Enrichment of Grapes with Zinc-Efficiency of Foliar Fertilization with ZnSO4 and ZnO and Implications on Winemaking
Abstract
:1. Introduction
2. Results
2.1. Fields Characteristics for Agronomic Enrichment with Zinc
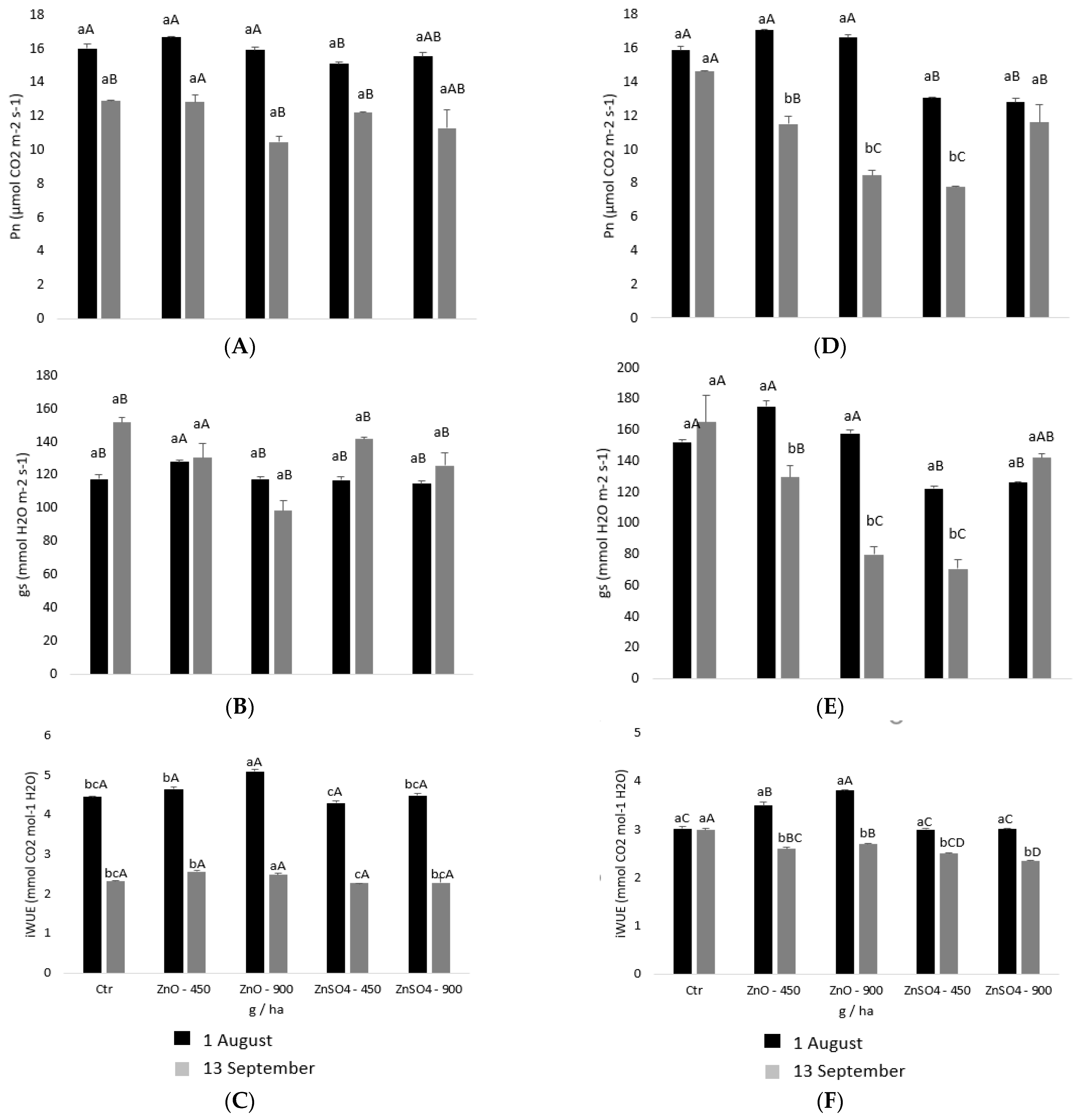
2.2. Physiological Monitoring of Photoassimilates during Zinc Enrichment
2.3. Nutrient Contents in Leaves and Grapes during Zinc Enrichment
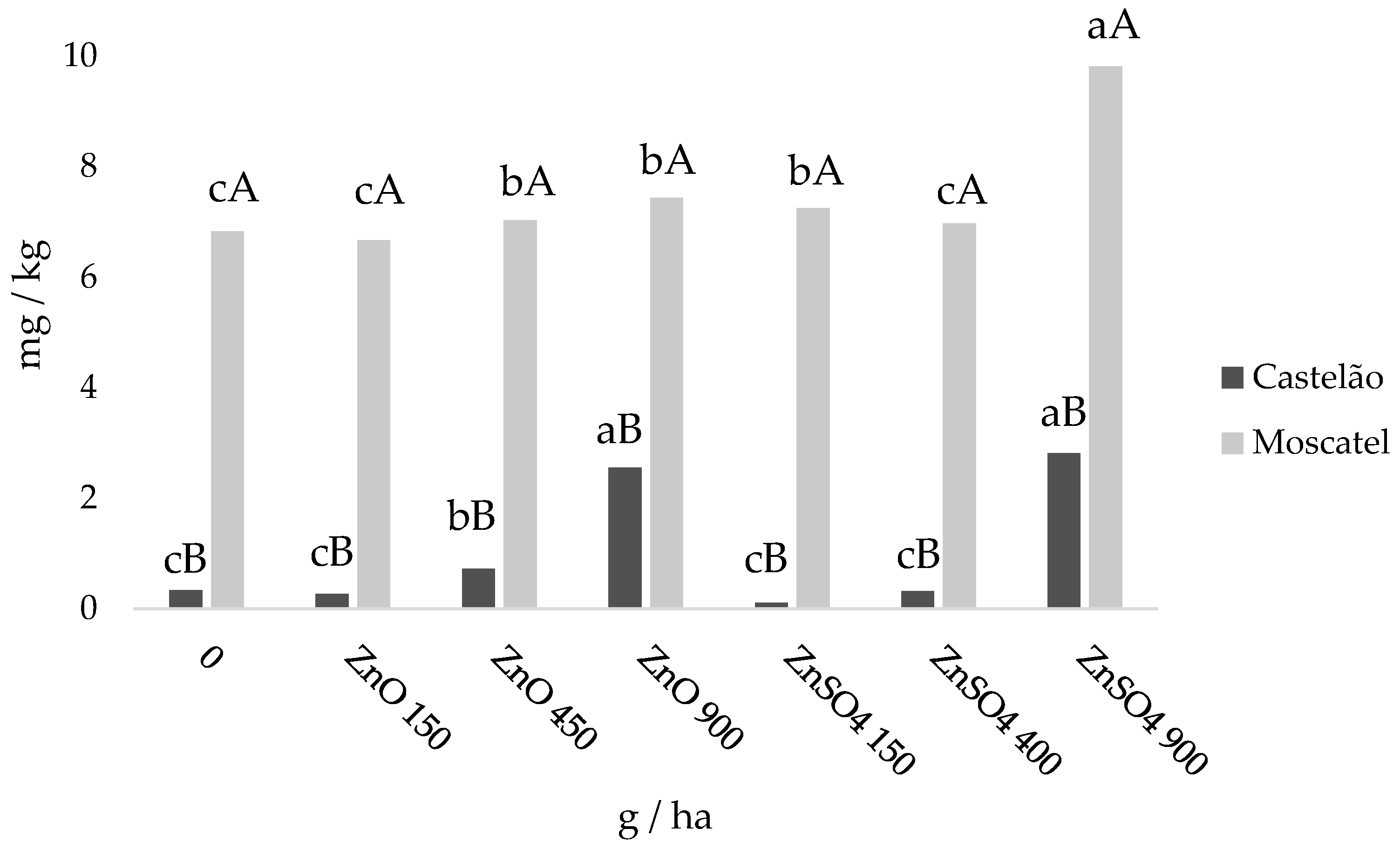
2.4. Zn Accumulation in Grapes at Harvest
2.5. Physicochemical Characteristics and Colorimetric Analysis of Grapes
2.6. Zn Accumulation in Wine
3. Discussion
4. Materials and Methods
4.1. Experimental Fields
4.2. Orthophotomap
4.3. Soil and Irrigation Water Analysis
4.4. Leaf Gas Exchange Measurements
4.5. Analysis of Nutrient Contents in Grapes and Leaves
4.6. Analysis of Total Zinc Content in Grapes and Wine
4.7. Analysis of Zinc Content in Grape Tissues
4.8. Morphometric and Colorimetric Analyses
4.9. Winemaking
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barak, P.; Helmke, P.A. The chemistry of zinc. In Zinc in Sil and Plants; Robson, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 1–13. [Google Scholar]
- Auld, D.S. Zinc coordination sphere in biochemical zinc sites. Biometals 2001, 14, 271–313. [Google Scholar] [CrossRef]
- Klug, A. Zinc finger peptides for the regulation of gene expression. J. Mol. Biol. 1999, 293, 215–218. [Google Scholar] [CrossRef]
- Englbrecht, C.C.; Schoof, H.; Böhm, S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Liu, Y.; Zhang, W.; Chen, X.; Zou, C. Agronomic approach of zinc biofortification can increase zinc bioavailability in wheat flour and thereby reduce zinc deficiency in humans. Nutrients 2017, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Webb, E.C. Enzyme Nomenclature, Recommendations of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology; Academic Press: New York, NY, USA, 1992; p. 862. [Google Scholar]
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- Uwitonze, A.M.; Ojeh, N.; Murererehe, J.; Atfi, A.; Razzaque, M.S. Zinc adequacy is essential for the maintenance of optimal oral health. Nutrients 2020, 12, 949. [Google Scholar] [CrossRef] [Green Version]
- IAEA—International Atomic Energy Agency. Available online: https://www.iaea.org/opic/annual-report-2018 (accessed on 7 February 2018).
- Rugeles-Reyes, S.M.; Cecílio, A.B.; Aguilar, M.A.L.; Silva, P.H.S. Foliar application of zinc in the agronomic biofortification of arugula. Food Sci. Technol. 2019, 39, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Chasapis, C.T.; Ntoupa, P.S.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Gottstein, T.; Reinhold, D. Zinc deficiency-An independent risk factor in the pathogenesis of haemorrhagic stroke? Nutrients 2020, 12, 3548. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Bouis, H.E.; Welch, R.M. Biofortification—A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010, 50, S20–S32. [Google Scholar] [CrossRef] [Green Version]
- Pal, V.; Singh, G.; Dhaliwal, S.S. Agronomic biofortification of chickpea with zinc and iron through application of zinc and urea. Commun. Soil Sci. Plant Anal. 2019, 50, 1864–1877. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Hussain, S.; Ishfaq, M.; Matloob, A.; Ali, N.; Ahmad, M.; Alyemeni, M.N.; Ahmad, P. Zinc-induced efects on productivity, zinc use eficiency, and grain biofortification of bread wheat under diferent tillage permutations. Agronomy 2020, 10, 1566. [Google Scholar] [CrossRef]
