Exogenous Postharvest Application of Calcium Chloride and Salicylic Acid to Maintain the Quality of Broccoli Florets
Abstract
1. Introduction
2. Materials and Methods
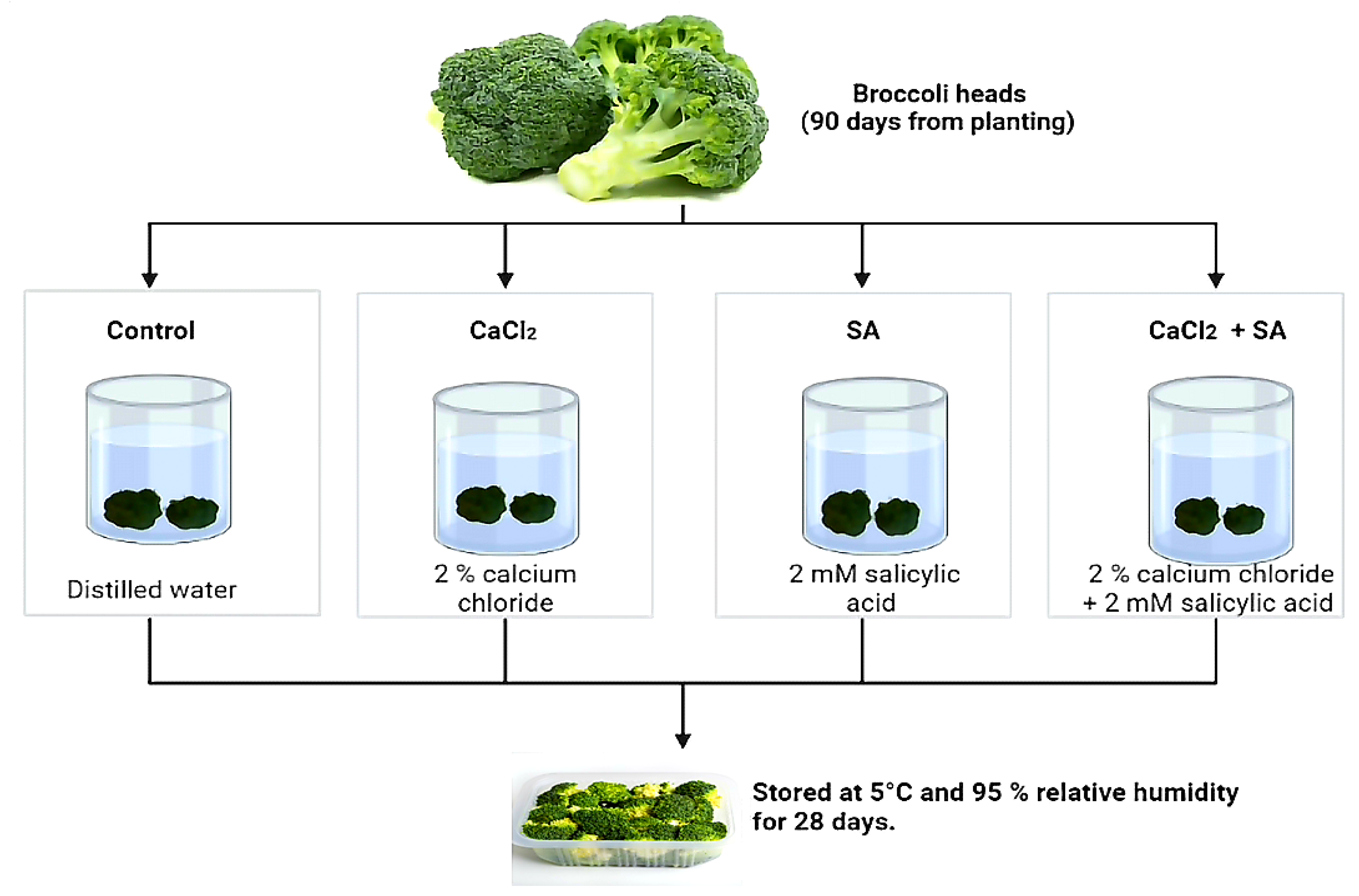
2.1. Plant Material, Treatments, and Storage Condition
2.2. Weight Loss, Appearance Score, Chlorophyll, and Carotenoids
2.3. Vitamin C, Total Phenolic, and Flavonoids
2.4. Glucosinolates, Sulforaphane, and Peroxidase
2.5. Antioxidant Activity (%) Using DPPH
2.6. Statistical Analysis
3. Results
3.1. Weight Loss, Appearance Score, Chlorophyll Content, and Carotenoids
3.2. Total Phenolic Compounds, Vitamin C, Flavonoids, and Glucosinolates
3.3. Sulforaphane, Peroxidase Activity, and Antioxidant Activity
3.4. Correlation Study
4. Discussion
4.1. Weight Loss, Appearance Score, Chlorophyll Content, and Carotenoids
4.2. Total Phenolic Compounds, Vitamin C, Flavonoids, and Glucosinolates
4.3. Sulforaphane, Peroxidase Activity, and Antioxidant Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Tang, Y.; Dong, S.; Shao, X.; Wang, H.; Zheng, Y.; Yang, Z. Reducing yellowing and enhancing antioxidant capacity of broccoli in storage by sucrose treatment. Postharvest Biol. Technol. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Mahmoud, A.W.M.; El-Sawy, M.B.I.; Parmar, A. Pre-Harvest Foliar Application of Mineral Nutrients to Retard Chlorophyll Degradation and Preserve Bio-Active Compounds in Broccoli. Agronomy 2019, 9, 711. [Google Scholar] [CrossRef]
- Jia, C.-G.; Xu, C.-J.; Wei, J.; Yuan, J.; Yuan, G.-F.; Wang, B.-L.; Wang, Q.-M. Effect of modified atmosphere packaging on visual quality and glucosinolates of broccoli florets. Food Chem. 2009, 114, 28–37. [Google Scholar] [CrossRef]
- Paulsen, E.; Barrios, S.; Baenas, N.; Moreno, D.A.; Heinzen, H.; Lema, P. Effect of temperature on glucosinolate content and shelf life of ready-to-eat broccoli florets packaged in passive modified atmosphere. Postharvest Biol. Technol. 2018, 138, 125–133. [Google Scholar] [CrossRef]
- Shi, J.; Gao, L.; Zuo, J.; Wang, Q.; Wang, Q.; Fan, L. Exogenous sodium nitroprusside treatment of broccoli florets extends shelf life, enhances antioxidant enzyme activity, and inhibits chlorophyll-degradation. Postharvest Biol. Technol. 2016, 116, 98–104. [Google Scholar] [CrossRef]
- Lemoine, M.L.; Civello, P.M.; Martínez, G.A.; Chaves, A.R. Influence of postharvest UV-C treatment on refrigerated storage of minimally processed broccoli (Brassica oleracea var. Italica). J. Sci. Food Agric. 2007, 87, 1132–1139. [Google Scholar] [CrossRef]
- Favre, N.; Bárcena, A.; Bahima, J.V.; Martínez, G.; Costa, L. Pulses of low intensity light as promising technology to delay postharvest senescence of broccoli. Postharvest Biol. Technol. 2018, 142, 107–114. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, J.; Fang, Y.; Yan, Z.; Shi, J.; Gao, L.; Wang, Q.; Jiang, A. Effect of folic acid on the postharvest physiology of broccoli during storage. Food Chem. 2021, 339, 127981. [Google Scholar] [CrossRef]
