An Improved Procedure for Agrobacterium-Mediated Transformation of ‘Carrizo’ Citrange
Abstract
:1. Introduction
2. Results
2.1. Explants from Light-Grown Seedlings Exhibited Higher Transformation Efficiency than Those from Etiolated Seedlings
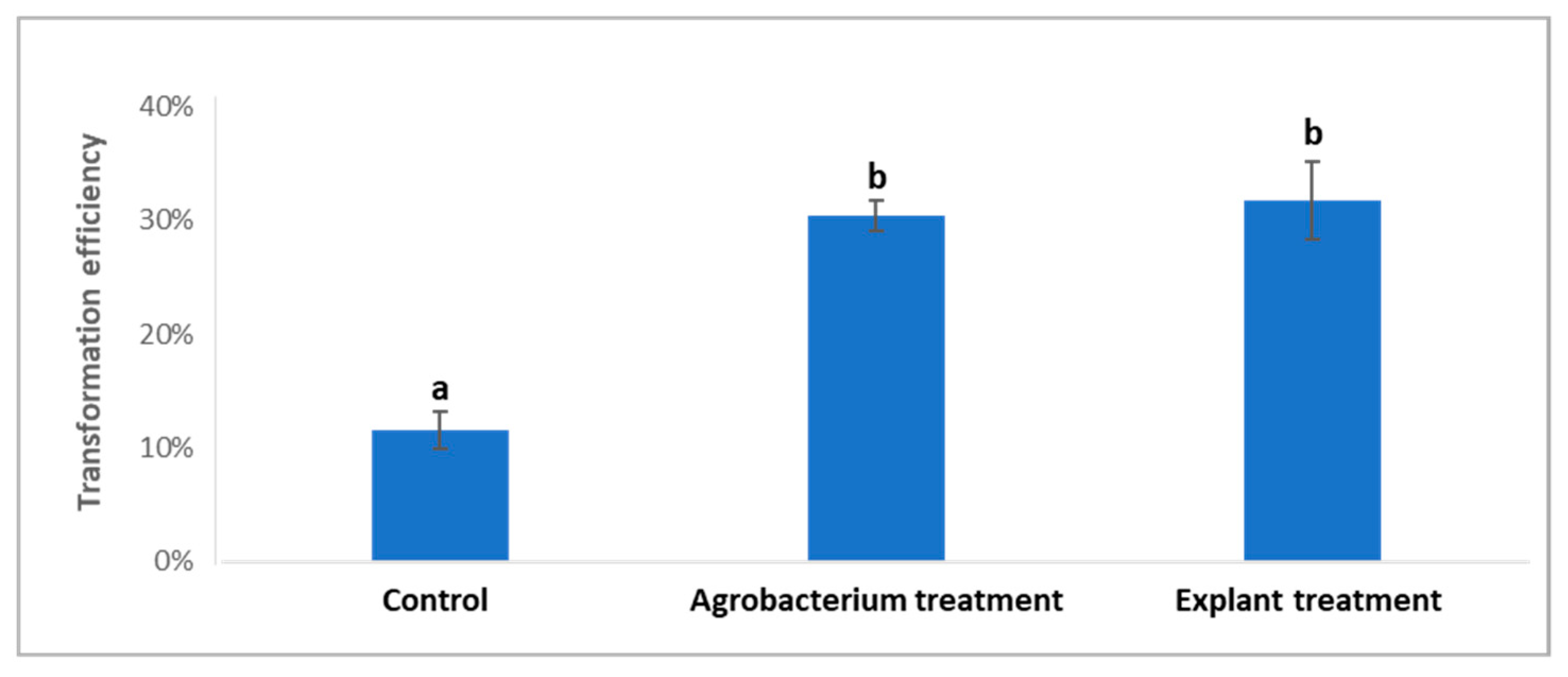
2.2. Treatments of Agrobacterium Cells and ‘Carrizo’ Citrange Explants Prior to and during Co-Cultivation on Citrus Transformation Efficiency
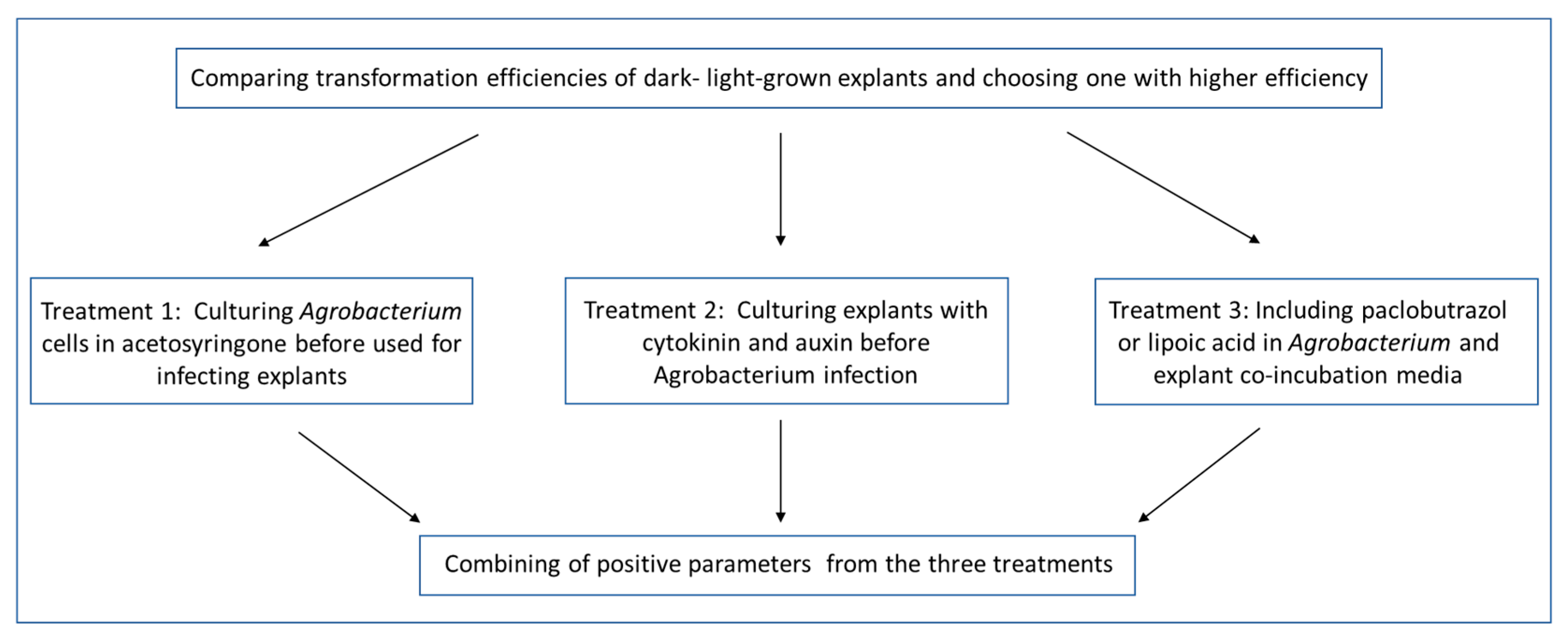
2.3. Incorporation of the Three Simple Treatments into Transformation Procedure and Analysis of Their Effects on Transformation Efficiency in ‘Carrizo’ Citrange
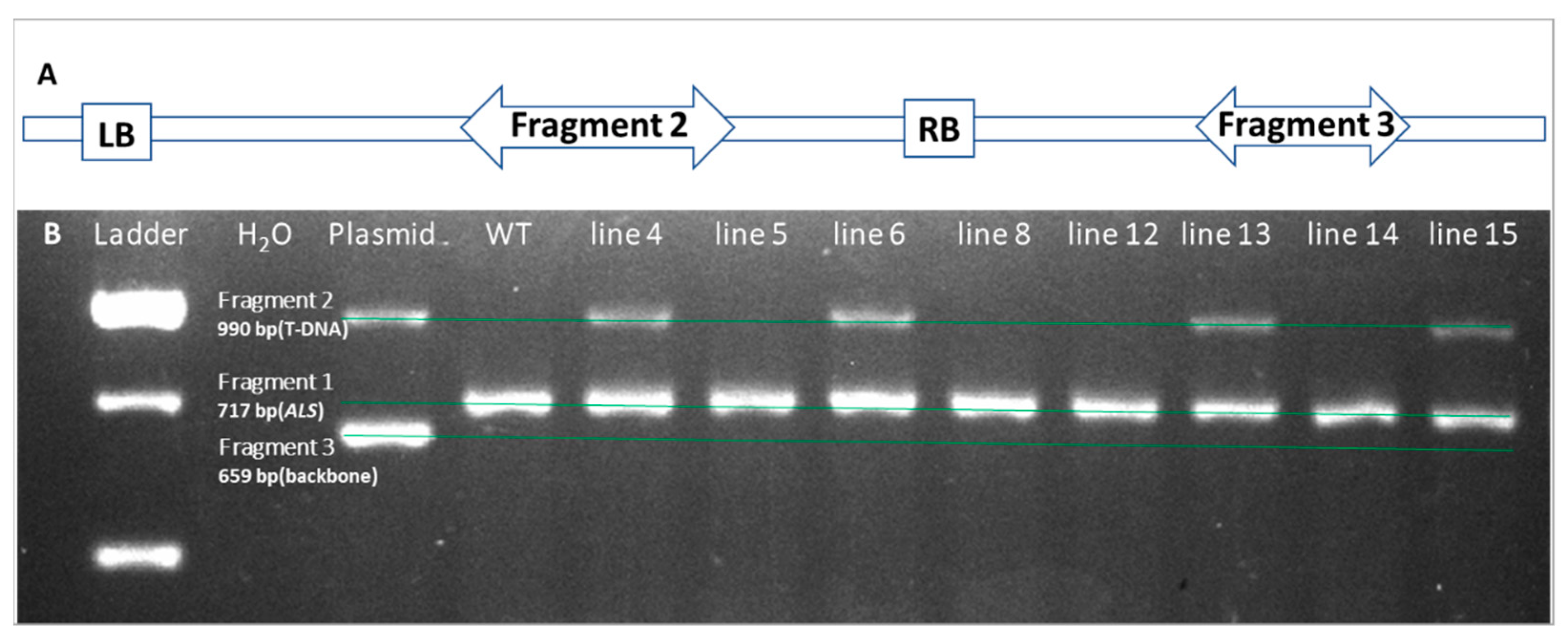
2.4. Confirmation of Transgenic Shoots by PCR Amplification
3. Discussion
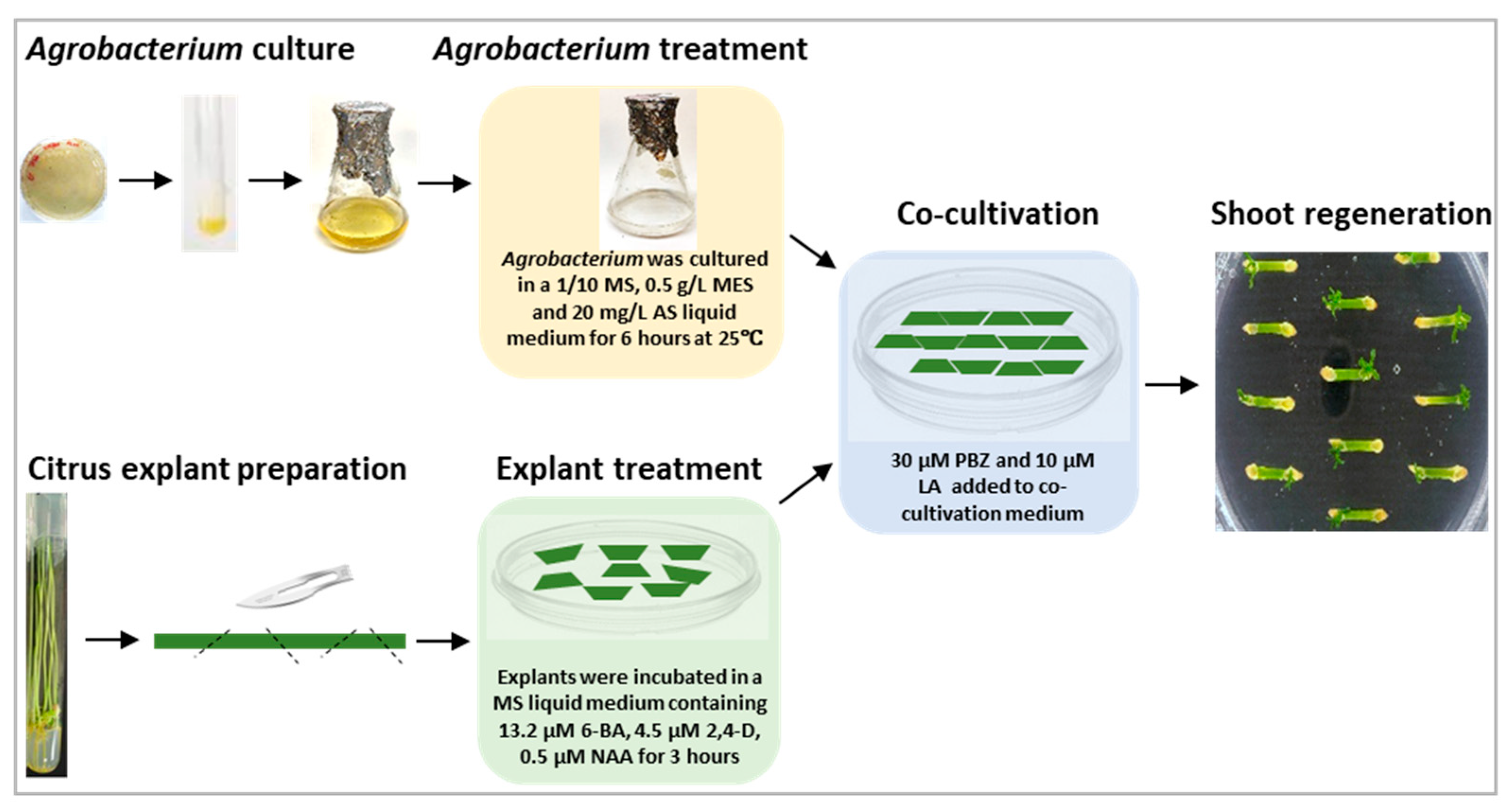
4. Materials and Methods
4.1. Plant Materials Preparation
4.2. Agrobacterium Preparation
4.3. Agrobacterium-Mediated Citrus Transformation and Citrus Shoot Regeneration
4.4. GUS Histochemical Assays
4.5. Molecular Confirmation of Transgenic Shoots
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raveh, E.; Goldenberg, L.; Porat, R.; Carmi, N.; Gentile, A.; La Malfa, S. Conventional Breeding of Cultivated Citrus Varieties. In The Citrus Genome; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020; pp. 33–48. [Google Scholar]
- Cuenca, J.; Garcia-Lor, A.; Navarro, L.; Aleza, P. Citrus genetics and breeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Cham, Switzerland, 2018; pp. 403–436. [Google Scholar]
- Poles, L.; Licciardello, C.; Distefano, G.; Nicolosi, E.; Gentile, A.; La Malfa, S. Recent advances of in vitro culture for the application of new breeding techniques in citrus. Plants 2020, 9, 938. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, P.; Germanà, M.A.; Froelicher, Y.; Cuenca, J.; Aleza, P.; Morillon, R.; Grosser, J.W.; Guo, W. Ploidy manipulation for citrus breeding, genetics, and genomics. In The Citrus Genome; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020; pp. 75–105. [Google Scholar]
