Vegetation Affecting Water Quality in Small Streams: Case Study in Hemiboreal Forests, Latvia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of Sample Plots
2.2. Field Survey
2.3. Stream Parameters
2.4. Data Analysis
3. Results
3.1. Species Composition
3.2. Bioindicator Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gundersen, P.; Laurén, A.; Finér, L.; Ring, E.; Koivusalo, H.; Sætersdal, M.; Weslien, J.O.; Sigurdsson, B.D.; Högbom, L.; Laine, J.; et al. Environmental Services Provided from Riparian Forests in the Nordic Countries. Ambio 2010, 39, 555–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelle, A.J.; Johnson, A.W.; Conolly, C. Wetland and stream buffer size requirements—A review. J. Environ. Qual. 1994, 23, 878–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luke, S.H.; Luckai, N.J.; Burke, J.M.; Prepas, E.E. Riparian areas in the Canadian boreal forest and linkages with water quality in streams. Environ. Rev. 2007, 15, 79–97. [Google Scholar] [CrossRef]
- Lowrance, R. Groundwater nitrate and denitrification in a coastal plain riparian forest. J. Environ. Qual. 1992, 21, 401–405. [Google Scholar] [CrossRef]
- Macdonald, J.S.; MacIsaac, E.A.; Herunter, H.E. The effect of variable-retention riparian buffer zones on water temperatures in small headwater streams in sub-boreal forest ecosystems of British Columbia. Can. J. For. Res. 2003, 33, 1371–1382. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, C.; Svedmark, M. Basic Principles and ecological Consequences of Changing water regimes: Riparian plant communities. Environ. Manag. 2002, 30, 468–480. [Google Scholar] [CrossRef]
- O’Hara, K.L. What is close-to-nature silviculture in a changing world? For. Int. J. For. Res. 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Naiman, R.J.; Décamps, H. The Ecology of Interfaces: Riparian Zones. Annu. Rev. Ecol. Evol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef] [Green Version]
- Howard, J.K.; Cuffey, K.M. The functional role of native freshwater mussels in the fluvial benthic environment. Freshw. Biol. 2006, 51, 460–474. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hakenkamp, C.C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef] [Green Version]
- Araujo, R.; Ramos, A. Action Plan for Margaritifera Margaritifera in Europe; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2000; p. 32. [Google Scholar]
- Astorga, A.; Heino, J.; Luoto, M.; Muotka, T. Freshwater biodiversity at regional extent: Determinants of macroinvertebrate taxonomic richness in headwater streams. Ecography 2011, 34, 705–713. [Google Scholar] [CrossRef]
- Broadmeadow, S.; Nisbet, T.R. The effects of riparian forest management on the freshwater environment: A literature review of best management practice. Hydrol. Earth Syst. Sci. 2004, 8, 286–305. [Google Scholar] [CrossRef]
- Hedström, P.; Bystedt, D.; Karlsson, J.; Bokma, F.; Byström, P. Brownification increases winter mortality in fish. Oecologia 2017, 183, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, K.; Besemer, K.; Burns, N.R.; Battin, T.J.; Bengtsson, M.M. Light availability affects stream biofilm bacterial community composition and function, but not diversity. Environ. Microbiol. 2015, 17, 5036–5047. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Elliott, J. Temperature Requirements of Atlantic Salmon Salmo Salar, Brown Trout Salmo Trutta and Arctic Charr Salvelinus Alpinus: Predicting the Effects of Climate change. J. Fish Biol. 2010, 77, 1793–1817. [Google Scholar] [CrossRef] [PubMed]
