T.; Parween, S.; Saito, Y.; Shigemitsu, T.; Yamakawa, H.; Nakazono, M.; Masumura, T.; Nishizawa, N.K.; Kondo, M.; Sreenivasulu, N. Laser microdissection-based tissue-specific transcriptome analysis reveals a novel regulatory network of genes involved in heat-induced grain chalk in rice endosperm. Plant Cell Physiol. 2019, 60, 626–642. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Takaiwa, F. Differences in transcriptional regulatory mechanisms functioning for free lysine content and seed storage protein accumulation in rice grain. Plant Cell Physiol. 2010, 51, 1964–1974. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.Q.; He, W.L.; Hu, S.D.; Wu, G.Y. Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 2019, 51, 1153–1165. [Google Scholar] [CrossRef]
- Hagen, S.R.; Frost, B.; Augustin, J. Precolumn phenylisothiocyanate derivatization and liquid chromatography of amino acids in food. J. Assoc. Off. Anal. Chem. 1989, 72, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Wu, H.Y.; Li, Q.F.; Duan, R.X.; Zhang, C.Q.; Sun, S.S.M.; Liu, Q.Q. Characterization of agronomy, grain physicochemical quality, and nutritional property of high-lysine 35R transgenic rice with simultaneous modification of lysine biosynthesis and catabolism. J. Agric. Food Chem. 2017, 65, 4296–4304. [Google Scholar]
- Tran, T.U.; Suzuki, K.; Okadome, H.; Ikezaki, H.; Homma, S.; Ohtsubo, K. Detection of changes in taste of japonica and indica brown and milled rice (Oryza sativa L.) during storage using physicochemical analyses and a taste sensing system. J. Agric. Food Chem. 2005, 53, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.U.; Suzuki, K.; Okadome, H.; Homma, S.; Ohtsubo, K. Analysis of the tastes of brown rice and milled rice with different milling yields using a taste sensing. Food Chem. 2004, 88, 557–566. [Google Scholar] [CrossRef]
- Teng, Z.N.; Chen, Y.K.; Yuan, Y.Q.; Peng, Y.Q.; Yi, Y.K.; Yu, H.H.; Yi, Z.X.; Yang, J.C.; Peng, Y.; Duan, M.J.; et al. Identification of microRNAs regulating grain filling of rice inferior spikelets in response to moderate soil drying post-anthesis. Crop J. 2022; in press. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Patel, R.; Sahu, S.K. Time of flowering affects grain quality and spikelet partitioning within the rice panicle. Aust. J. Plant Physiol. 1993, 20, 231–242. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H. Grain-filling problem in “super” rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Peng, S.B.; Visperas, R.M.; Sanico, A.L.; Zhu, Q.S.; Gu, S.L. Grain filling pattern and cytokinin content in the grains and roots of rice plants. Plant Growth Regul. 2000, 30, 261–270. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Panigrahi, R.; Turner, N.C. Physiology of spikelet development on the rice panicle: Is manipulation of apical dominance crucial for grain yield improvement? Adv. Agron. 2011, 110, 333–359. [Google Scholar]
- Ishimaru, T.; Hirose, T.; Matsuda, T.; Goto, A.; Takahashi, K.; Sasaki, H.; Terao, T.; Ishii, R.; Ohsugi, R.; Yamagishi, T. Expression patterns of genes encoding carbohydrate-metabolizing enzymes and their relationship to grain filling in rice (Oryza sativa L.): Comparison of caryopses located at different positions in a panicle. Plant Cell Physiol. 2005, 46, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.T.; Xu, G.W.; Wang, Z.Q.; Zhang, H.; Yang, J.C.; Zhang, J.H. Expression of proteins in superior and inferior spikelets of rice during grain filling under different irrigation regimes. Proteomics 2016, 16, 102–121. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhao, H.; Huang, F.L.; Long, J.F.; Song, G.; Lin, W.X. The 14-3-3 protein GF14f negatively affects grain filling of inferior spikelets of rice (Oryza sativa L.). Plant J. 2019, 99, 344–358. [Google Scholar] [PubMed]
- Zhang, X.C.; Lei, J.C.; Zheng, D.Y.; Liu, Z.H.; Li, G.H.; Wang, S.H.; Ding, Y.F. Amino acid composition of leaf, grain and bracts of japonica rice (Oryza sativa ssp. japonica) and its response to nitrogen. Plant Growth Regul. 2017, 82, 1–9. [Google Scholar]