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.J.; Zuo, H.; Xu, Q. Genomic insights into citrus domestication and its important agronomic traits. Plant Commun. 2021, 2, 100138. [Google Scholar] [CrossRef] [PubMed]
- Mujjassim, N.E.; Mallik, M.; Rathod, N.K.K.; Nitesh, S.D. Cisgenesis and intragenesis a new tool for conventional plant breeding: A review. J. Pharmacogn. Phytochem. 2019, 8, 2485–2489. [Google Scholar]
- Rai, K.K.; Aamir, M.; Zehra, A.; Rai, A.C. Research Trends in Genetically Modified (GM) Plants. In Policy Issues in Genetically Modified Crops; Singh, P., Borthakur, A., Singh, A.A., Kumar, A., Singh, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 453–480. [Google Scholar]
- Sun, L.; Ke, F.; Nie, Z.; Wang, P.; Xu, J. Citrus genetic engineering for disease resistance: Past, present and future. Int. J. Mol. Sci. 2019, 20, 5256. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.; Wang, L. CRISPR/Cas9 technology for improving agronomic traits and future prospective in agriculture. Planta 2021, 254, 68. [Google Scholar] [CrossRef]
- Gill, K.; Kumar, P.; Kumar, A.; Kapoor, B.; Sharma, R.; Joshi, A.K. Comprehensive mechanistic insights into the citrus genetics, breeding challenges, biotechnological implications, and omics-based interventions. Tree Genet. Genomes 2022, 18, 9. [Google Scholar] [CrossRef]
- Vardi, A.; Bleichman, S.; Aviv, D. Genetic transformation of Citrus protoplasts and regeneration of transgenic plants. Plant Sci. 1990, 69, 199–206. [Google Scholar] [CrossRef]
- Hidaka, T.; Omura, M. Transformation of citrus protoplasts by electroporation. J. Jpn. Soc. Hortic. Sci. 1993, 62, 371–376. [Google Scholar] [CrossRef]