- Hussain, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad, M.; Dar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanistic impact of zinc deficiency in human development. Front. Nutr. 2022, 9, 717064. [Google Scholar] [CrossRef]
- Erenoglu, E.B.; Kutman, U.B.; Ceylan, Y.; Yildiz, B.; Cakmak, I. Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc (65Zn) in wheat. New Phytol. 2011, 189, 438–448. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; dos Reis, A.R. The molecular genetics of zinc uptake and utilization efficiency in crop plants. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burrit, D.J., Tran, L.P., Fujiwara, T., Eds.; Elsevier Science: London, UK, 2018; pp. 87–108. [Google Scholar] [CrossRef]
- Rose, T.J.; Impa, S.M.; Rose, M.T.; Pariasca-Tanaka, J.; Mori, A.; Heuer, S.; Johnson-Beebout, S.E.; Wissuwa, M. Enhancing phosphorus and zinc acquisition efficiency in rice: A critical review of root traits and their potential utility in rice breeding. Ann. Bot. 2013, 112, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Y.; Liu, Y.M.; Zhang, W.; Chen, X.P.; Zou, C.Q. Zinc Uptake, Translocation, and remobilization in winter wheat as affected by soil application of Zn fertilizer. Front. Plant Sci. 2019, 10, 426. [Google Scholar] [CrossRef]
- Xue, Y.; Yue, S.; Zhang, W.; Liu, D.; Cui, Z.; Chen, X.; Ye, Y.; Zou, C. Zinc, iron, manganese and copper uptake requirement in response to nitrogen supply and the increased grain yield of summer maize. PLoS ONE 2014, 9, e93895. [Google Scholar] [CrossRef]
- Dwivedi, R.S.; Randhawa, N.S.; Bansal, R.L. Phosphorus-zinc interaction: I. Sites of immobilization of zinc in maize at a high level of phosphorus. Plant Soil 1975, 43, 639–648. [Google Scholar] [CrossRef]
- Impa, S.M.; Morete, M.J.; Ismail, A.M.; Schulin, R.; Johnson-Beebout, S.E. Zn uptake, translocation and grain Zn loading in rice (Oryza sativa L.) genotypes selected for Zn deficiency tolerance and high grain Zn. J. Exp. Bot. 2013, 64, 2739–2751. [Google Scholar] [CrossRef]
- Stomph, T.J.; Jiang, W.; Van Der Putten, P.E.L.; Struik, P.C. Zinc allocation and re-allocation in rice. Front. Plant Sci. 2014, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Rengel, Z.; Batten, G.D.; Crowley, D.E. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crop. Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Sandhu, P.S.; Behera, S.K.; Singh, P.; Kaur, J.; Singh, H.; Abdel-Hafez, S.H.; et al. Interactive effects of foliar application of zinc, iron and nitrogen on productivity and nutritional quality of Indian mustard (Brassica juncea L.). Agronomy 2021, 11, 2333. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Schulin, R.; Chaney, R.L.; Daneshbakhsh, B.; Afyuni, M. Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture: A review. Agron. Sustain. Dev. 2010, 30, 83–107. [Google Scholar] [CrossRef] [Green Version]
- Pendias, K.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: London, UK, 2001. [Google Scholar]
- Ohnishi, M.; Furutani, R.; Sohtome, T.; Suzuki, T.; Wada, S.; Tanaka, S.; Ifuku, K.; Ueno, D.; Miyake, C. Photosynthetic parameters show specific responses to essential mineral deficiencies. Antioxidants 2021, 10, 996. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelkp, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Ahmad, P.; Alymeni, M.N.; Al-Hugail, A.A.; Algahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Jalal, A.; Shah, S.; Filho, M.C.M.T.; Khan, A.; Shah, T.; Ilyas, M.; Rosa, P.A.L. Agro-Biofortification of zinc and iron in wheat grains. Gesunde Pflanz. 2020, 72, 227–236. [Google Scholar] [CrossRef]