- Rudzīte, M. Distribution of the freshwater pearl mussel Margaritifera margaritifera (Linnaeus 1758) in Latvia. Acta Univ. Latv. Biol. 2004, 676, 79–85. [Google Scholar]
- Rudzīte, M. Strategy for conservation of the Freshwater Pearl Mussel Margaritifera margaritifera L. populations in Latvia. Acta Biol. Univ. Daugavpil. 2001, 1, 38–44. [Google Scholar]
- Bauer, G. The adaptive value of offspring size among freshwater mussels (Bivalvia: Unionoidea). J. Anim. Ecol. 1994, 63, 933–944. [Google Scholar] [CrossRef]
- Young, M.R.; Williams, J.C. The reproductive biology of the freshwater pearl mussel Margaritifera margaritifera (Linn.) in Scotland. I. Field studies. Arch. Hydrobiol. 1984, 99, 405–422. [Google Scholar]
- Fiebig, D.M.; Lock, M.A.; Neal, C. Soil water in the riparian zone as a source of carbon for a headwater stream. J. Hydrol. 1990, 116, 217–237. [Google Scholar] [CrossRef]
- Mayer, P.M.; Reynolds, S.K.; McMutchen, M.D.; Canfield, T.J. Meta-analysis of nitrogen removal in riparian buffers. J. Environ. Qual. 2007, 36, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Goudarzian, P.; Yazdani, M.; Matinkhah, S.H. Greenhouse and field evaluation of phytoremediation for nitrogen and phosphorus in a riparian buffer strip. Appl. Ecol. Environ. Res. 2021, 19, 933–952. [Google Scholar] [CrossRef]
- Lidman, F.; Köhler, S.J.; Mörth, C.M.; Laudon, H. Metal transport in the boreal landscape—The role of wetlands and the affinity for organic matter. Environ. Sci. Technol. 2014, 48, 3783–3790. [Google Scholar] [CrossRef] [PubMed]
- Pinay, G.; Fabre, A.; Vervier, P.; Gazelle, F. Control of C, N, P distribution in soils of riparian forests. Landsc. Ecol. 1992, 6, 121–132. [Google Scholar] [CrossRef]
- Klopfenstein, N.B.; Chun, Y.W.; Kim, M.-S.; Ahuja, M.A.; Dillon, M.C.; Carman, R.C.; Eskew, L.G. Micropropagation, Genetic Engineering, and Molecular Biology of Populus; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 1997; p. 326.
- Aronsson, P.; Perttu, K. Willow vegetation filters for wastewater treatment and soil remediation combined with biomass production. For. Chron. 2001, 77, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Ericsson, T. Growth and nutrition in three Salix clones grown in low conductivity solutions. Physiol. Plant. 1981, 52, 239–244. [Google Scholar] [CrossRef]
- Gray, D.H.; Sotir, R.B. Biotechnical and Soil Bioengineering Slope Stabilization: A Practical Guide for Erosion Control; John Wiley & Sons: Toronto, ON, Canada, 1996; p. 365. [Google Scholar]
- Fustec, J.; Lode, T.; Le Jacques, D.; Cormier, J.P. Colonization, riparian habitat selection and home range size in a reintroduced population of European beavers in the Loire. Freshw. Biol. 2001, 46, 1361–1371. [Google Scholar] [CrossRef]
- Naiman, R.J.; Johnston, C.A.; Kelley, V. Alteration of North American streams by beaver. BioScience 1998, 38, 753–762. [Google Scholar] [CrossRef]
- Collen, P.; Gibson, R.J. The general ecology of beavers (Castor spp.), as related to their influence on stream ecosystems and riparian habitats, and the subsequent effects on fish—A review. Rev. Fish Biol. Fish 2000, 10, 439–461. [Google Scholar] [CrossRef]