- Yao, J.; Wu, J.; Gleave, A.P.; Morris, B.A.M. Transformation of citrus embryogenic cells using particle bombardment and production of transgenic embryos. Plant Sci. 1996, 113, 175–183. [Google Scholar] [CrossRef]
- Moore, G.A.; Febres, V.J.; Niblett, C.L.; Luth, D.; McCaffery, M.; Garnsey, S.M. Agrobacterium-mediated transformation of grapefruit (Citrus paradisi Macf.) with genes from citrus tristeza virus. In Proceedings of the First International Symposium on Citrus Biotechnology, First International Citrus Biotechnology Symposium, Eilat, Israel, 29 November–3 December 1998; Goren, R., Goldschmidt, E.E., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2000; Volume 535, pp. 237–244. [Google Scholar]
- Salonia, F.; Ciacciulli, A.; Poles, L.; Pappalardo, H.D.; La Malfa, S.; Licciardello, C. New plant breeding techniques in citrus for the improvement of important agronomic traits. A Review. Front. Plant Sci. 2020, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Huang, S.; Chen, C.; Deng, Z.; Ling, P.; Gmitter, F.G. Factors affecting Agrobacterium-mediated transformation and regeneration of sweet orange and citrange. Plant Cell Tissue Organ Cult. 2002, 71, 147–155. [Google Scholar] [CrossRef]
- Dutt, M.; Grosser, J.W. Evaluation of parameters affecting Agrobacterium-mediated transformation of citrus. Plant Cell Tissue Organ Cult. 2009, 98, 331–340. [Google Scholar] [CrossRef]
- Hu, W.; Li, W.; Xie, S.; Fagundez, S.; McAvoy, R.; Deng, Z.; Li, Y. Kn1 gene overexpression drastically improves genetic transformation efficiencies of citrus cultivars. Plant Cell Tissue Organ Cult. 2016, 125, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Orbović, V.; Grosser, J.W. Citrus transformation using juvenile tissue explants. In Agrobacterium Protocols; Wang, K., Ed.; Springer: New York, NY, USA, 2015; Volume 1224, pp. 245–257. [Google Scholar]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef]