- Dutta, T.; Neelapu, N.R.R.; Surekha, C. Iron, zinc, and copper application in overcoming environmental stress. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives, 1st ed.; Roychoudhury, A., Tripathi, D.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 582–596. [Google Scholar]
- Kandoliya, R.U.; Sakarvadiya, H.L.; Kunjadia, B.B. Effect of zinc and iron application on leaf chlorophyll, carotenoid, grain yield and quality of wheat in calcareous soil of Saurashtra region. Int. J. Chem. Stud. 2018, 6, 2092–2096. [Google Scholar]
- McKevith, B. Nutritional aspects of cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Ozturk, L.; Yazici, M.A.; Yucel, C.; Torun, A.; Cekic, C.; Bagci, A.; Ozkan, H.; Braun, H.J.; Sayers, Z.; Cakmak, I. Concentration and localization of zinc during seed development and germination in wheat. Plant Physiol. 2006, 128, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Aisbitt, B.; Caswell, H.; Lunn, J. Cereals–current and emerging nutritional issues. Nutr. Bull. 2008, 33, 169–185. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.W.; Tian, X.H.; Wang, S.X.; Chen, Y.L. Effect of nitrogen fertilizer and foliar zinc application at different growth stages on zinc translocation and utilization efficiency in winter wheat. Cereal Res. Commun. 2013, 42, 81–90. [Google Scholar] [CrossRef]
- Abdoli, M.; Esfandiari, E.; Mousavi, S.B.; Sadeghzadeh, B. Effects of foliar application of zinc sulfate at different phenological stages on yield formation and grain zinc content of bread wheat (cv. Kohdasht). Azarian J. Agric. 2014, 1, 11–16. [Google Scholar]
- Aisha, I.; Muhammad, Y.A.; Mumtaz, H.; Muhammad, A.; Rashid, A.; Ali, K. Effect of micronutrients (zn, cu and b) on photosynthetic and fruit yield attributes of citrus reticulata blanco variety kinnow. Pak. J. Bot. 2015, 47, 1241–1247. [Google Scholar]
- Teixeira, R.F.M.; Domingos, T.; Costa, A.P.S.V.; Oliveira, R.; Farropas, L.; Calouro, F.; Barradas, A.M.; Carneiro, J.P.B.G. Soil organic matter dynamics in Portuguese natural and sown rainfed grasslands. Ecol. Model. 2011, 222, 993–1001. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soils to Humans; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; ISBN 3-540-32713-4. [Google Scholar]
- Racena, R.; Garcia-Lopez, A.M.; Delgado, A. Zinc uptake by plants as affected by fertilization with Zn sulfate, phosphorous availability and soil properties. Plants 2021, 11, 390. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of Zinc Nutrition for Increasing Zinc Availability, Uptake, Yield, and Quality of Maize (Zea mays L.) Grains: An Overview. Commun. Soil Sci. Plant Anal. 2020, 51, 2001–2021. [Google Scholar] [CrossRef]
- Luís, I.C.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Coelho, A.R.F.; Simões, M.; Patanita, M.; Dôres, J.; Ramalho, J.C.; Silva, M.M.; et al. Zinc enrichment in two contrasting genotypes of Triticum aestivum L. grains: Interactions between edaphic conditions and foliar fertilizers. Plants 2021, 10, 204. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmad, F.; Abid, M.; Ullah, M.A. Impact of zinc fertilization on gas exchange characteristics and water use efficiency of cotton crop under arid environment. Pak. J. Bot. 2009, 41, 2189–2197. [Google Scholar]
- Saboor, A.; Ali, M.A.; Ahmed, N.; Skalicky, M.; Danish, S.; Fahad, S.; Hassan, F.; Hassan, M.M.; Brestic, M.; Sabagh, A.E.; et al. Biofertilizer-based zinc application enhances maize growth, gas exchange attributes, and yield in zinc-deficient soil. Agriculture 2021, 11, 310. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Moutinho-Pereira, J.M.; Correia, C.M.; Gonçalves, B.M.; Bacelar, E.A.; Torres-Pereira, J.M. Leaf Gas Exchange and Water Relations of Grapevines Grown in Three Different Conditions. Photosynthetica 2004, 42, 81–86. [Google Scholar] [CrossRef]
