Secondary Succession Altered the Diversity and Co-Occurrence Networks of the Soil Bacterial Communities in Tropical Lowland Rainforests
Abstract
:1. Introduction
2. Results
2.1. Plant Community Composition and Diversity
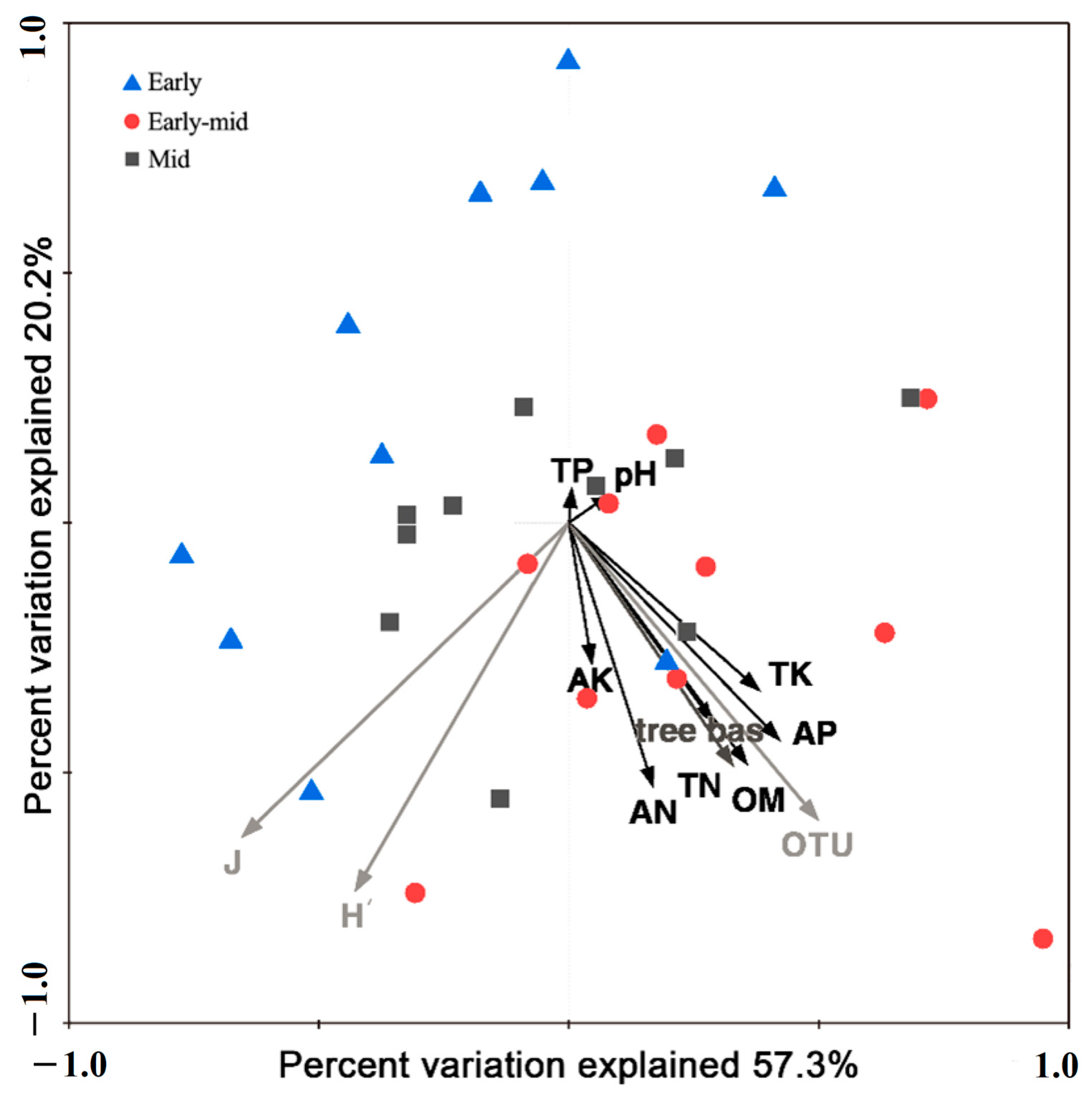
2.2. Soil Properties among Different Successional Stages
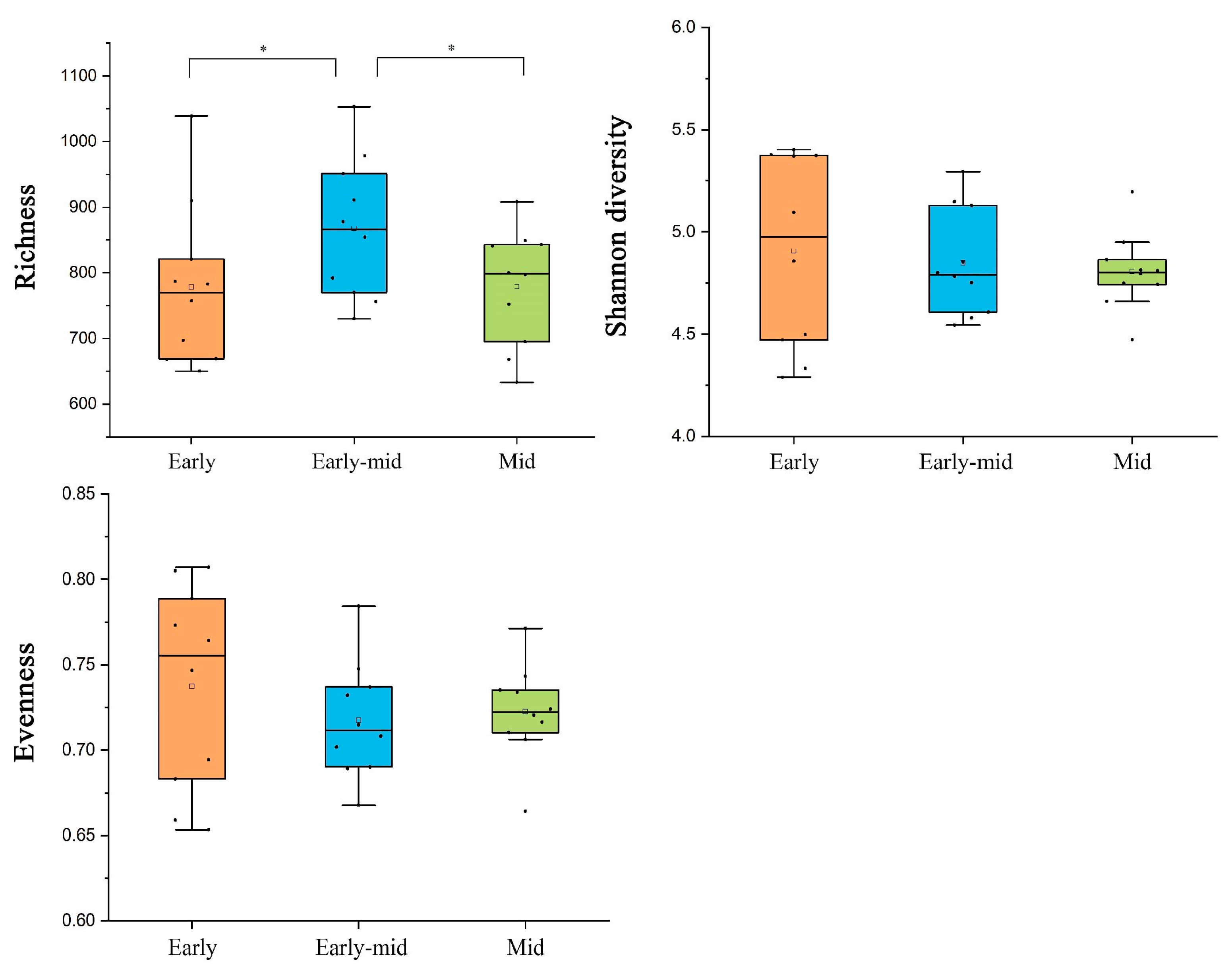
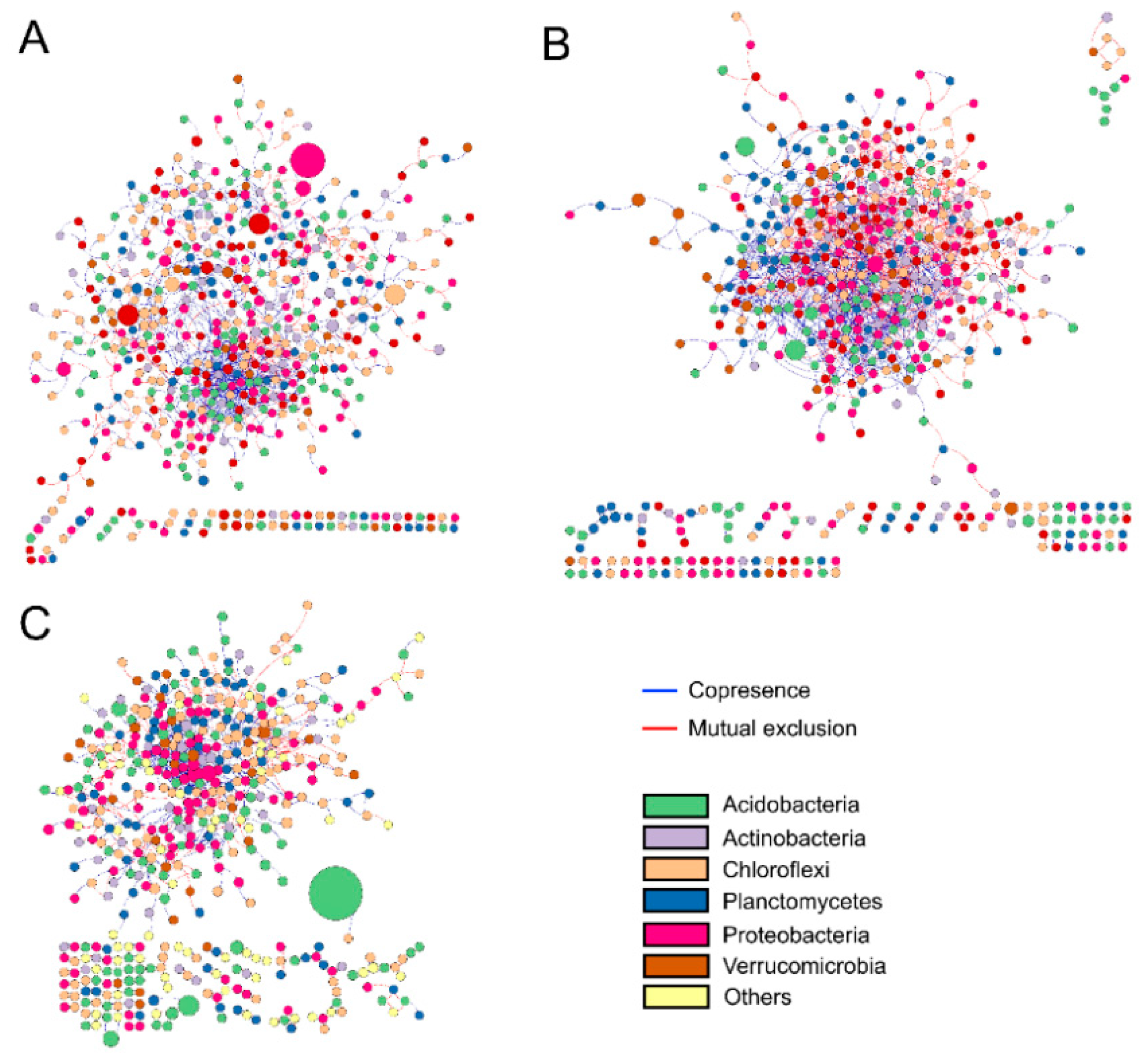
2.3. Soil Bacterial Diversity and Networks
2.4. Niche Overlap and Interspecific Linkages of Leguminous Trees
3. Discussion
3.1. The Development Direction of Plant Community Diversity and Interspecific Relationship of Leguminous Trees
3.2. Soil Properties and Soil Bacteria Community Characteristics along the Succession
3.3. Effects of Natural Succession on Bacterial Co-Occurrence Network Interactions
4. Materials and Methods
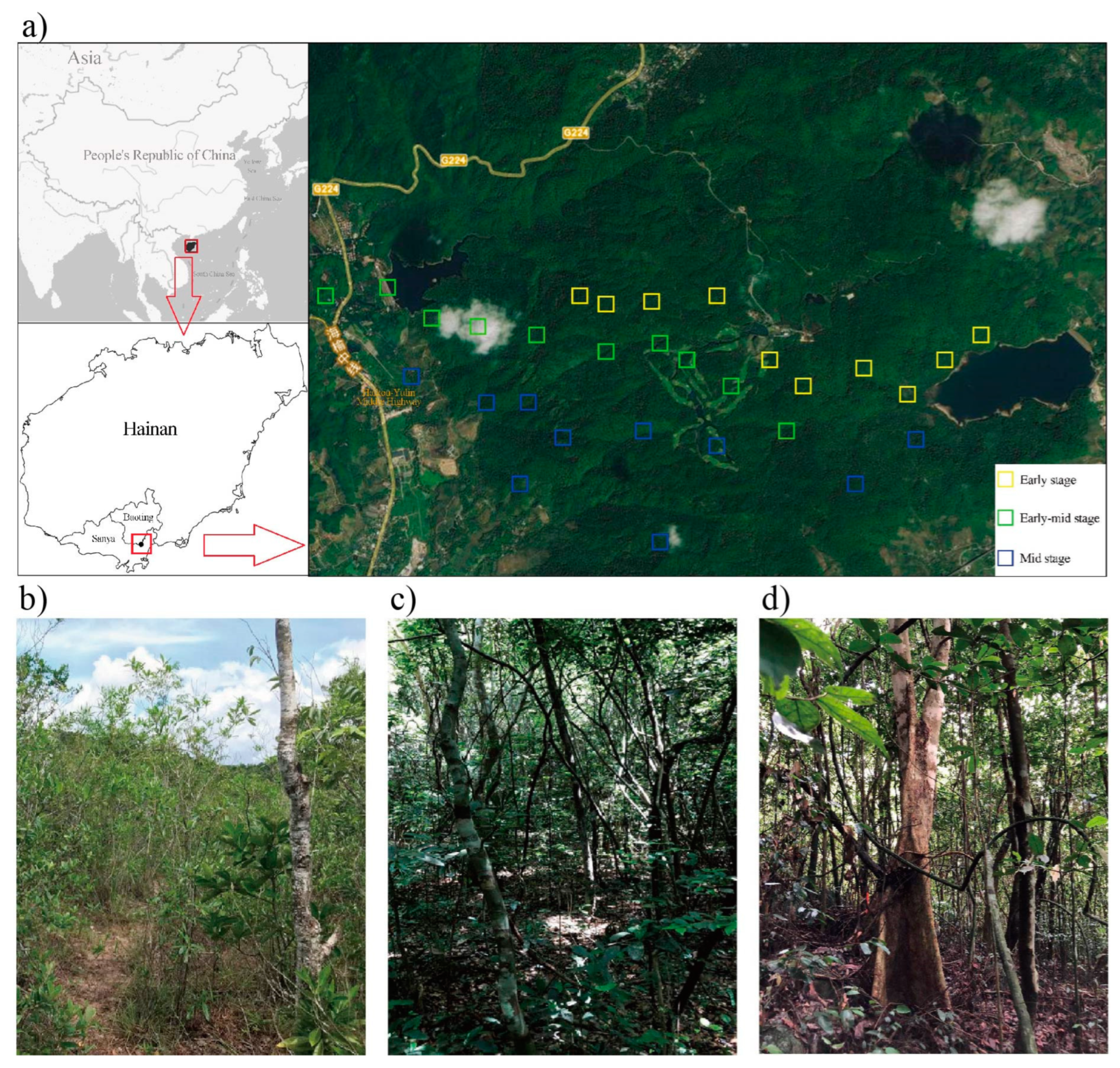
4.1. Study Site and Experimental Design
4.2. Plant Community Surveying and Soil Sampling
4.3. Analysis of Soil Physical and Chemical Properties
4.4. Soil Samples DNA Extraction, PCR Amplification, and Sequencing
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quijas, S.; Romero-Duque, L.P.; Trilleras, J.M.; Conti, G.; Kolb, M.; Brignone, E.; Dellafiore, C. Linking biodiversity, ecosystem services, and beneficiaries of tropical dry forests of Latin America: Review and new perspectives. Ecosyst. Serv. 2019, 36, 100909. [Google Scholar] [CrossRef]