- Shao, C.; Shen, L.; Qiu, C.; Wang, Y.; Qian, Y.; Chen, J.; Ouyang, Z.; Zhang, P.; Guan, X.; Xie, J. Characterizing the impact of high temperature during grain filling on phytohormone levels, enzyme activity and metabolic profiles of an early indica rice variety. Plant Biol. 2021, 23, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Zhang, C.Q.; Liu, Q.Q.; Gu, M.H. Expression characteristics of asparagine synthetase gene among rice varieties with different seed storage protein content. Genom. Appl. Biol. 2009, 28, 39–45. [Google Scholar]
- Azevedo, R.A.; Lancien, M.; Lea, P.J. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids 2006, 30, 143–162. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Lea, P.J. Lysine metabolism in higher plants. Amino Acids 2001, 20, 261–279. [Google Scholar] [CrossRef]
- Tang, G.L.; Zhu, X.H.; Gakiere, B.; Levanony, H.; Kahana, A.; Galili, G. The bifunctional LKR/SDH locus of plants also encodes a highly active monofunctional lysine-ketoglutarate reductase using a polyadenylation signal located within an intron. Plant Physiol. 2002, 130, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Cao, Y.Y.; Zhang, H.; Liu, L.J.; Zhang, J.H. Involvement of polyamines in the post-anthesis development of inferior and superior spikelets in rice. Planta 2008, 228, 137–149. [Google Scholar] [CrossRef]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortalotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Beltrano, J.; Carbone, A.; Montaldi, E.R.; Guiamet, J.J. Ethylene as promoter of wheat grain maturation and ear senescence. Plant Growth Regul. 1994, 15, 107–112. [Google Scholar] [CrossRef]
- Kendrick, M.D.; Chang, C. Ethylene signaling: New levels of complexity and regulation. Curr. Opin. Plant Biol. 2008, 5, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.T.; Xu, Y.J.; Wang, J.C.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Polyamines and ethylene interact in rice grains in response to soil drying during grain filling. J. Exp. Bot. 2013, 64, 2523–2538. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A. Plant polyamines-metabolism and function. In Polyamines and Ethylene: Biochemistry, Physiology, and Interactions; Flores, H.E., Arteca, R.N., Shannon, J.C., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 1990; pp. 1–23. [Google Scholar]
- Xu, Y.J.; Tang, S.P.; Jian, C.Q.; Cai, W.L.; Zhang, W.Y.; Wang, Z.Q.; Yang, J.C. Roles of polyamines and ethylene in grain filling, grain weight and quality of rice. Chin. J. Rice Sci. 2022, 36, 253–269. [Google Scholar]
- Sen, K.; Choudhuri, M.M.; Ghosh, B. Changes in polyamine contents during development and germination of rice seeds. Phytochemistry 1981, 20, 631–633. [Google Scholar] [CrossRef]
- Lin, P.P.C.; Egli, D.B.; Li, G.M.; Meckel, L. Polyamine titer in the embryonic axis and cotyledons of Glycine max (L.) during seed growth and maturation. Plant Physiol. 1984, 103, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.Y.; Wang, Z.M.; Kong, F.N.; Zhang, M.J.; Zhou, S.L. Roles of carbohydrate supply and ethylene, polyamines in maize kernel set. J. Integr. Plant Biol. 2011, 53, 388–398. [Google Scholar] [CrossRef]
- Xu, Y.J.; Jian, C.Q.; Li, K.; Tian, Y.F.; Zhu, K.Y.; Zhang, W.Y.; Wang, W.L.; Wang, Z.Q.; Yang, J.C. The role of polyamines in regulating amino acid biosynthesis in rice grains. Food Energy Secur. 2021, 10, e306. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, D.P.; Peng, X.L.; Ma, B.J.; Shao, S.M.; Jing, W.J.; Gu, J.F.; Liu, L.J.; Wang, Z.Q.; Liu, Y.Y.; et al. Optimizing integrative cultivation management improves grain quality while increasing yield and nitrogen use efficiency in rice. J. Integr. Agric. 2019, 18, 2716–2731. [Google Scholar] [CrossRef]