- Kesminas, V.; Andrius, S.; Virginija, P.; Tomas, V. Ecological Impact of Eurasian Beaver (Castor fiber) Activity on Fish Communities in Lithuanian Trout Streams. Rocz. Ochr. Srodowiska 2013, 15, 59–80. [Google Scholar]
- Langendoen, E.J.; Lowrance, R.R.; Williams, R.G.; Pollen, N.; Simon, A. Impacts of Global Climate Change—Modeling the Impact of Riparian Buffer Systems on Bank Stability of an Incised Stream. In Proceedings of the American Society of Civil Engineers World Water and Environmental Resources Congress 2005, Anchorage, AK, USA, 15–19 May 2005. [Google Scholar] [CrossRef]
- Thorne, C.R. Effects of Vegetation on Riverbank Erosion and Stability; Vegetation and Erosion, Chichester; Thornes, J.B., Ed.; Wiley: Hoboken, NJ, USA, 1990; pp. 125–144. [Google Scholar]
- Wu, T.; Watson, A. In situ shear tests of soil blocks with roots. Can. Geotech. J. 1998, 35, 579–590. [Google Scholar] [CrossRef]
- Bagella, S. Does cross-taxon analysis show similarity in diversity patterns between vascular plants and bryophytes? Some answers from a literature review. Comptes Rendus Biol. 2014, 337, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Longton, R.E. Bryophytes in terrestrial ecosystems. Bot. J. Linn. 1990, 104, 1. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lang, S.I.; Soundzilovskaia, N.A.; During, H.J. Comparative cryptogam ecology: A review of bryophyte and lichen traits that drive biogeochemistry. Ann. Bot. 2007, 99, 987–1001. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, T.H.; Zackrisson, O.; Nilsson, M.C.; Sellstedt, A. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 2002, 419, 917–920. [Google Scholar] [CrossRef]
- Hylander, K.; Dynesius, M.; Jonsson, B.G.; Nilsson, C. Substrate form determines the fate of bryophytes in riparian buffer strips. Ecol. Appl. 2005, 15, 674–688. [Google Scholar] [CrossRef]
- Blanquet, B.J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Wien, Austria, 1964. [Google Scholar]
- Āboliņa, A. List of bryophytes of Latvia. Latv. Veģetācija 2001, 3, 47–87. (In Latvian) [Google Scholar]
- Gavrilova, Ģ.; Šulcs, V. The flora of vascular plants in Latvia. In The List of Taxa; The Institute of Biology at University of Latvia: Rīga, Latvia, 1999. (In Latvian) [Google Scholar]
- Latvian Geology and Meteorology Centre. Latvian Environment. Available online: https://www.meteo.lv/lapas/vide/udens/udens-kvalitate/udens-kvalitates-novertejums?id=1100&nid=433 (accessed on 2 February 2022).
- Veidemane, K. Kopsavilkums Par Galvenajām Mazo Upju Pārvaldības un Apsaimniekošanas Problēmām Latvijā un Ieteiktie Rīcības Virzieni Situācijas Uzlabošanā; Baltijas Vides Forums: Rīga, Latvia, 2018; p. 19. [Google Scholar]
- Moore, I.D.; Grayson, R.B.; Ladson, A.R. Digital terrain modelling: A review of hydrological, geomorphological and biological applications. Hydrol. Process. 1991, 5, 3–30. [Google Scholar] [CrossRef]
- Ghunowa, K.; MacVicar, B.J.; Ashmore, P. Stream power index for networks (SPIN) toolbox for decision support in urbanizing watersheds. Environ. Model. Softw. 2021, 144, 105185. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 March 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package version 2.5-7. Acessoem 2020, 23, 2010. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 March 2022).