- Teixeira, H.M.; Cardoso, I.M.; Bianchi, F.J.J.A.; Silva, A.D.C.; Jamme, D.; Peña-Claros, M. Linking vegetation and soil functions during secondary forest succession in the Atlantic forest. For. Ecol. Manag. 2020, 457, 117696. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Kim, D.G.; Peng, C.; Shangguan, Z. Controls of soil and aggregate-associated organic carbon variations following natural vegetation restoration on the Loess Plateau in China. Land Degrad. Dev. 2018, 29, 3974–3984. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Bakker, M.G.; Bradeen, J.M.; Kinkel, L.L. Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 2015, 96, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satdichanh, M.; Ma, H.; Yan, K.; Dossa, G.G.O.; Winowiecki, L.; Vågen, T.G.; Gassner, A.; Xu, J.; Harrison, R.D. Phylogenetic diversity correlated with above-ground biomass production during forest succession: Evidence from tropical forests in southeast asia. J. Ecol. 2019, 107, 1419–1432. [Google Scholar] [CrossRef]
- Qu, Z.L.; Liu, B.; Ma, Y.; Xu, J.; Sun, H. The response of the soil bacterial community and function to forest succession caused by forest disease. Funct. Ecol. 2020, 34, 2548–2559. [Google Scholar] [CrossRef]
- Matsuo, T.; Martínez-Ramos, M.; Bongers, F.; Sande, M.T.V.D.; Poorter, L. Forest structure drives changes in light heterogeneity during tropical secondary forest succession. J. Ecol. 2021, 109, 2871–2884. [Google Scholar] [CrossRef]