- Yang, J.C.; Chang, E.H.; Zhang, W.J.; Wang, Z.Q.; Liu, L.J. Relationship between root chemical signals and grain quality of rice. Agric. Sci. China 2007, 6, 101–105. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Liu, K.; Wang, Z.Q.; Liu, L.J. Involvement of polyamines in the drought resistance of rice. J. Exp. Bot. 2007, 58, 1545–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tan, G.L.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Ethylene and ACC levels in developing grains are related to the poor appearance and milling quality of rice. Plant Growth Regul. 2009, 58, 85–96. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Liu, K.; Wang, P. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 2006, 57, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.J.; Jian, C.Q.; Li, K.; Tian, Y.F.; Zhu, K.Y.; Zhang, W.Y.; Wang, W.L.; Wang, Z.Q.; Yang, J.C. High ethylene level impedes amino acid biosynthesis in rice grains. Plant Growth Regul. 2022, 96, 51–65. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Ye, Y.X.; Wang, Z.Q.; Zhu, Q.S.; Liu, L.J. Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant Cell Environ. 2004, 27, 1055–1064. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Liu, K.; Wang, Z.Q.; Liu, L.J. Abscisic acid and ethylene interact in wheat grains in response to soil drying during grain filling. New Phytol. 2006, 271, 293–303. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H. Grain filling of cereals under soil drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, W.Y.; Yang, J.C. Physiological mechanism underlying spikelet degeneration in rice. J. Integr. Agric. 2018, 17, 1475–1481. [Google Scholar] [CrossRef] [Green Version]
- Martinez, C.; Espinosa-Ruiz, A.; de Lucas, M.; Bernardo-Garcia, S.; Franco-Zorrilla, J.M.; Prat, S. PIF4-induced BR synthesis is critical to diurnal and thermomorphogenic growth. EMBO J. 2018, 37, e99552. [Google Scholar] [CrossRef]
- Vriet, G.; Russinova, E.; Reuzeau, C. From squalene to brassinolide: The steroid metabolic and signaling pathways across the plant kingdom. Mol. Plant 2013, 6, 1738–1757. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wu, C.; Yuan, S.; Yin, L.; Sun, W.; Zhao, Q.; Zhao, B.; Li, X. Kinase activity of OsBRI1 is essential for brassinosteroids to regulate rice growth and development. Plant Sci. 2013, 199–200, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fei, K.Q.; Zhang, W.Y.; Wang, Z.Q.; Zhang, J.H.; Yang, J.C. Brassinosteroids mediate the effect of high temperature during anthesis on the pistil activity of photo-thermosensitive genetic male-sterile rice lines. Crop J. 2021, 9, 109–119. [Google Scholar] [CrossRef]
- Jiang, W.B.; Huang, H.Y.; Hu, Y.W.; Zhu, S.W.; Wang, Z.Y.; Lin, W.H. Brassinosteroid regulates seed size and shape in Arabidopsis. Plant Physiol. 2013, 162, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Li, C.Q.; Jiang, Y. Contents and compositions of amino acids in rice grains and their regulation: A review. Acta Agron. Sin. 2022, 48, 1037–1051, (In Chinese with English Abstract). [Google Scholar]
- Yang, J.C.; Liu, L.J.; Zhang, H. Principle and Technology of Efficient Utilization of Fertilizer Nitrogen in High-Yielding Rice; Science Press: Beijing, China, 2022; pp. 236–258. [Google Scholar]
- Zhang, W.Y.; Zhu, K.Y.; Wang, Z.Q.; Zhang, H.; Gu, J.F.; Liu, L.J.; Yang, J.C.; Zhang, J.H. Brassinosteroids function in spikelet differentiation and degeneration in rice. J. Integr. Plant Biol. 2019, 61, 943–963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.Y.; Sheng, J.Y.; Fu, L.D.; Xu, Y.J.; Xiong, F.; Wu, Y.F.; Wang, W.L.; Wang, Z.Q.; Zhang, J.H.; Yang, J.C. Brassinosteroids mediate the effect of soil-drying during meiosis on spikelet degeneration in rice. Environ. Exp. Bot. 2020, 169, 103887. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Fu, L.D.; Men, C.B.; Yu, J.X.; Yao, J.Y.; Shen, J.Y.; Xu, Y.J.; Wang, Z.Q.; Liu, L.J.; Yang, J.C.; et al. Response of brassinosteroids to nitrogen rates and their regulation on rice spikelet degeneration during meiosis. Food Energy Secur. 2020, 9, e201. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Miao, W.Q.; Chen, J. Roles of jasmonates and brassinosteroids in rice responses to high temperature stress—A review. Crop J. 2021, 9, 977–985. [Google Scholar] [CrossRef]
- Sun, L.; Di, D.W.; Li, G.J.; Kronzucker, H.J.; Shi, W.M. Spatiotemporal dynamics in global rice gene expression (Oryza sativa L.) in response to high ammonium stress. J. Plant Physiol. 2017, 212, 94–104. [Google Scholar] [CrossRef]