- Ballester, A.; Cervera, M.; Pena, L. Evaluation of selection strategies alternative to nptII in genetic transformation of citrus. Plant Cell Rep. 2008, 27, 1005–1015. [Google Scholar] [CrossRef]
- Gelvin, S.B. Plant DNA Repair and Agrobacterium T-DNA Integration. Int. J. Mol. Sci. 2021, 22, 8458. [Google Scholar] [CrossRef]
- Engström, P.; Zambryski, P.; Van Montagu, M.; Stachel, S. Characterization of Agrobacterium tumefaciens virulence proteins induced by the plant factor acetosyringone. J. Mol. Biol. 1987, 197, 635–645. [Google Scholar] [CrossRef]
- Dutt, M.; Vasconcellos, M.; Grosser, J.W. Effects of antioxidants on Agrobacterium-mediated transformation and accelerated production of transgenic plants of Mexican lime (Citrus aurantifolia Swingle). Plant Cell Tissue Organ Cult. 2011, 107, 79–89. [Google Scholar] [CrossRef]
- Dan, Y.; Armstrong, C.L.; Dong, J.; Feng, X.; Fry, J.E.; Keithly, G.E.; Martinell, B.J.; Roberts, G.A.; Smith, L.A.; Tan, L.J.; et al. Lipoic Acid—An unique plant transformation enhancer. In Vitr. Cell. Dev. Biol. Plant 2009, 45, 630–638. [Google Scholar] [CrossRef]
- Nudin, N.F.H.; van Kronenburg, B.; Tinnenbroek, I.; Krens, F. The importance of salicylic acid and an improved plant condition in determining success in Agrobacterium-mediated transformation. In Proceedings of the 25th International EUCARPIA Symposium Section Ornamentals: Crossing Borders, XXV International EUCARPIA Symposium Section Ornamentals: Crossing Borders, Melle, Belgium, 28 June–2 July 2015; Van Huylenbroeck, J., Dhooghe, E., Eds.; International Society for Horticultural Science: Melle, Belgium, 2015; Volume 1087, pp. 65–69. [Google Scholar]