- Uroy, L.; Ernoult, A.; Mony, C. Effect of landscape connectivity on plant communities: A review of response patterns. Landsc. Ecol. 2019, 34, 203–225. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Kessler, A.; Bauerle, T.L. Phenolic root exudate and tissue compounds vary widely among temperate forest tree species and have contrasting effects on soil microbial respiration. New Phytol. 2018, 218, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Santalahti, M.; Sun, H.; Jumpponen, A.; Pennanen, T.; Heinonsalo, J. Vertical and seasonal dynamics of fungal communities in boreal Scots pine forest soil. FEMS Microbiol. Ecol. 2016, 92, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herridge, D.F.; Giller, K.E.; Jensen, E.S.; Peoples, M.B. Quantifying country-to-global scale nitrogen fixation for grain legumes II. Coefficients, templates and estimates for soybean, groundnut and pulses. Plant Soil 2021, 469, 1–14. [Google Scholar] [CrossRef]
- Schwember, A.R.; Schulze, J.; Del Pozo, A.; Cabeza, R.A. Regulation of symbiotic nitrogen fixation in legume root nodules. Plants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakraliya, S.K.; Singh, U.; Bohra, A.; Choudhary, K.K.; Kumar, S.; Meena, R.S.; Jat, M.L. Nitrogen and legumes: A meta-analysis. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 277–314. [Google Scholar]
- Roesch, L.F.W.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.M.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.O.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef]
