Exogenous Application of Melatonin and Methyl Jasmonate as a Pre-Harvest Treatment Enhances Growth of Barhi Date Palm Trees, Prolongs Storability, and Maintains Quality of Their Fruits under Storage Conditions
Abstract
:1. Introduction
2. Results
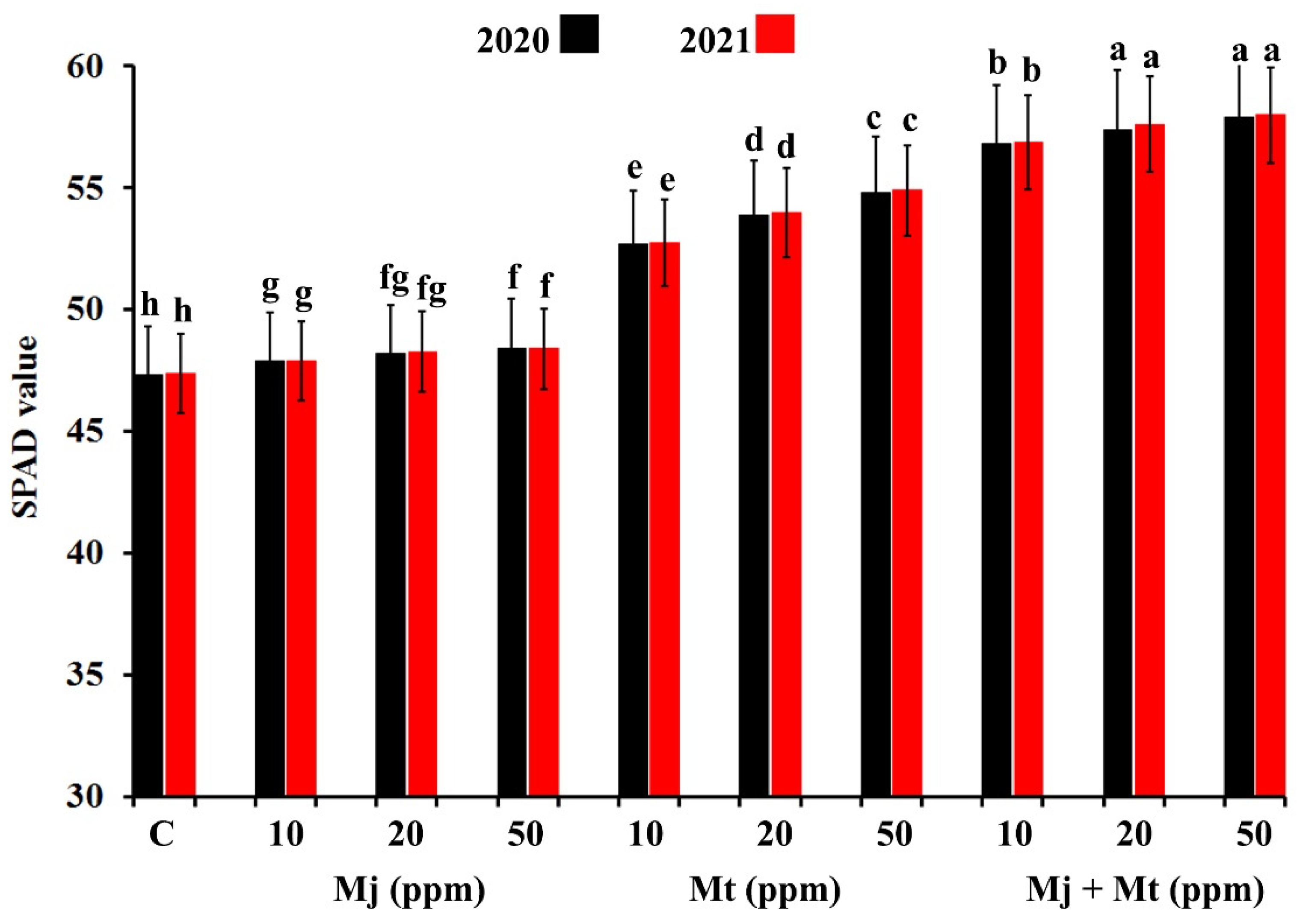
2.1. Effects on Relative Chlorophyll Pigments
2.2. Effects on the Nutrient Contents
2.3. Effects on the Yield and Its Components
2.4. Effect on the Fruit Weight Loss
2.5. Effect on the Fruit Decay