- Wang, J.; Allan, A.C.; Wang, W.-Q.; Yin, X.-R. The effects of salicylic acid on quality control of horticultural commodities. N. Z. J. Crop Hortic. Sci. 2022, 1–19. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on postharvest physiology of horticultural crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S.; Fard, J.R.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hortic. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Kumari, P.; Barman, K.; Patel, V.B.; Siddiqui, M.W.; Kole, B. Reducing postharvest pericarp browning and preserving health promoting compounds of litchi fruit by combination treatment of salicylic acid and chitosan. Sci. Hortic. 2015, 197, 555–563. [Google Scholar] [CrossRef]
- Supapvanich, S.; Promyou, S. Hot water incorporated with salicylic acid dips maintaining physicochemical quality of Holland papaya fruit stored at room temperature. Emir. J. Food Agric. 2017, 29, 18–24. [Google Scholar] [CrossRef]
- Adhikary, T.; Gill, P.S.; Jawandha, S.K.; Bhardwaj, R.D.; Anurag, R.K. Browning and quality management of pear fruit by salicylic acid treatment during low temperature storage. J. Sci. Food Agric. 2021, 101, 853–862. [Google Scholar] [CrossRef]
- Iijima, T.; Yamaguchi, T. K2CO3-catalyzed direct synthesis of salicylic acid from phenol and supercritical CO2. Appl. Catal. A Gen. 2008, 345, 12–17. [Google Scholar] [CrossRef]
- Barman, K.; Sharma, S.; Siddiqui, M.W. (Eds.) Emerging Postharvest Treatment of Fruits and Vegetables, 1st ed.; Apple Academic Press: Palm Bay, FL, USA, 2018. [Google Scholar] [CrossRef]
- Cid-López, M.L.; Soriano-Melgar, L.d.A.A.; García-González, A.; Cortéz-Mazatán, G.; Mendoza-Mendoza, E.; Rivera-Cabrera, F.; Peralta-Rodríguez, R.D. The benefits of adding calcium oxide nanoparticles to biocompatible polymeric coatings during cucumber fruits postharvest storage. Sci. Hortic. 2021, 287, 110285. [Google Scholar] [CrossRef]
- Amiri, S.; Rezazad Bari, L.; Malekzadeh, S.; Amiri, S.; Mostashari, P.; Ahmadi Gheshlagh, P. Effect of Aloe vera gel-based active coating incorporated with catechin nanoemulsion and calcium chloride on postharvest quality of fresh strawberry fruit. J. Food Process. Preserv. 2021, e15960. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Ziane, N.; Salmieri, S.; Lacroix, M. Combined Post-harvest Treatments for Improving Quality and Extending Shelf-Life of Minimally Processed Broccoli Florets (Brassica oleracea var. italica). Food Bioprocess. Technol. 2018, 11, 84–95. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Parmar, A.; Ali, M.R.; Abdel-Aziz, M.E.; Abdeldaym, E.A. Improving postharvest storage of fresh artichoke bottoms by an edible coating of Cordia myxa gum. Postharvest Biol. Technol. 2020, 163, 111143. [Google Scholar] [CrossRef]