- Banning, N.C.; Gleeson, D.B.; Grigg, A.H.; Grant, C.D.; Andersen, G.L.; Brodie, E.L.; Murphy, D.V. Soil microbial community successional patterns during forest ecosystem restoration. Appl. Environ. Microbiol. 2011, 77, 6158–6164. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.Z.; Bai, L.; Wang, J.Y.; Deng, J.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J. Change in soil bacterial community during secondary succession depend on plant and soil characteristics. Catena 2019, 173, 246–252. [Google Scholar] [CrossRef]
- Hu, X.; Shu, Q.; Shang, Z.; Guo, W.; Qi, L. Secondary Succession in the Tropical Lowland Rainforest Reduced the Stochasticity of Soil Bacterial Communities through the Stability of Plant Communities. Forests 2022, 13, 348. [Google Scholar] [CrossRef]
- Powers, J.S.; Marín-Spiotta, E. Ecosystem processes and biogeochemical cycles in secondary tropical forest succession. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 497–519. [Google Scholar] [CrossRef] [Green Version]
- Alamgir, M.; Turton, S.M.; Macgregor, C.J.; Pert, P.L. Assessing regulating and provisioning ecosystem services in a contrasting tropical forest landscape. Ecol. Indic. 2016, 64, 319–334. [Google Scholar] [CrossRef]