2.6. Effect on the Fruit Firmness
2.7. Effect on the Total Soluble Solids Content (TSS)
2.8. Effect on the Total Sugars Content (TS)
2.9. Effect on the Total Acidity (TA)
2.10. Effect on the Total Phenolic Content and Activity of Peroxidase and Polyphenol Oxidase Enzymes
2.11. Correlations between the Recorded Variables from the Date Palm Trees
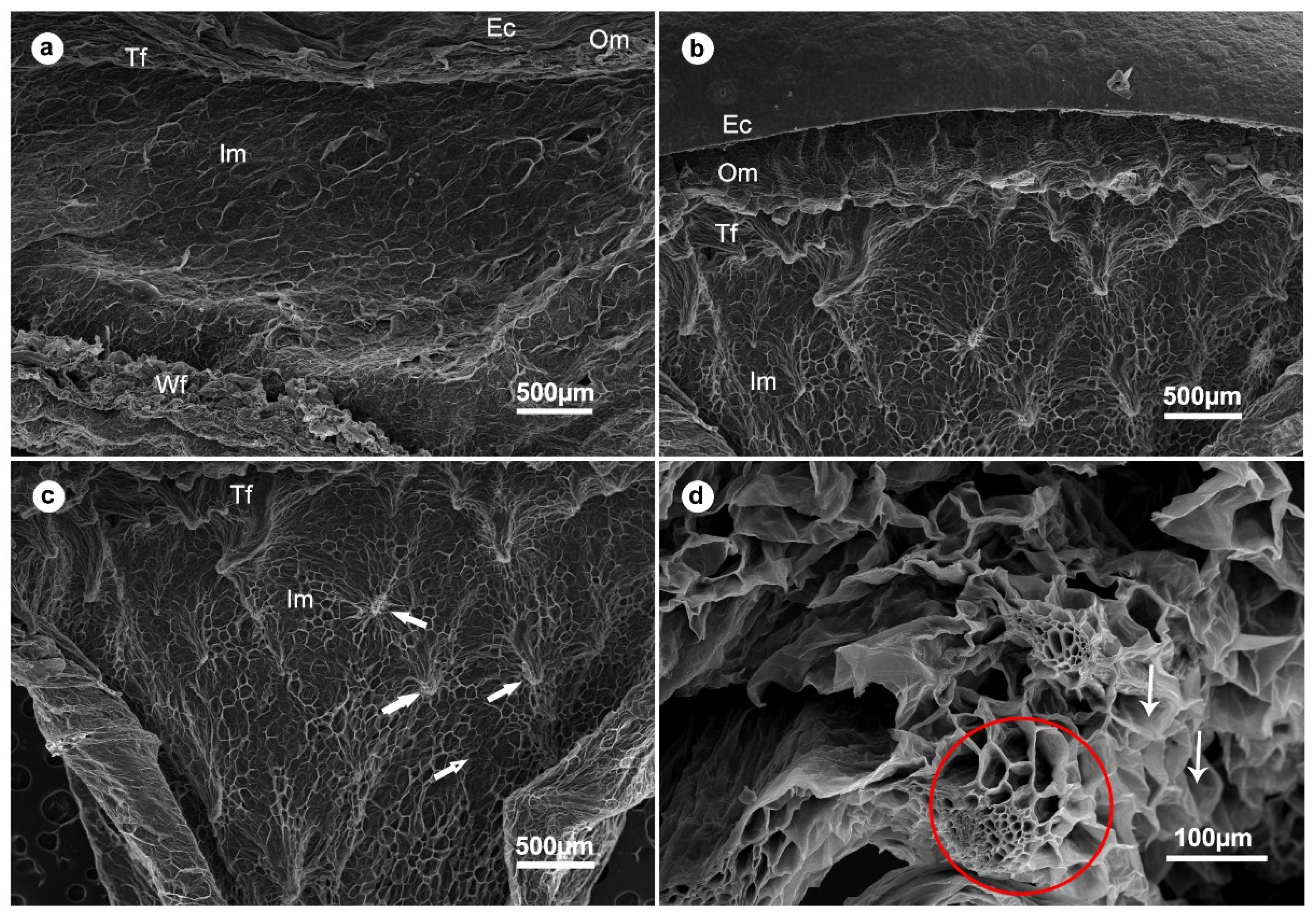
2.12. Scanning Electron Microscopy (SEM)
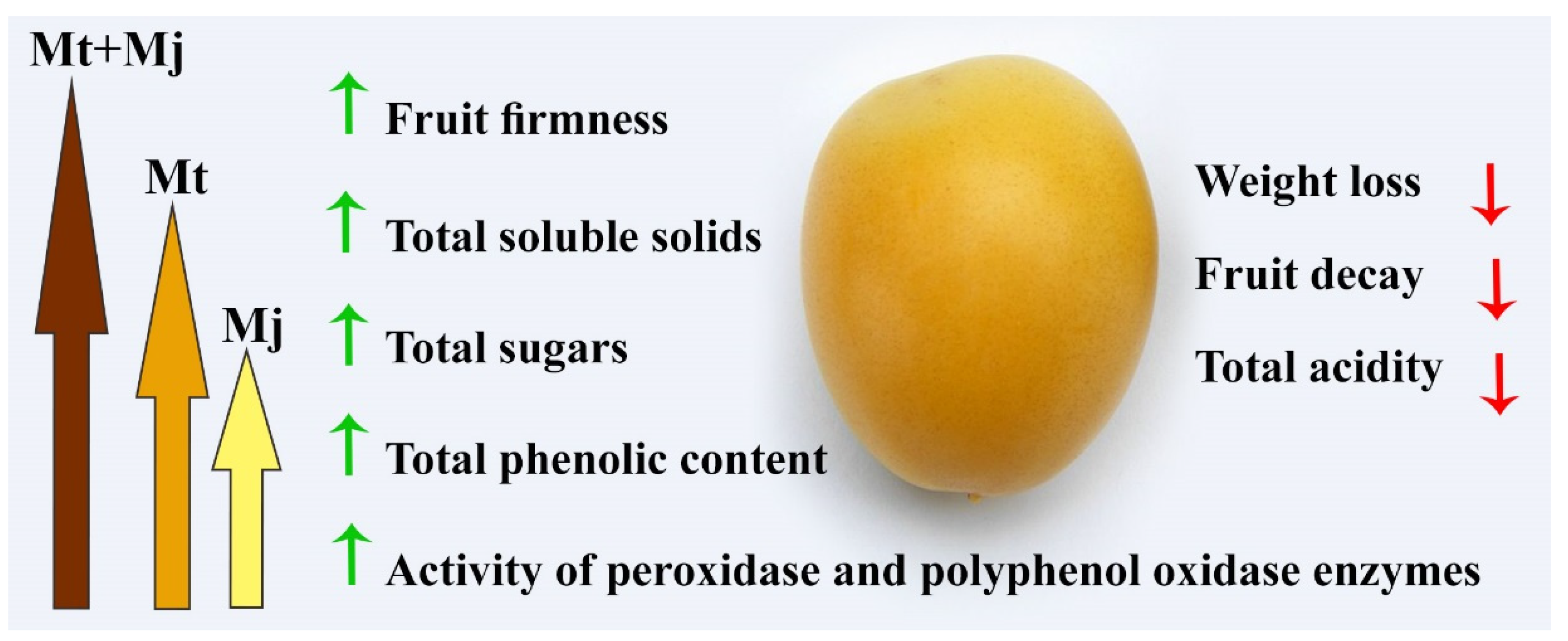
3. Discussion
4. Materials and Methods
4.1. Chemicals and Date Palm Cultivar
4.2. Field Experiment
4.2.1. Biochemical Analyses of Date Palm Leaves
4.2.2. Yield and Its Components
4.2.3. Postharvest Physical Properties of Date Palm Fruits
4.2.4. Postharvest Chemical Properties of Date Palm Fruits
4.2.5. Biochemical Analyses of Date Palm Fruits
4.2.6. SEM Observations
4.3. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Statistics Division. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 16 October 2021).