- Senevirathna, P.A.W.A.N.K.; Daundasekera, W.A.M. Effect of postharvest calcium chloride vacuum infiltration on the shelf life and quality of tomato (cv. ‘Thilina‘). Ceylon J. Sci. 2010, 39, 35–44. [Google Scholar] [CrossRef]
- Mazumder, M.N.; Misran, A.; Ding, P.; Wahab, P.E.; Mohamad, A. Effect of Harvesting Stages and Calcium Chloride Application on Postharvest Quality of Tomato Fruits. Coatings 2021, 11, 1445. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Dokhanieh, A.Y.; Hassanpour, H.; Rezapour Fard, J. Enhancement of antioxidant capacity of cornelian cherry (Cornus mas) fruit by postharvest calcium treatment. Sci. Hortic. 2013, 161, 160–164. [Google Scholar] [CrossRef]
- Supapvanich, S.; Arkajak, R.; Yalai, K. Maintenance of postharvest quality and bioactive compounds of fresh-cut sweet leaf bush (Sauropus androgynus L. Merr.) through hot CaCl2 dips. Int. J. Food Sci. Technol. 2012, 47, 2662–2670. [Google Scholar] [CrossRef]
- Ali, M.R.; Abdel-Aziz, M.E. Application of edible film and coating based on Aloe vera gel for preservation of physico-chemical properties of Physalis peruviana L. fruits. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e1574. [Google Scholar] [CrossRef]
- Gao, J.; Si, Y.; Zhu, Y.; Luo, F.; Yan, S. Temperature abuse timing affects the rate of quality deterioration of postharvest broccoli during different pre-storage stages. Sci. Hortic. 2018, 227, 207–212. [Google Scholar] [CrossRef]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Shehata, S.A.; El-Mogy, M.M.; Mohamed, H.F.Y. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2019, 25, 196–209. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical Characterization and Utilization of Dried Red Beetroot (Beta vulgaris) Peel Extract in Maintaining the Quality of Nile Tilapia Fish Fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Bjerg, B.; Olsen, O.; Rasmussen, K.W.; Sørensen, H. New Principles of Ion-Exchange Techniques Suitable to Sample Preparation and Group Separation of Natural Products Prior to Liquid Chromatography. J. Liq. Chromatogr. 1984, 7, 691–707. [Google Scholar] [CrossRef]
- Bjerg, B.; Sørensen, H. Quantitative analysis of glucosinolates in oilseed rape based on HPLC of desulfoglucosinolates and HPLC of intact glucosinolates. In Glucosinolates in Rapeseeds: Analytical Aspects, Proceedings of the Seminar in the CEC Programme of Research on Plant Productivity, Gembloux, Belgium, 1–3 October 1987; Wathelet, J.P., Ed.; Springer: Dordrecht, The Netherlands, 1987; pp. 125–150. [Google Scholar]
- Gu, Z.-X.; Guo, Q.-H.; Gu, Y.-J. Factors Influencing Glucoraphanin and Sulforaphane Formation in Brassica Plants: A Review. J. Integr. Agric. 2012, 11, 1804–1816. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Separation and Purification of Sulforaphane from Broccoli by Solid Phase Extraction. Int. J. Mol. Sci. 2011, 12, 1854–1861. [Google Scholar] [CrossRef]