- Nyirambangutse, B.; Zibera, E.; Uwizeye, F.K.; Nsabimana, D.; Bizuru, E.; Pleijel, H.; Uddling, J.; Wallin, G. Carbon stocks and dynamics at different successional stages in an Afromontane tropical forest. Biogeosciences 2017, 14, 1285–1303. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.L.; Edwards, D.P.; Galbraith, D. Increasing human dominance of tropical forests. Science 2015, 349, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Baccini, A.; Walker, W.; Carvalho, L.; Farina, M.; Sulla-Menashe, D.; Houghton, R.A. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 2017, 358, 230–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, L.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.A. The Study on Evolutional History of Hainan Island’s Ecological Environment-From the Point of Animal and Plant; Nanjing Agricultural University: Nanjing, China, 2006. [Google Scholar]
- Brum, H.D.; Souza, A.F. Flood disturbance and shade stress shape the population structure of açaí palm Euterpe precatoria, the most abundant Amazon species. Botany 2020, 98, 147–160. [Google Scholar] [CrossRef]
- Machacova, K.; Borak, L.; Agyei, T.; Schindler, T.; Soosaar, K.; Mander, U.; Ah-Peng, C. Trees as net sinks for methane (CH4) and nitrous oxide (N2O) in the lowland tropical rain forest on volcanic Réunion Island. New Phytol. 2021, 229, 1983–1994. [Google Scholar] [CrossRef]
- Lin, Q.; Dini-Andreote, F.; Li, L.; Umari, R.; Novotny, V.; Kukla, J.; Heděnec, P.; Frouz, J. Soil microbial interconnections along ecological restoration gradients of lowland forests after slash-and-burn agriculture. FEMS Microbiol. Ecol. 2021, 97, fiab063. [Google Scholar] [CrossRef]