- De Oliveira, A.C.; Pegoraro, C.; Viana, V.E. The Future of Rice Demand: Quality Beyond Productivity, 1st ed.; Springer International Publishing: Cham, Switzerland, 2020; p. 541. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Zlatev, Z.S.; Leitão, A.E.; Pais, I.P.; Fortunato, A.S.; Lidon, F.C. Moderate water stress causes different stomatal and non-stomatal changes in the photosynthetic functioning of Phaseolus vulgaris L. genotypes. Plant Biol. 2013, 16, 133–146. [Google Scholar] [CrossRef]
- Christensen, P.; Jensen, F. Foliar uptake of zinc nutritional sprays: A study of application methods, timing, and materials. In Report of Research for Fresh Table Grapes; University of California: Oakland, CA, USA, 1976; Volume 5. [Google Scholar]
- Christensen, P.; Jensen, F.L. Grapevine response to concentrate and dilute application of two zinc compounds. Am. J. Enol. Vitic. 1978, 29, 213–216. [Google Scholar]
- Christensen, L.P.; Kasimatis, A.N.; Jensen, F.L. Grapevine Nutrition and Fertilization in the San Joaquin Valley; University of California: Oakland, CA, USA, 1982. [Google Scholar]
- Christensen, P. Additives don’t improve zinc uptake in grapevines. In California Agriculture; University of California: Oakland, CA, USA, 1986; Volume 40, pp. 22–23. [Google Scholar]
- Moyer, M.M.; Singer, S.D.; Davenport, J.R.; Hoheisel, G.-A. Vineyard Nutrient Management in Washington State; Pullman: Washington, DC, USA, 2018; p. 45. [Google Scholar]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition, 2nd ed.; Commonwealth Agriculture Bureaux: Bucks, UK, 1966; p. 547. [Google Scholar]
- Moore, D.P. Mechanisms of micronutrient uptake by plants. In Micronutrients in Agriculture; Mortvedt, J.J., Giordano, P.M., Lindsay, W.L., Eds.; Soil Science Society of America: Madison, WI, USA, 1972; pp. 171–192. [Google Scholar]
- Loneragan, J.F. The availability and absorption of trace elements in soil-plant systems and their relation to movement and concentration of trace elements in plants. In Trace Elements in Soil-Plant Animal Systems; Nicholas, D.J.D., Egan, A.R., Eds.; Academic Press: New York, NY, USA, 1975; pp. 109–134. [Google Scholar]
- Olsen, S.R. Micronutrient interactions. In Micronutrients in Agriculture; Mortvedt, J.J., Giordano, P.M., Lindsay, W.L., Eds.; Soil Science Society of America: Madison, WI, USA, 1972; pp. 243–261. [Google Scholar]
- Fageria, V.D. Nutrient interactions in crop plant. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Growth and nutrition concentrations of common bean, lowland rice, corn soybean, and wheat at different soil pH an an inceptisol. J. Pant Nutr. 1999, 22, 1495–1507. [Google Scholar] [CrossRef]
- Shukla, U.C.; Mukhi, A.K. Ameliorative role of Zn, K, and gypsum on maize: Growth under alkali soil conditions. Agron. J. 1980, 72, 85–88. [Google Scholar] [CrossRef]
- Reich, M.; Shahbaz, M.; Prajapati, D.H.; Parmar, S.; Hawkesford, M.J.; De Kok, L.J. Interactions of sulfate with other nutrients as revealed by H2S fumigation of chinese cabbage. Front. Plant Sci. 2016, 7, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, M.; Fox, R.I. Influence of phosphate fertilization on zinc adsorption by tropical soils. Soil Sci. Soc. Am. J. 1979, 43, 683–686. [Google Scholar] [CrossRef]
- Mandal, L.N. Influence of phosphorus and zinc application in the availability of zinc, copper, iron, manganese and phosphorus in waterlogged rice soils. Soil Sci. 1980, 130, 251–257. [Google Scholar] [CrossRef]