- Yang, L.; Hu, W.; Xie, Y.; Li, Y.; Deng, Z. Factors affecting Agrobacterium-mediated transformation efficiency of kumquat seedling internodal stem segments. Sci. Hortic. 2016, 209, 105–112. [Google Scholar] [CrossRef]
- Sassi, M.; Ruberti, I.; Vernoux, T.; Xu, J. Shedding light on auxin movement: Light-regulation of polar auxin transport in the photocontrol of plant development. Plant Signal. Behav. 2013, 8, e23355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokawa, K.; Koshiba, T.; Baluška, F. Light-dependent control of redox balance and auxin biosynthesis in plants. Plant Signal. Behav. 2014, 9, e29522. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Fagundez, S.; Katin-Grazzini, L.K.; Li, Y.; Li, W.; Chen, Y.; Wang, X.; Deng, Z.; Xie, S.; McAvoy, R.J.; et al. Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Hortic. Res. 2017, 4, 17071. [Google Scholar] [CrossRef] [Green Version]
- Gelvin, S.B. Agrobacterium virulence gene induction. In Agrobacterium Protocols Methods in Molecular Biology; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; Volume 343, pp. 77–85. [Google Scholar]
- Alt-Mörbe, J.; Kühmann, H.; Schröder, J. Differences in induction of Ti-plasmid virulence genes virG and virD and continued control of virD expression by four external factors. Mol. Plant-Microbe Interact. 1989, 2, 301–308. [Google Scholar] [CrossRef]
- Salas, M.; Park, S.; Srivatanakul, M.; Smith, R. Temperature influence on stable T-DNA integration in plant cells. Plant Cell Rep. 2001, 20, 701–705. [Google Scholar] [CrossRef]
- Su, G.; Park, S.; Lee, S.; Murai, N. Low co-cultivation temperature at 20 °C resulted in the reproducible maximum increase in both the fresh weight yield and stable expression of GUS activity after Agrobacterium tumefaciens-mediated transformation of tobacco leaf disks. Am. J. Plant Sci. 2012, 3, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Birch, R.G. Plant transformation: Problems and strategies for practical application. Annu. Rev. Plant Physiol. 