- Cole, L.E.S.; Bhagwat, S.A.; Willis, K.J. Recovery and resilience of tropical forests after disturbance. Nat. Commun. 2014, 5, 3906. [Google Scholar] [CrossRef] [Green Version]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.A.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2011, 9, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Letcher, S.G. Phylogenetic structure of angiosperm communities during tropical forest succession. Proc. R. Soc. B Biol. Sci. 2010, 277, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Han, S.F. Research on nitrogen-fixing leguminous species and rhizobia resources of leguminous species. Sci. Silvae Sin. 1996, 32, 8. [Google Scholar]
- Hairiah, K.; Van Noordwijk, M.; Cadisch, G. Quantification of biological N2 fixation of hedgerow trees in Northern Lampung. NJAS-Wagening. J. Life Sci. 2000, 48, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Wanthongchai, K.; Bauhus, J.; Goldammer, J.G. Nutrient losses through prescribed burning of aboveground litter and understorey in dry dipterocarp forests of different fire history. Catena 2008, 74, 321–332. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Ji, M.; Kong, W.; Yue, L.; Wang, J.; Deng, Y.; Zhu, L. Salinity reduces bacterial diversity, but increases network complexity in Tibetan Plateau lakes. FEMS Microbiol. Ecol. 2019, 95, fiz190. [Google Scholar] [CrossRef]