- De Caceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. Available online: http://sites.google.com/site/miqueldecaceres/ (accessed on 10 March 2022). [CrossRef] [PubMed]
- Bragina, A.; Berg, C.; Müller, H.; Moser, D.; Berg, G. Insights into functional bacterial diversity and its effects on Alpine bog ecosystem functioning. Sci. Rep. 2013, 3, 1955. [Google Scholar] [CrossRef] [PubMed]
- Putkinen, A.; Larmola, T.; Tuomivirta, T.; Siljanen, H.M.P.; Bodrossy, L.; Tuittila, E.S.; Fritze, H. Water dispersal of methanotrophic bacteria maintains functional methane oxidation in Sphagnum mosses. Front. Microbiol. 2012, 3, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, M.; Kardol, P.; Gundale, M.J.; Bansal, S.; Nilsson, M.C.; Metcalfe, D.B.; Wardle, D.A. Direct and indirect drivers of moss community structure, function, and associated microfauna across a successional gradient. Ecosystems 2015, 18, 154–169. [Google Scholar] [CrossRef]
- Gagic, V.; Bartomeus, I.; Jonsson, T.; Taylor, A.; Winqvist, C.; Fischer, C.; Slade, E.M.; Steffan-Dewenter, I.; Emmerson, M.; Potts, S.G.; et al. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B 2015, 282, 20142620. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Compton, J.E.; Church, M.R.; Larned, S.T.; Hogsett, W.E. Nitrogen export from forested watershed in the Oregon Coast Range: The role of N2-fixing red alder. Ecosystems 2003, 6, 773–785. [Google Scholar] [CrossRef]
- Van Miegroet, H.; Homann, P.S.; Cole, D.W. Soil nitrogen dynamics following harvesting and conversion of red alder and Douglas fir stands. Soil Sci. Soc. Am. J. 1992, 56, 1311–1318. [Google Scholar] [CrossRef] [Green Version]
- Fiala, K.; Tuma, I.; Holub, P.; Jandák, J. The role of Calamagrostis communities in preventing soil acidification and base cation losses in a deforested mountain area affected by acid deposition. Plant Soil 2005, 268, 35–49. [Google Scholar] [CrossRef]
- Aerts, R.; Berendse, F.; de Caluwe, H.; Schmitz, M. Competition in heathland along an experimental gradient of nutrient availability. Oikos 1990, 57, 310–318. [Google Scholar] [CrossRef]
- Boston, H.L.; Hill, W.R. Photosynthesis light relations of stream periphyton communities. Limnol. Oceanogr. 1991, 36, 644–656. [Google Scholar] [CrossRef] [Green Version]
- Von Schiller, D.; Marti, E.; Riera, J.L.; Sabater, F. Effects of nutrients and light on periphyton biomass and nitrogen uptake in Mediterranean streams with contrasting land uses. Freshw. Biol. 2007, 52, 891–906. [Google Scholar] [CrossRef]
- Hasselquist, E.M.; Kuglerová, L.; Sjögren, J.; Hjältén, J.; Ring, E.; Sponseller, R.A.; Andersson, E.; Lundström, J.; Mancheva, I.; Nordin, A.; et al. Moving towards multi-layered, mixed-species forests in riparian buffers will enhance their long-term function in boreal landscapes. For. Ecol. Manag. 2021, 493, 119254. [Google Scholar] [CrossRef]
- Moore, R.D.; Spittlehouse, D.L.; Story, A.; Dan Moore, R.; Spittlehouse, D.L.; Story, A. Riparian microclimate and stream temperature response to forest harvesting: A review. JAWRA J. Am. Water Resour. Assoc. 2005, 41, 813–834. [Google Scholar] [CrossRef]

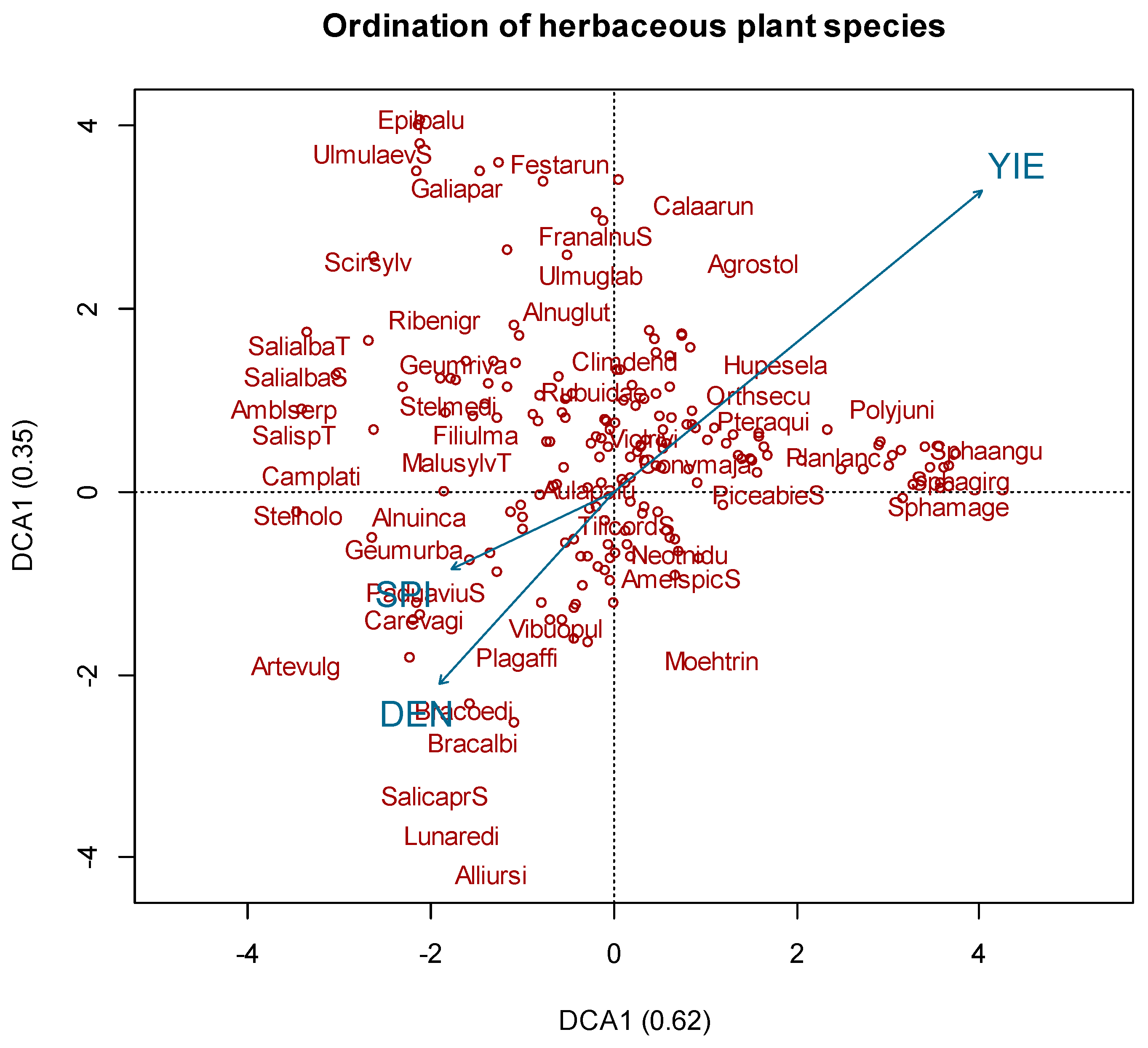
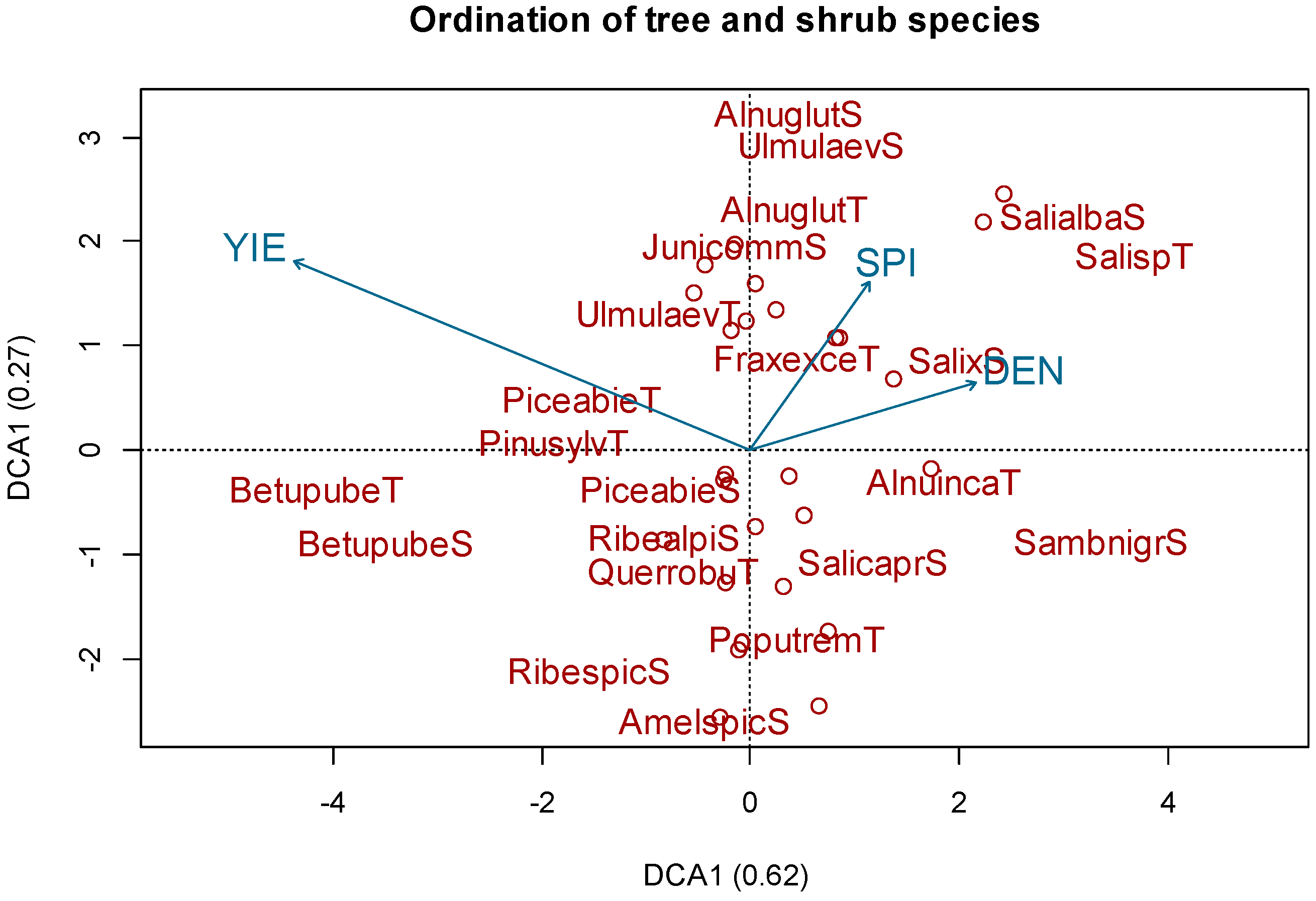
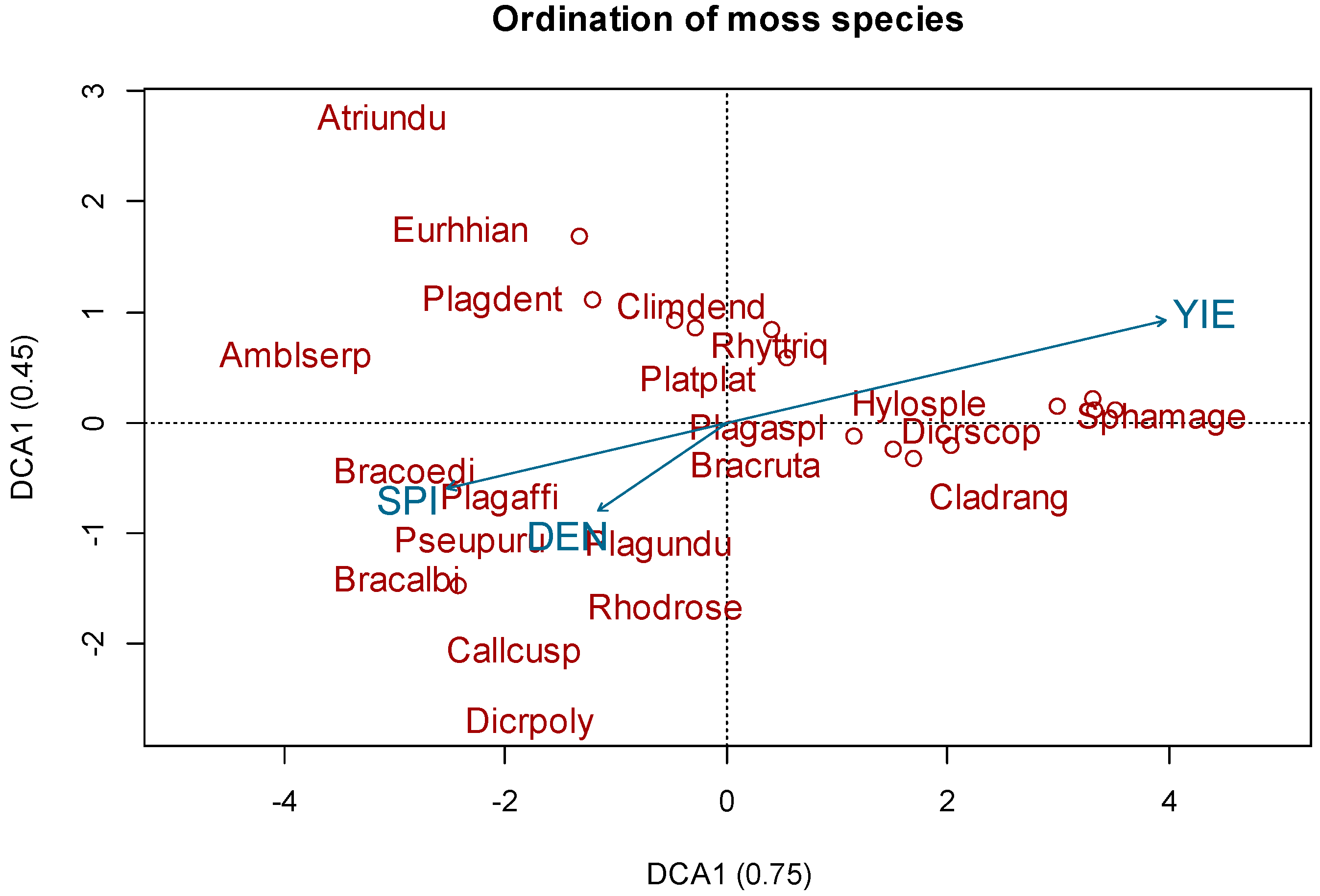
| Factor | Df | SumOfSqs | R² | F Value | p-Value |
|---|---|---|---|---|---|
| Total yield | 1 | 3.22 | 0.12 | 11.93 | 0.001 |
| Total density | 1 | 1.35 | 0.05 | 5.00 | 0.001 |
| Deadwood | 1 | 0.29 | 0.01 | 1.08 | 0.344 |
| Stream power index | 1 | 0.25 | 0.01 | 0.94 | 0.459 |
| Residuals | 84 | 22.69 | 0.82 | ||