1997, 48, 297–326. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Dias, J.M.; Molina, R.V.; BordÓN, Y.; Guardiola, J.L.; GarcÍA-Luis, A. Direct and indirect shoot organogenic pathways in epicotyl cuttings of Troyer citrange differ in hormone requirements and in their response to light. Ann. Bot. 2000, 85, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Soumya, P.R.; Kumar, P.; Pal, M. Paclobutrazol: A novel plant growth regulator and multi-stress ameliorant. Indian J. Plant Physiol. 2017, 22, 267–278. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Taha, N.A.; Taher, D.I.; Metwaly, M.M.; El-Beltagi, H.S.; Rezk, A.A.; El-Ganainy, S.M.; Shehata, W.F.; El-Ramady, H.R.; Bayoumi, Y.A. Paclobutrazol improves the quality of tomato seedlings to be resistant to Alternaria solani Blight disease: Biochemical and histological perspectives. Plants 2022, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Hamama, L.; Voisine, L.; Naouar, A.; Gala, R.; Cesbron, D.; Michel, G.; Dorion, N. Effect of GA3 and paclobutrazol on adventitious shoot regeneration of two Pelargonium sp. Acta Hortic. 2012, 961, 187–194. [Google Scholar] [CrossRef]
- Moradi, S.; Dianati Daylami, S.; Arab, M.; Vahdati, K. Direct somatic embryogenesis in Epipactis veratrifolia, a temperate terrestrial orchid. J. Hortic. Sci. Biotechnol. 2017, 92, 88–97. [Google Scholar] [CrossRef]
- Dan, Y.; Zhang, S.; Zhong, H.; Yi, H.; Sainz, M.B. Novel compounds that enhance Agrobacterium-mediated plant transformation by mitigating oxidative stress. Plant Cell Rep. 2015, 34, 291–309. [Google Scholar] [CrossRef]