- Lima-Mendez, G.; Faust, K.; Henry, N.; Decelle, J.; Colin, S.; Carcillo, F.; Chaffron, S.; Ignacio-Espinosa, J.C.; Roux, S.; Vincent, F.; et al. Determinants of community structure in the global plankton interactome. Science 2015, 348, 9. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Or, D. Hydration dynamics promote bacterial coexistence on rough surfaces. ISME J. 2013, 7, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, X.; Han, X.; Deng, Y. Higher precipitation strengthens the microbial interactions in semi-arid grassland soils. Glob. Ecol. Biogeogr. 2018, 27, 570–580. [Google Scholar] [CrossRef]
- Xu, R.J.; Hu, X.; Qi, L.H.; Liu, G.L.; Peng, C.; Shu, Q. Effects of forest restoration on distribution and growth of rattan in lowland rainforest, Hainan Island. Chin. J. Ecol. 2019, 38, 3313–3319. [Google Scholar]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [Green Version]
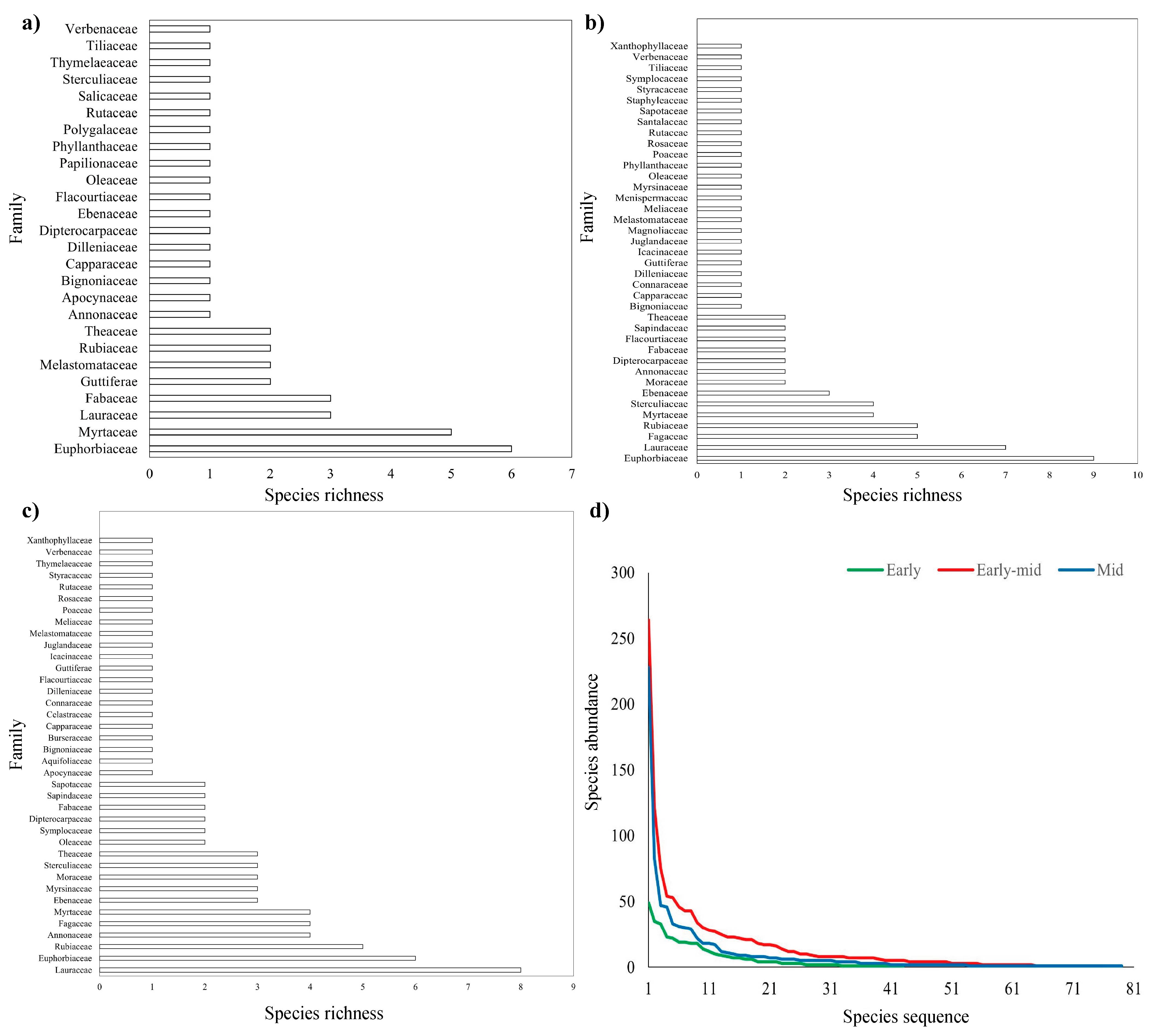
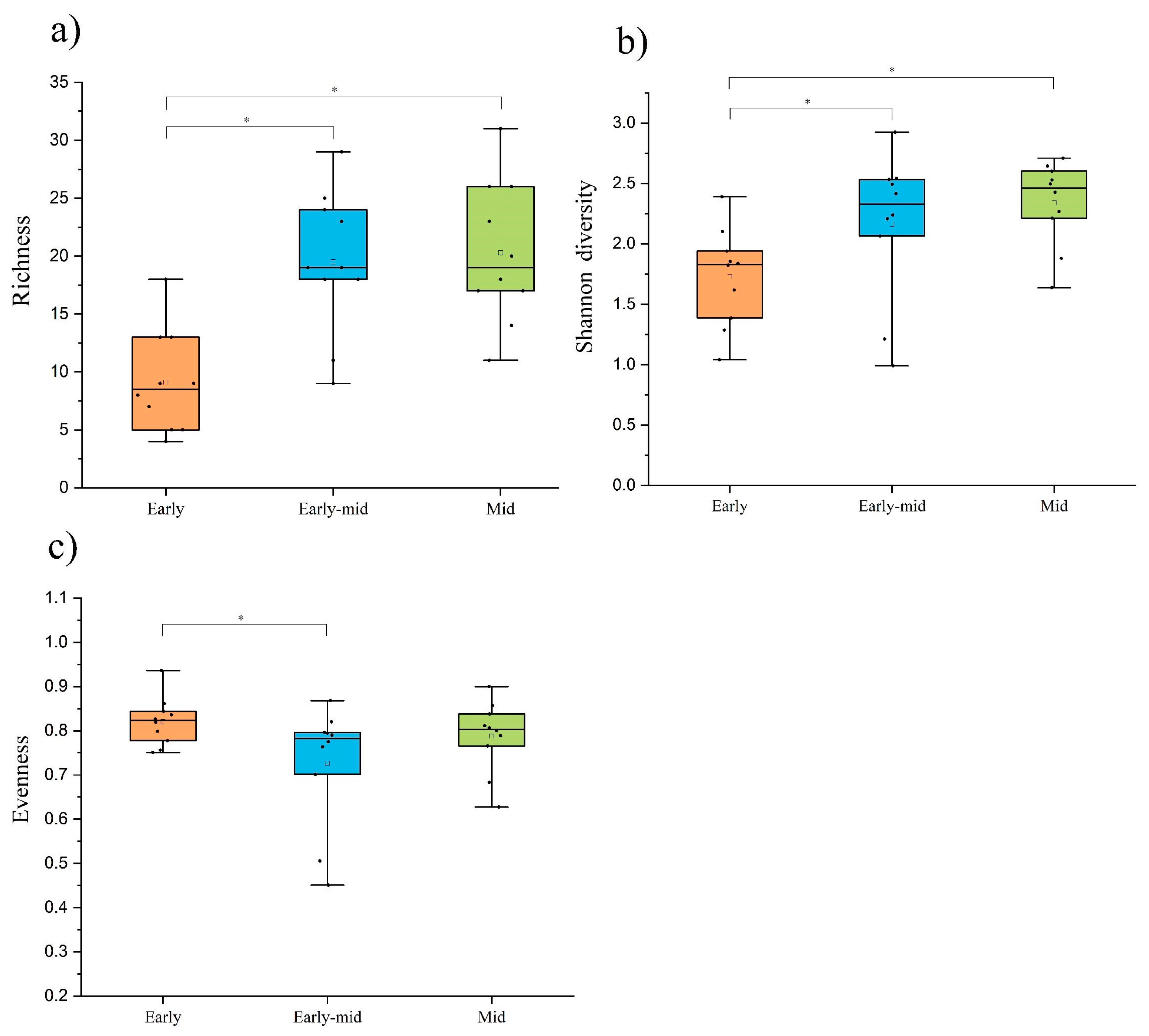
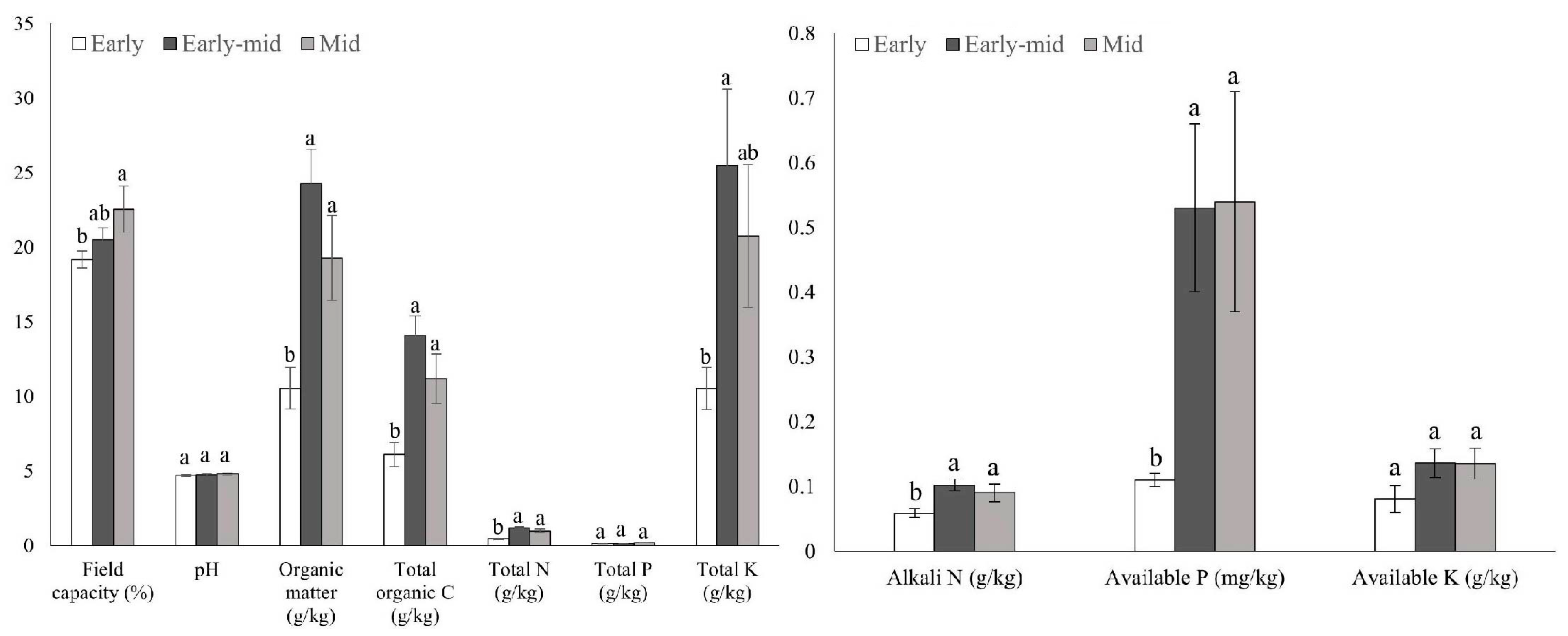
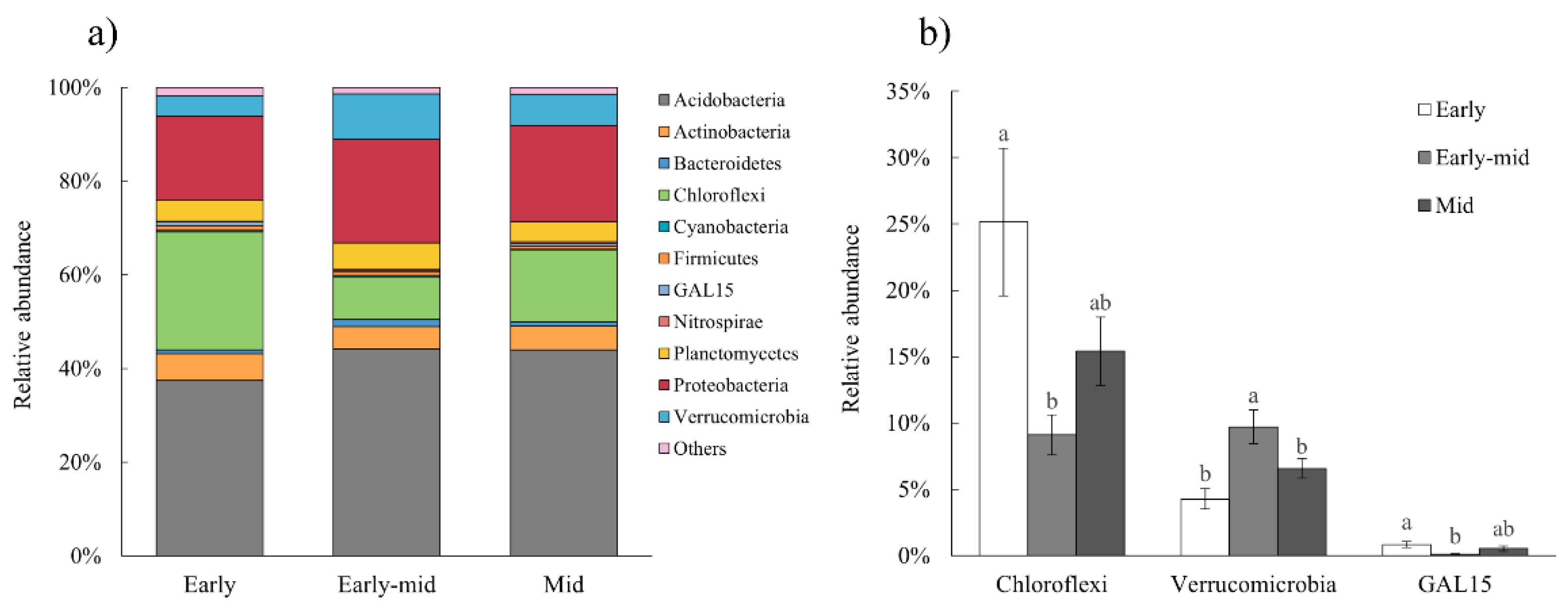
| Succession Stage | Early | Early-Mid | Mid |
|---|---|---|---|
| Basal area (m2/ha) | 4.74 ± 1.05 c | 36.21 ± 2.52 b | 42.99 ± 2.22 a |
| Stem density (tree/ha) | 917.50 ± 143.47 c | 3165.00 ± 247.91 a | 2057.50 ± 200.52 b |
| Mean height (m) | 3.75 ± 0.22 b | 10.52 ± 0.30 a | 10.60 ± 0.43 a |
| Maximum height (m) | 6.46 ± 0.50 b | 22.07 ± 1.11 a | 23.25 ± 1.58 a |
| Early | Early-Mid | Mid | |
|---|---|---|---|
| Total degree | 532 | 539 | 477 |