| Total | 88 | 27.81 | 1.00 |
| Factor | R Value | p-Value |
|---|---|---|
| Stream | 0.520 | 0.001 |
| Distance from the stream margins | 0.002 | 0.089 |
| Water quality | 0.150 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saklaurs, M.; Dubra, S.; Liepa, L.; Jansone, D.; Jansons, Ā. Vegetation Affecting Water Quality in Small Streams: Case Study in Hemiboreal Forests, Latvia. Plants 2022, 11, 1316. https://doi.org/10.3390/plants11101316
Saklaurs M, Dubra S, Liepa L, Jansone D, Jansons Ā. Vegetation Affecting Water Quality in Small Streams: Case Study in Hemiboreal Forests, Latvia. Plants. 2022; 11(10):1316. https://doi.org/10.3390/plants11101316
Chicago/Turabian StyleSaklaurs, Mārcis, Stefānija Dubra, Līga Liepa, Diāna Jansone, and Āris Jansons. 2022. "Vegetation Affecting Water Quality in Small Streams: Case Study in Hemiboreal Forests, Latvia" Plants 11, no. 10: 1316. https://doi.org/10.3390/plants11101316
APA StyleSaklaurs, M., Dubra, S., Liepa, L., Jansone, D., & Jansons, Ā. (2022). Vegetation Affecting Water Quality in Small Streams: Case Study in Hemiboreal Forests, Latvia. Plants, 11(10), 1316. https://doi.org/10.3390/plants11101316