- Haldar, M.; Mandal, L.N. Effect of phosphorus and zinc on the growth and phosphorus, zinc, copper iron and manganese nutrition of rice. Plant Sci. 1981, 59, 415–425. [Google Scholar] [CrossRef]
- Mandal, B.; Mandal, L.N. Effect of phosphorus application on transformation of zinc fraction in soil and on the zinc nutrition of lowland rice. Plant Soil 1990, 121, 115–123. [Google Scholar] [CrossRef]
- Alloway, B.J. Fundamental aspects. In Zinc in Soils and Crop Nutrition; International Zinc Association: Brussels, Belgium, 2004; pp. 30–35. [Google Scholar]
- Bavaresco, L.; Gatti, M.; Fregoni, M. Nutritional deficiencis. In Methodologies and Results in Grapevine Research; Springer: Berlin/Heidelberg, Germany, 2010; pp. 165–191. [Google Scholar]
- Iyengar, S.S.; Martens, D.C.; Miller, W.P. Distribution and plant availability of soil zinc fractions. Soil Sci. Soc. Am. J. 1981, 105, 681–689. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Goor, B.J.; Wiersma, D. Chemical form of manganese and zinc in phloem exudates. Physiol. Plant. 1976, 36, 213–216. [Google Scholar] [CrossRef]
- Tiffin, L.O. The form and distribution of metals in plants: An overview. In Proceedings of the Hanford Life Sciences Symposium; Symposium Series; USA Department of Energy: Washington, DC, USA, 1977; pp. 315–334. [Google Scholar]
- Loneragan, J.F.; Snowgall, K.; Robson, A.D. Remobilization of nutrients and its significance in plant nutrition. In Transport and Transfer Process in Plants; Wardlaw, I.F., Passioura, J.B., Eds.; Academic Press: New York, NY, USA, 1976; pp. 463–469. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Zhang, Q.; Brown, P.H. Distribution and transport of foliar applied zinc in pistachio. J. Am. Soc. Hortic. Sci. 1999, 124, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano 2020, 7, 2411–2502. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Wang, J.H.; Mao, H.; Zhao, H.; Huang, D.; Wang, Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in loess plateau, China. Field Crop. Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Cruz, T.N.M.; Savassa, S.M.; Gomes, M.H.F.; Rodrigues, E.S.; Duran, N.M.; Almeida, E.; Martinelli, A.P.; Carvalho, H.W.P. Shedding light on the mechanisms of absorption and transport of ZnO nanoparticles by plants via in vivo X-ray spectroscopy. Environ. Sci. Nano 2017, 4, 2367–2376. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Lv, Z.; Cui, L.; Mao, H.; Kopittke, P.M. Using synchrotron based approaches to examine the foliar application of ZnSO4 and ZnO nanoparticles for field-grown winter wheat. J. Agric. Food Chem. 2018, 66, 2572–2579. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Kanellis, A.K. Grape. In Biochemistry of Fruit Ripening; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Magalhães, N. Tratado de Viticultura—A Videira, a Vinha e o “Terroir”; Publicações Chaves Ferreira: Lisboa, Portugal, 2008. [Google Scholar]
- Chang, E.H.; Jung, S.M.; Hur, Y.Y. Changes in the aromatic composition of grape cv. Cheongsoo wine depending on the degree of grape ripening. Food Sci. Biotechnol. 2014, 23, 1761–1771. [Google Scholar] [CrossRef]
- Trad, M.; Boge, M.; Hamda, H.B.; Renard, C.M.G.C.; Harbi, M. The Glucose-Fructose ratio of wild Tunisian grapes. Cogent Food Agric. 2017, 3, 1374156. [Google Scholar] [CrossRef]
- Liang, Z.; Sang, M.; Fan, P.; Wu, B.; Wang, L.; Yang, S.; Li, S. CIELAB coordinates in response to berry skin anthocyanins and their composition in Vitis. J. Food Sci. 2011, 76, C490–C497. [Google Scholar] [CrossRef]