- Dan, Y.; Zhang, S.; Matherly, A. Regulation of hydrogen peroxide accumulation and death of Agrobacterium-transformed cells in tomato transformation. Plant Cell Tissue Organ Cult. 2016, 127, 229–236. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Miller, J. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1972; p. 433. [Google Scholar]


| Transformation Efficiency (%) 1 | Compared with Control | |
|---|---|---|
| Control | 11.53 ± 1.68 a | 100% |
| Agrobacterium + explant treatment | 32.80 ± 5.45 b | 258% |
| Agrobacterium + explant treatment + PBZ + LA 2 | 52.34 ± 1.10 c | 452% |
| PCR Experiment | No. of Tested Shoots | No. of PCR Confirmed Transgenic Shoots | Confirmation Rate (%) | |||
|---|---|---|---|---|---|---|
| GUS Positive | GUS Negative | From GUS Positive | From GUS Negative | From GUS Positive | From GUS Negative | |
| 1 | 15 | 3 | 15 | 0 | 100 | 0 |
| 2 | 14 | 2 | 14 | 0 | 100 | 0 |
| 3 | 13 | 2 | 13 | 0 | 100 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tang, D.; Liu, Z.; Chen, J.; Cheng, B.; Kumar, R.; Yer, H.; Li, Y. An Improved Procedure for Agrobacterium-Mediated Transformation of ‘Carrizo’ Citrange. Plants 2022, 11, 1457. https://doi.org/10.3390/plants11111457
Li Y, Tang D, Liu Z, Chen J, Cheng B, Kumar R, Yer H, Li Y. An Improved Procedure for Agrobacterium-Mediated Transformation of ‘Carrizo’ Citrange. Plants. 2022; 11(11):1457. https://doi.org/10.3390/plants11111457
Chicago/Turabian StyleLi, Yanjun, Dan Tang, Zongrang Liu, Jianjun Chen, Baoping Cheng, Rahul Kumar, Huseyin Yer, and Yi Li. 2022. "An Improved Procedure for Agrobacterium-Mediated Transformation of ‘Carrizo’ Citrange" Plants 11, no. 11: 1457. https://doi.org/10.3390/plants11111457
APA StyleLi, Y., Tang, D., Liu, Z., Chen, J., Cheng, B., Kumar, R., Yer, H., & Li, Y. (2022). An Improved Procedure for Agrobacterium-Mediated Transformation of ‘Carrizo’ Citrange. Plants, 11(11), 1457. https://doi.org/10.3390/plants11111457