| Total correlation | 911 | 1180 | 732 |
| Positive correlation | 657 (72.1%) | 713 (60.4%) | 480 (65.6%) |
| Negative correlation | 254 (27.9%) | 467 (39.6%) | 252 (34.4%) |
| Average correlation per node | 1.71 | 2.19 | 1.53 |
| Average path length | 7.57 | 4.7 | 6.33 |
| Average clustering coefficient | 0.3 | 0.21 | 0.29 |
| Modularity | 0.751 | 0.626 | 0.789 |
| Number of modules | 47 | 66 | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Shu, Q.; Guo, W.; Shang, Z.; Qi, L. Secondary Succession Altered the Diversity and Co-Occurrence Networks of the Soil Bacterial Communities in Tropical Lowland Rainforests. Plants 2022, 11, 1344. https://doi.org/10.3390/plants11101344
Hu X, Shu Q, Guo W, Shang Z, Qi L. Secondary Succession Altered the Diversity and Co-Occurrence Networks of the Soil Bacterial Communities in Tropical Lowland Rainforests. Plants. 2022; 11(10):1344. https://doi.org/10.3390/plants11101344
Chicago/Turabian StyleHu, Xuan, Qi Shu, Wen Guo, Zean Shang, and Lianghua Qi. 2022. "Secondary Succession Altered the Diversity and Co-Occurrence Networks of the Soil Bacterial Communities in Tropical Lowland Rainforests" Plants 11, no. 10: 1344. https://doi.org/10.3390/plants11101344
APA StyleHu, X., Shu, Q., Guo, W., Shang, Z., & Qi, L. (2022). Secondary Succession Altered the Diversity and Co-Occurrence Networks of the Soil Bacterial Communities in Tropical Lowland Rainforests. Plants, 11(10), 1344. https://doi.org/10.3390/plants11101344