- Regulation EU nº 251/2014 of the European Parliament and of the Council, 2014. Available online: https://eur-lex.europa.eu/homepage.html (accessed on 14 January 2022).
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Direcção Geral de Agricultura Desenvolvimento Rural. Carta de Capacidade de Uso do Solo de Portugal—Bases e Normas Adoptadas na Sua Elaboração, 6th ed.; Ministério da Economia, Secretaria de Estado da Agricultura, Serviço de Reconhecimento e de Ordenamento Agrário: Lisboa, Portugal, 1972; pp. 25–26. [Google Scholar]
- Pessoa, M.F.; Scotti-Campos, P.; Pais, I.; Feteiro, A.; Canuto, D.; Simões, M.; Pelica, J.; Pataco, I.; Ribeiro, V.; Reboredo, F.H.; et al. Nutritional profile of the Portuguese cabbage (Brassica oleracea L var. costata) and its relationship with the elemental soil analysis. Emir. J. Food Agric. 2016, 28, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Pelica, J.; Barbosa, S.; Lidon, F.; Pessoa, M.F.; Reboredo, F.; Calvão, T. The paradigm of high concentration of metals of natural or anthropogenic origin in soils—The case of Neves-Corvo mine area (Southern Portugal). J. Geochem. Explor. 2018, 186, 12–23. [Google Scholar] [CrossRef]
- Rodier, J.; Legube, B.; Merlet, N. L’Analyse de l’Eau, 9th ed.; Dunod: Paris, France, 2009; p. 1579. ISBN 9782100072460. [Google Scholar]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water analyses. EOS Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar] [CrossRef]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwell, F.; Goulão, L.; et al. Long-term air [CO2] strenghtens photosymthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Glob. Change Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef]
- Mangueze, A.V.J.; Pessoa, M.F.G.; Silva, M.J.; Ndayiragije, A.; Magaia, H.E.; Cossa, V.S.I.; Reboredo, F.H.; Carvalho, M.L.; Santos, J.P.; Guerra, M.; et al. Simultaneous zinc and selenium biofortification in rice. Accumulation, localization and implications on the overall mineral content of the flour. J. Cereal Sci. 2018, 82, 34–41. [Google Scholar] [CrossRef]
- Reboredo, F.H.S.; Ribeiro, C.A.G. Vertical distribution of Al, Cu, Fe and Zn in soil salt marshes of the Sado estuary, Portugal. Int.J. Environ. Stud. 1984, 23, 249–253. [Google Scholar] [CrossRef]
- Cardoso, P.; Mateus, T.C.; Velu, G.; Singh, R.P.; Santos, J.P.; Carvalho, M.L.; Lourenço, V.M.; Lidon, F.; Reboredo, F.; Guerra, M. Localization and distribution of Zn and Fe in grains of biofortified bread wheat lines through micro- and triaxial-X-ray fluorescence spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2018, 141, 70–79. [Google Scholar] [CrossRef]
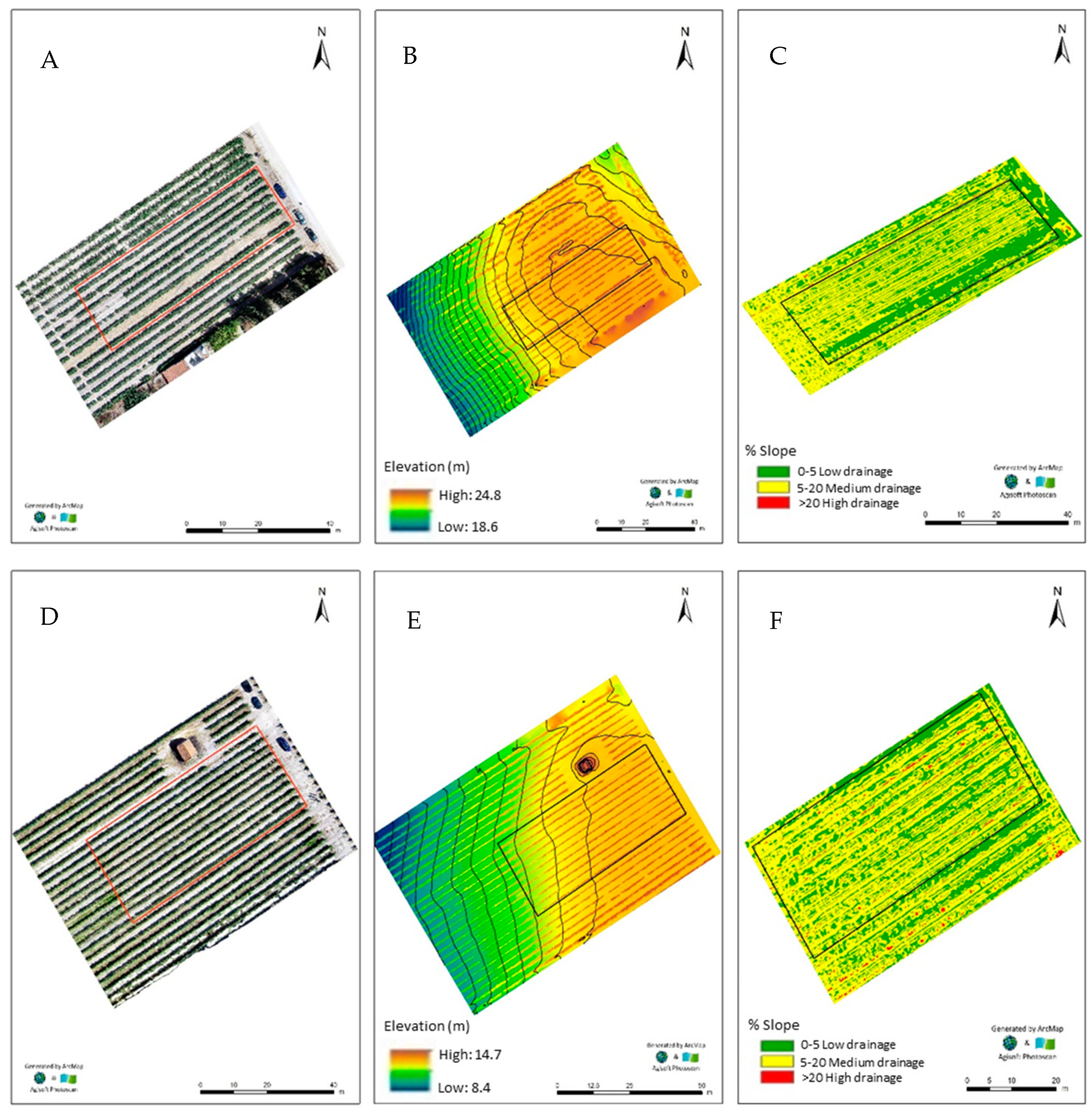
| Field | Ability to Accumulate or Drain Surface Water | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope Class (%) | Surface Drainage | Area (m2) | Area (%) | ||||||||
| Lagameças | 1—(0–5%) | Low | 437.7 | 49.38 | |||||||
| 2—(5–20%) | Moderate | 448.4 | 50.59 | ||||||||
| 3—>20% | High | 0.2 | 0.02 | ||||||||
| Lau Novo | 1—(0–5%) | Low | 589.9 | 34.87 | |||||||
| 2—(5–20%) | Moderate | 1080.5 | 63.86 | ||||||||
| 3—>20% | High | 21.4 | 1.27 | ||||||||
| Soil analysis (0–30 cm deep) (n = 28) | |||||||||||
| pH | Electrical Conductivity | Organic Matter | Ca | K | Mg | P | Fe | S | Zn | Mn | |
| μS cm−1 | % | mg/kg | |||||||||
| Lagameças | 7.08 ± 0.08 a | 100.83 ± 7.11 a | 1.48 ± 0.10 a | 0.28 ± 0.03 a | 2.53 ± 0.05 b | 0.07 ± 0.04 a | 0.14 ± 0.00 b | 0.47 ± 0.03 a | 36.82 ± 2.28 a | 34.65 ± 3.42 a | 191.41 ± 13.90 a |