- In, B.-C.; Motomura, S.; Inamoto, K.; Doi, M.; Mori, G. Multivariate Analysis of Relations between Preharvest Environmental Factors, Postharvest Morphological and Physiological Factors, and Vase Life of Cut’Asami Red’Roses. J. Jpn. Soc. Hortic. Sci. 2007, 76, 66–72. [Google Scholar] [CrossRef][Green Version]
- Awad, A.H.R.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef]
- Nath, A.; Bagchi, B.; Misra, L.K.; Deka, B.C. Changes in postharvest phytochemical qualities of broccoli florets during ambient and refrigerated storage. Food Chem. 2011, 127, 1510–1514. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Ali, M.R.; Darwish, O.S.; Rogers, H.J. Impact of salicylic acid, abscisic acid, and methyl jasmonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Res. 2019, 9, 333–348. [Google Scholar] [CrossRef]
- Kazemi, M.; Aran, M.; Zamani, S. Effect of calcium chloride and salicylic acid treatments on quality characteristics of kiwifruit (Actinidia deliciosa cv. Hayward) during storage. Am. J. Plant Physiol. 2011, 6, 183–189. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.S.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Q. Effects of polyamines and salicylic acid on postharvest storage of ‘Ponkan’ mandarin. Acta Hortic. 2004, 632, 317–320. [Google Scholar] [CrossRef]
- Turmanidze, T.; Gulua, L.; Jgenti, M.; Wicker, L. Potential antioxidant retention and quality maintenance in raspberries and strawberries treated with calcium chloride and stored under refrigeration. Braz. J. Food Technol. 2017, 20. [Google Scholar] [CrossRef]
- Ul Haq, A.; Lone, M.L.; Farooq, S.; Parveen, S.; Altaf, F.; Tahir, I.; Kaushik, P.; El-Serehy, H.A. Efficacy of salicylic acid in modulating physiological andbiochemical mechanisms to improve postharvest longevity in cut spikes of Consolida ajacis (L.) Schur. Saudi J. Biol. Sci. 2022, 29, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, Z.; Su, Y.; Liu, D.; Ye, X. Effect of Salicylic Acid Treatment on Postharvest Quality, Antioxidant Activities, and Free Polyamines of Asparagus. J. Food Sci. 2011, 76, S126–S132. [Google Scholar] [CrossRef]
- Turkyylmaz, B.; Akta, L.Y.; Guven, A. Salicylic acid induced some biochemical and physiological changes in Phaseolus vulgaris L. Sci. Eng. J. Firat Univ. 2005, 17, 319–326. [Google Scholar]
- Saftner, R.A.; Conway, W.S.; Sams, C.E. Effects of Postharvest Calcium and Fruit Coating Treatments on Postharvest Life, Quality Maintenance, and Fruit-Surface Injury in ‘Golden Delicious’ Apples. J. Am. Soc. Hortic. Sci. 1998, 123, 294–298. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Robert, A.S.; William, S.C.; Carl, E.S. Postharvest Calcium Infiltration Alone and Combined with Surface Coating Treatments Influence Volatile Levels, Respiration, Ethylene Production, and Internal Atmospheres of ‘Golden Delicious’ Apples. J. Am. Soc. Hortic. Sci. 1999, 124, 553–558. [Google Scholar]