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K.S.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; et al. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2017, 90, 61–78. [Google Scholar] [CrossRef]
- Chanclud, E.; Kisiala, A.; Emery, N.R.J.; Chalvon, V.; Ducasse, A.; Romiti-Michel, C.; Gravot, A.; Kroj, T.; Morel, J.B. Cytokinin production by the rice blast fungus is alpha pivotal requirement for full virulence. PLoS Pathog. 2016, 12, e1005457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, B.; Kong, H.; Li, Y.; Wang, L.; Zhong, M.; Sun, L.; Gao, G.; Zhang, Q.; Luo, L.; Wang, G.; et al. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat. Commun. 2014, 5, 5847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegeder, M. Transporters for amino acids in plant cells: Some functions and many unknowns. Curr. Opin. Plant Biol. 2012, 15, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Alqudah, A.M. Key hormonal components regulate agronomically important traits in barley. Int. J. Mol. Sci. 2018, 19, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prerostova, S.; Kramna, B.; Dobrev, P.I.; Gaudinova, A.; Marsik, P.; Fialad, R.; Knirsch, V.; Vanek, T.; Kuresova, G.; Vankova, R. Organ–specific hormonal cross-talk in phosphate deficiency. Environ. Exp. Bot. 2018, 153, 198–208. [Google Scholar] [CrossRef]
- Basunia, M.A.; Nonhebel, H.M. Hormonal regulation of cereal endosperm development with a focus on rice (Oryza sativa). Funct. Plant Biol. 2019, 46, 493–506. [Google Scholar] [CrossRef]
- Norton, G.J.; Shafaei, M.; Travis, A.J.; Deacon, C.M.; Danku, J.; Pond, D.; Cochrane, N.; Lockhart, K.; Salt, D.; Zhang, H.; et al. Impact of alternate wetting and drying on rice physiology, grain production, and grain quality. Field Crops Res. 2017, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.L.; Xi, M.; Zhang, X.C.; Lin, Z.M.; Ding, C.Q.; Tang, S.; Liu, Z.H.; Wang, S.H.; Ding, Y.F. Nitrogen effect on amino acid composition in leaf and grain of japonica rice during grain filling stage. J. Cereal Sci. 2015, 64, 29–33. [Google Scholar] [CrossRef]
- Mallikarjuna, S.B.P.; Marathi, B.; Ribeiro-Barros, A.I.F.; Ricachenevsky, F.K. Editorial: Development of healthy and nutritious cereals: Recent insights on molecular advances in breeding. Front. Genet. 2021, 12, 635006. [Google Scholar]
- Wu, K.; Xu, X.; Zhong, N.; Huang, H.; Yu, J.; Ye, Y.; Wu, Y.; Fu, X. The rational design of multiple molecular module-based assemblies for simultaneously improving rice yield and grain quality. J. Genet. Genom. 2018, 46, 337–341. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Botella, J.R.; Zhu, J. Generation of new glutinous rice by CRISPR/Cas9-targeted mutagenesis of the Waxy gene in elite rice varieties. J. Integr. Plant Biol. 2018, 60, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Q.; Zhang, C.; Chu, R.; Gu, Z.; Tan, H.; Zhao, D.; Fan, X.; Liu, Q. Creating novel Wx alleles with fine-tuned amylose levels and improved grain quality in rice by promoter editing using CRISPR/Cas9 system. Plant Biotech. J. 2020, 18, 2164–2166. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, Q.; Chen, Z.; Yue, Y.; Liu, Y.; Zhang, Y.; Zhou, D.X. Histone deacetylases control lysine acetylation of ribosomal proteins in rice. Nucleic Acids Res. 2021, 49, 4613–4628. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhou, Y.; Jiang, Y. Amino Acids in Rice Grains and Their Regulation by Polyamines and Phytohormones. Plants 2022, 11, 1581. https://doi.org/10.3390/plants11121581
Yang J, Zhou Y, Jiang Y. Amino Acids in Rice Grains and Their Regulation by Polyamines and Phytohormones. Plants. 2022; 11(12):1581. https://doi.org/10.3390/plants11121581
Chicago/Turabian StyleYang, Jianchang, Yujiao Zhou, and Yi Jiang. 2022. "Amino Acids in Rice Grains and Their Regulation by Polyamines and Phytohormones" Plants 11, no. 12: 1581. https://doi.org/10.3390/plants11121581
APA StyleYang, J., Zhou, Y., & Jiang, Y. (2022). Amino Acids in Rice Grains and Their Regulation by Polyamines and Phytohormones. Plants, 11(12), 1581. https://doi.org/10.3390/plants11121581