| Lau Novo | 72.05 ± 2.90 b | 6.80 ± 0.06 b | 1.09 ± 0.04 b | 0.17 ± 0.01 b | 3.20 ± 0.05 a | 0.07 ± 0.00 a | 0.20 ± 0.02 a | 0.26 ± 0.01 b | 25.03 ± 8.12 a | 23.77 ± 1.88 b | 145.11 ± 6.98 b |
| Water analysis | |||||||||||
| pH | Electrical Conductivity | Ca2+ | K+ | Mg2+ | Na+ | Cl− | HCO3− | SO42− | NO3− | PO43− | |
| μS cm−1 | mg L−1 (meq L−1) | ||||||||||
| Lau Novo | 6.27 | 252.01 | 8.51 (0.40) | 3.98 (0.12) | 4.39 (0.31) | 21.73 (0.93) | 34.70 (0.90) | 34.77 (0.51) | 33.10 (0.63) | 17.40 (0.21) | <1.5 (<0.04) |
| Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Zn | Cu | Fe | Ca | K | S | P | |
| mg/kg | mg/kg | mg/kg | % | % | % | % | ||
| Castelão | Control | 33.87 ± 1.35 eA | 61.91 ± 4.51 bB | 110.47 ± 9.38 aA | 2.78 ± 0.06 dB | 2.60 ± 0.05 c,dA | 0.65 ± 0.02 aA | 0.25 ± 0.01 bA |
| ZnO (150 g ha−1) | 98.89 ± 2.03 dB | 81.20 ± 0.80 aB | 74.97 ± 0.90 aA | 3.60 ± 0.09 aB | 2.95 ± 0.07 bA | 0.61 ± 0.01 a,bA | 0.31 ± 0.00 aA | |
| ZnO (450 g ha−1) | 309.44 ± 1.49 bA | 72.08 ± 3.01 a,bB | 111.62 ± 5.94 aA | 3.21 ± 0.10 b,cB | 3.68 ± 0.06 aA | 0.65 ± 0.02 aA | 0.31 ± 0.01 aB | |
| ZnO (900 g ha−1) | 490.55 ± 12.32 aB | 40.27 ± 0.72 cB | 100.54 ± 12.19 aA | 3.71 ± 0.06 aA | 2.39 ± 0.06 dA | 0.54 ± 0.03 b,cA | 0.31 ± 0.02 aA | |
| ZnSO4 (150 g ha−1) | 284.22 ± 8.59 bA | 61.99 ± 5.26 bB | 103.69 ± 12.73 aB | 3.12 ± 0.03 cB | 3.01 ± 0.04 bA | 0.52 ± 0.01 cA | 0.24 ± 0.01 bA | |
| ZnSO4 (450 g ha−1) | 113.26 ± 1.49 dB | 66.47 ± 2.19 a,bB | 115.80 ± 6.36 aA | 3.51 ± 0.03 a,bB | 2.47 ± 0.01 c,dA | 0.58 ± 0.01 a,b,cA | 0.29 ± 0.01 a,bA | |
| ZnSO4 (900 g ha−1) | 196.35 ± 3.40 cB | 58.72 ± 2.78 bB | 96.85 ± 5.69 aA | 3.09 ± 0.02 cB | 2.68 ± 0.05 cA | 0.55 ± 0.01 b,cA | 0.26 ± 0.00 bB | |
| Moscatel | Control | 17.47 ± 3.09 eB | 1883.46 ± 4.81 b,cA | 60.29 ± 4.47 bB | 3.22 ± 0.06 eA | 2.35 ± 0.05 a,bA | 0.42 ± 0.00 cB | 0.20 ± 0.00 dB |
| ZnO (150 g ha−1) | 135.64 ± 3.72 cA | 2121.86 ± 67.14 a,bA | 74.42 ± 4.77 bA | 4.19 ± 0.11 b,cA | 2.48 ± 0.04 a,bB | 0.50 ± 0.02 bB | 0.31 ± 0.01 b,cA | |
| ZnO (450 g ha−1) | 289.95 ± 11.98 bA | 1492.61 ± 45.03 cA | 67.58 ± 9.79 bB | 4.53 ± 0.07 a,bA | 2.65 ± 0.06 aB | 0.57 ± 0.01 aB | 0.37 ± 0.01 aA | |
| ZnO (900 g ha−1) | 584.25 ± 6.39 aA | 2217.30 ± 20.80 a,bA | 93.73 ± 10.83 bA | 3.63 ± 0.03 d,eA | 2.30 ± 0.06 bA | 0.43 ± 0.00 b,cB | 0.37 ± 0.01 a,bA | |
| ZnSO4 (150 g ha−1) | 73.22 ± 3.83 dB | 2264.28 ± 158.37 a,bA | 168.73 ± 2.91 aA | 3.89 ± 0.05 c,dA | 2.27 ± 0.01 bB | 0.46 ± 0.03 b,cA | 0.26 ± 0.02 cA | |
| ZnSO4 (450 g ha−1) | 183.94 ± 5.87 cA | 2445.83 ± 67.57 aA | 96.46 ± 3.36 bA | 3.98 ± 0.10 c,dA | 2.41 ± 0.09 a,bA | 0.49 ± 0.01 b,cB | 0.31 ± 0.02 cA | |
| ZnSO4 (900 g ha−1) | 323.64 ± 22.52 bA | 1593.96 ± 144.08 cA | 98.19 ± 15.91 bA | 4.70 ± 0.15 aA | 2.30 ± 0.11 bB | 0.48 ± 0.02 b,cB | 0.29 ± 0.01 cA | |
| Grapes | ||||||||
| Treatments | Zn | Cu | Fe | Ca | K | S | P | |
| mg/kg | mg/kg | mg/kg | % | % | % | % | ||
| Castelão | Control | 7.19 ± 0.73 cB | n.d. | 0.53 ± 0.01 b,cB | 2.02 ± 0.07 aA | 0.16 ± 0.00 b,cA | 0.18 ± 0.01 dB | |
| ZnO (150 g ha−1) | 9.29 ± 0.36 b,cB | 0.81 ± 0.06 aA | 2.64 ± 0.13 aA | 0.21 ± 0.01 aA | 0.28 ± 0.01 aA | |||
| ZnO (450 g ha−1) | 9.99 ± 1.59 b,cA | 0.77 ± 0.06 a,bA | 2.36 ± 0.26 aA | 0.17 ± 0.01 b,cA | 0.21 ± 0.00 c,dA | |||
| ZnO (900 g ha−1) | 16.03 ± 0.34 aB | 0.81 ± 0.05 aA | 2.18 ± 0.01 aA | 0.20 ± 0.01 a,bA | 0.26 ± 0.01 a,bA | |||
| ZnSO4 (150 g ha−1) | 8.45 ± 0.75 b,cB | 0.63 ± 0.07 a,b,cA | 2.20 ± 0.27 aA | 0.20 ± 0.01 a,b,cA | 0.23 ± 0.01 b,cA | |||
| ZnSO4 (450 g ha−1) | 7.84 ± 0.41 b,cB | 0.66 ± 0.05 a,b,cA | 2.05 ± 0.04 aA | 0.19 ± 0.00 a,b,cA | 0.23 ± 0.01 b,cA | |||
| ZnSO4 (900 g ha−1) | 11.44 ± 1.08 bA | 0.48 ± 0.03 cB | 2.18 ± 0.02 aA | 0.16 ± 0.01 cA | 0.20 ± 0.00 c,dB | |||