- Vallejo, F.; Tomas-Barberan, F.; Garcia-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef]
- Supapvanich, S.; Promyou, S. Efficiency of salicylic acid application on postharvest perishable crops. In Salicylic Acid: Plant Growth and Development; Hayat, S., Ahmad, A., Alyemeni, M.N., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 339–355. [Google Scholar]
- Huang, R.-H.; Liu, J.-H.; Lu, Y.-M.; Xia, R.-X. Effect of salicylic acid on the antioxidant system in the pulp of ‘Cara cara’ navel orange (Citrus sinensis L. Osbeck) at different storage temperatures. Postharvest Biol. Technol. 2008, 47, 168–175. [Google Scholar] [CrossRef]
- Sarikhani, H.; Sasani-Homa, R.; Bakhshi, D. Effect of salicylic acid and SO2 generator pad on storage life and phenolic contents of grape (Vitis vinifera L. ‘Bidaneh Sefid’ and ‘Bidaneh Ghermez’). Acta Hortic. 2010, 877, 1623–1630. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. In Vitro Cell. Dev. Biol. Plant 2022, 1–15. [Google Scholar] [CrossRef]
- Divya, P.; Puthusseri, B.; Neelwarne, B. The effect of plant regulators on the concentration of carotenoids and phenolic compounds in foliage of coriander. LWT Food Sci. Technol. 2014, 56, 101–110. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Rivero, R.M.; López-Cantarero, I.; Romero, L. Role of Ca2+ in the metabolism of phenolic compounds in tobacco leaves (Nicotiana tabacum L.). Plant Growth Regul. 2003, 41, 173–177. [Google Scholar] [CrossRef]
- Perucka, I.; Olszówka, K. Effect of foliar calcium chloride treatment on the level of chlorogenic acid, b carotene, lutein and tocopherols in lettuce (Lactuca sativa L.). Acta Agrobot. 2011, 64, 65–72. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Kong, W.; Li, S.; Archbold, D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006, 41, 244–251. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Ocio, M.J.; Gavara, R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.R.; Tyagi, S.K. Pre-harvest foliar application of calcium and boron influences physiological disorders, fruit yield and quality of strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2007, 112, 215–220. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Ji, H.; Zhang, Z.; Chen, J.; Tan, Y.; Wintergerst, K.; Zheng, Y.; Sun, J.; Cai, L. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hui, Q.; Gu, Z.; Zhou, Y.; Guo, L.; Shen, C.; Zhang, W. Effects of CaCl2 on the metabolism of glucosinolates and the formation of isothiocyanates as well as the antioxidant capacity of broccoli sprouts. J. Funct. Foods 2016, 24, 156–163. [Google Scholar] [CrossRef]