| Moscatel | Control | 12.58 ± 0.49 cA | n.d. | 0.77 ± 0.05 a,bA | 2.06 ± 0.03 aA | 0.16 ± 0.00 a,bA | 0.29 ± 0.00 aA | |
| ZnO (150 g ha−1) | 11.38 ± 0.56 cA | 0.73 ± 0.05 a,bA | 1.83 ± 0.05 a,bB | 0.16 ± 0.00 a,bB | 0.23 ± 0.00 b,cB | |||
| ZnO (450 g ha−1) | 13.42 ± 0.09 b,cA | 0.64 ± 0.02 bA | 1.88 ± 0.07 a,bA | 0.16 ± 0.00 a,bA | 0.20 ± 0.01 c,dA | |||
| ZnO (900 g ha−1) | 21.16 ± 1.74 aA | 0.89 ± 0.03 aA | 1.87 ± 0.09 a,bB | 0.15 ± 0.01 bB | 0.22 ± 0.01 b,c,dA | |||
| ZnSO4 (150 g ha−1) | 11.47 ± 0.13 cA | 0.81 ± 0.06 a,bA | 1.56 ± 0.10 bA | 0.15 ± 0.01 a,bB | 0.19 ± 0.01 dA | |||
| ZnSO4 (450 g ha−1) | 16.73 ± 0.86 bA | 0.75 ± 0.04 a,bA | 1.83 ± 0.06 a,bB | 0.18 ± 0.01 aA | 0.25 ± 0.01 a,bA | |||
| ZnSO4 (900 g ha−1) | 14.41 ± 0.31 b,cA | 0.65 ± 0.03 bA | 1.58 ± 0.07 bB | 0.16 ± 0.00 a,bA | 0.23 ± 0.01 b,c,dA | |||
| Treatments | Zn (mg/kg) | |||
|---|---|---|---|---|
| Skin | Seeds | |||
| Castelão | Moscatel | Castelão | Moscatel | |
| Control | 20.06 ± 1.00 cB | 38.34 ± 1.92 aA | 16.02 ± 0.80 cA | 13.57 ± 0.68 cB |
| ZnO (150 g ha−1) | 16.61 ± 0.83 cB | 31.12 ± 1.56 bA | 10.96 ± 0.55 dB | 15.64 ± 0.78 cA |
| ZnO (450 g ha−1) | 21.78 ± 1.09 cB | 27.49 ± 1.37 bcA | 20.87 ± 1.04 bA | 14.59 ± 0.73 cB |
| ZnO (900 g ha−1) | 54.37 ± 2.72 aA | 22.68 ± 1.13 cB | 27.79 ± 1.39 aA | 16.82 ± 0.84 bcB |
| ZnSO4 (150 g ha−1) | 21.48 ± 1.07 cA | 21.17 ± 2.71 cA | 10.63 ± 0.53 dA | 9.82 ± 0.49 dA |
| ZnSO4 (450 g ha−1) | 17.53 ± 0.88 cB | 32.16 ± 1.61 abA | 15.48 ± 0.77 cB | 19.70 ± 0.99 bA |
| ZnSO4 (900 g ha−1) | 30.16 ± 1.51 bA | 23.25 ± 1.16 cB | 15.73 ± 0.79 cB | 28.59 ± 1.43 aA |
| Treatments | Wine | |
|---|---|---|
| Zn (µg L−1) | ||
| Castelão | Moscatel | |
| Control | 0.68 ± 0.27 aA | 0.54 ± 0.26 cA |
| ZnO (450 g ha−1) | 0.77 ± 0.10 aB | 1.20 ± 0.08 bA |
| ZnO (900 g ha−1) | 0.91 ± 0.08 aA | 1.05 ± 0.02 b,cA |
| ZnSO4 (450 g ha−1) | 0.89 ± 0.02 aB | 1.17 ± 0.06 bA |
| ZnSO4 (900 g ha−1) | 0.82 ± 0.14 aB | 1.92 ± 0.10 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daccak, D.; Lidon, F.C.; Pessoa, C.C.; Luís, I.C.; Coelho, A.R.F.; Marques, A.C.; Ramalho, J.C.; Silva, M.J.; Rodrigues, A.P.; Guerra, M.; et al. Enrichment of Grapes with Zinc-Efficiency of Foliar Fertilization with ZnSO4 and ZnO and Implications on Winemaking. Plants 2022, 11, 1399. https://doi.org/10.3390/plants11111399
Daccak D, Lidon FC, Pessoa CC, Luís IC, Coelho ARF, Marques AC, Ramalho JC, Silva MJ, Rodrigues AP, Guerra M, et al. Enrichment of Grapes with Zinc-Efficiency of Foliar Fertilization with ZnSO4 and ZnO and Implications on Winemaking. Plants. 2022; 11(11):1399. https://doi.org/10.3390/plants11111399
Chicago/Turabian StyleDaccak, Diana, Fernando C. Lidon, Cláudia Campos Pessoa, Inês Carmo Luís, Ana Rita F. Coelho, Ana Coelho Marques, José C. Ramalho, Maria José Silva, Ana Paula Rodrigues, Mauro Guerra, and et al. 2022. "Enrichment of Grapes with Zinc-Efficiency of Foliar Fertilization with ZnSO4 and ZnO and Implications on Winemaking" Plants 11, no. 11: 1399. https://doi.org/10.3390/plants11111399
APA StyleDaccak, D., Lidon, F. C., Pessoa, C. C., Luís, I. C., Coelho, A. R. F., Marques, A. C., Ramalho, J. C., Silva, M. J., Rodrigues, A. P., Guerra, M., Leitão, R. G., Campos, P. S., Pais, I. P., Semedo, J. N., Silva, M. M., Kullberg, J. C., Brito, M., Galhano, C., Legoinha, P., ... Reboredo, F. H. (2022). Enrichment of Grapes with Zinc-Efficiency of Foliar Fertilization with ZnSO4 and ZnO and Implications on Winemaking. Plants, 11(11), 1399. https://doi.org/10.3390/plants11111399


