- Zhuang, L.; Xu, K.; Zhu, Y.; Wang, F.; Xiao, J.; Guo, L. Calcium affects glucoraphanin metabolism in broccoli sprouts under ZnSO4 stress. Food Chem. 2021, 334, 127520. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Lu, X.H.; Sun, D.Q.; Mo, Y.W.; Xi, J.G.; Sun, G.M. Effects of postharvest salicylic acid treatment on fruit quality and antioxidant metabolism in pineapple during cold storage. J. Hortic. Sci. Biotechnol. 2010, 85, 454–458. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Li, J. Effects of 6-Benzylaminopurine–Calcium Chloride–Salicylic Acid on Yellowing and Reactive Oxygen Metabolism of Broccoli. Trans. Tianjin Univ. 2018, 24, 318–325. [Google Scholar] [CrossRef]
- Cui, K.; Shu, C.; Zhao, H.; Fan, X.; Cao, J.; Jiang, W. Preharvest chitosan oligochitosan and salicylic acid treatments enhance phenol metabolism and maintain the postharvest quality of apricots (Prunus armeniaca L.). Sci. Hortic. 2020, 267, 109334. [Google Scholar] [CrossRef]







| Weight Loss | Vit. C | Chlorophyll | Phenolic | Carotenoids | Glucosinolates | Flavonoids | Peroxidase | |
|---|---|---|---|---|---|---|---|---|
| Vit. C | −0.853 ** | |||||||
| Chlorophyll | −0.954 ** | 0.887 ** | ||||||
| Phenolic | −0.864 ** | 0.904 ** | 0.834 ** | |||||
| Carotenoids | −0.872 ** | 0.904 ** | 0.834 ** | 0.897 ** | ||||
| Glucosinolates | −0.960 ** | 0.894 ** | 0.916 ** | 0.885 ** | 0.948 ** | |||
| Flavonoids | −0.972 ** | 0.907 ** | 0.925 ** | 0.915 ** | 0.934 ** | 0.985 ** | ||
| Peroxidase | 0.016 | −0.340 * | −0.089 | −0.174 | −0.383 ** | −0.223 | −0.155 | |
| Antioxidant | −0.814 ** | 0.911 ** | 0.823 ** | 0.919 ** | 0.857 ** | 0.847 ** | 0.871 ** | −0.242 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; Ali, M.R.; Ramadan, K.M.A.; Anwar, R.; Shalaby, T.A.; Rezk, A.A.; El-Ganainy, S.M.; Mahmoud, S.F.; Alkafafy, M.; El-Mogy, M.M. Exogenous Postharvest Application of Calcium Chloride and Salicylic Acid to Maintain the Quality of Broccoli Florets. Plants 2022, 11, 1513. https://doi.org/10.3390/plants11111513
El-Beltagi HS, Ali MR, Ramadan KMA, Anwar R, Shalaby TA, Rezk AA, El-Ganainy SM, Mahmoud SF, Alkafafy M, El-Mogy MM. Exogenous Postharvest Application of Calcium Chloride and Salicylic Acid to Maintain the Quality of Broccoli Florets. Plants. 2022; 11(11):1513. https://doi.org/10.3390/plants11111513
Chicago/Turabian StyleEl-Beltagi, Hossam S., Marwa Rashad Ali, Khaled M. A. Ramadan, Raheel Anwar, Tarek A. Shalaby, Adel A. Rezk, Sherif Mohamed El-Ganainy, Samy F. Mahmoud, Mohamed Alkafafy, and Mohamed M. El-Mogy. 2022. "Exogenous Postharvest Application of Calcium Chloride and Salicylic Acid to Maintain the Quality of Broccoli Florets" Plants 11, no. 11: 1513. https://doi.org/10.3390/plants11111513
APA StyleEl-Beltagi, H. S., Ali, M. R., Ramadan, K. M. A., Anwar, R., Shalaby, T. A., Rezk, A. A., El-Ganainy, S. M., Mahmoud, S. F., Alkafafy, M., & El-Mogy, M. M. (2022). Exogenous Postharvest Application of Calcium Chloride and Salicylic Acid to Maintain the Quality of Broccoli Florets. Plants, 11(11), 1513. https://doi.org/10.3390/plants11111513