- Paszke, M.Z. Date palm and date palm inflorescences in the late uruk period (C. 3300 B.C.): Botany and archaic script. Iraq 2019, 81, 221–239. [Google Scholar] [CrossRef]
- Al-Shahib, W.; Marshall, R. The fruit of the date palm: It’s possible use as the best food for the future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A. Antioxidant capacity, antioxidant compounds and antioxidant enzyme activities in five date cultivars during development and ripening. Sci. Hortic. 2011, 129, 688–693. [Google Scholar] [CrossRef]
- Alsaed, A.K.; Mehyar, G.F.; Arar, A. Effect of harvesting time and storage temperature on the duration of Balah stage of “Barhi” dates. Ital. J. Food Sci. 2013, 25, 345–353. [Google Scholar]
- Hardeland, R. Melatonin in plants–Diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Zhong, L.; Lin, L.; Yang, L.; Liao, M.; Wang, X.; Wang, J.; Lv, X.; Deng, H.; Liang, D.; Xia, H.; et al. Exogenous melatonin promotes growth and sucrose metabolism of grape seedlings. PLoS ONE 2020, 15, e0232033. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef] [Green Version]
- Rashad, Y.M.; Fekry, W.M.E.; Sleem, M.M.; Elazab, N.T. Effects of mycorrhizal colonization on transcriptional expression of the responsive factor JERF3 and stress-responsive genes in banana plantlets in response to combined biotic and abiotic stresses. Front. Plant Sci. 2021, 12, 2391. [Google Scholar] [CrossRef]
- Rashad, Y.M.; El-Sharkawy, H.H.A.; Belal, B.E.A.; Razik, E.S.A.; Galilah, D.A. Silica nanoparticles as a probable anti-oomycete compound against downy mildew, and yield and quality enhancer in grapevines: Field evaluation, molecular, physiological, ultrastructural, and toxicity investigations. Front. Plant Sci. 2021, 12, 763365. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Zuo, J.; Gao, L.; Lv, J.; Wang, Q. Methyl jasmonate delays postharvest ripening and senescence in the non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2016, 120, 76–83. [Google Scholar] [CrossRef]
- Saniewski, M.; Dziurka, M.; Dziurka, K.; Góraj-Koniarska, J.; Ueda, J.; Miyamoto, K. Methyl jasmonate induces leaf senescence of Ginkgo biloba L.: Relevance to endogenous levels of plant hormones. Plant Growth Regul. 2020, 91, 383–396. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Lazar, D.; Murch, S.J.; Beilby, M.J.; Al Khazaaly, S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal. Behav. 2013, 8, e23279. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Sirhindi, G.; Mushtaq, R.; Gill, S.S.; Sharma, P.; Allah, E.F.A.; Ahmad, P. Jasmonic acid and methyl jasmonate modulate growth, photosynthetic activity and expression of photosystem II subunit genes in Brassica oleracea L. Sci. Rep. 2020, 10, 9322. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, A.; Yu, H.; Li, W.; Liang, C.; Guo, S.; Zhang, R.; Chu, C. Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant Sci. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnao, M.B.; Ruiz, J.H. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Giménez, M.J.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Preharvest application of methyl jasmonate increases crop yield, fruit quality and bioactive compounds in pomegranate ‘Mollar de Elche’ at harvest and during postharvest storage. J. Sci. Food Agric. 2020, 100, 145–153. [Google Scholar] [CrossRef]
- Pérez, A.C.; Goossens, A. Jasmonate signalling: A copycat of auxin signalling? Plant Cell Environ. 2013, 36, 2071–2084. [Google Scholar] [CrossRef]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.-J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- García, A.; Aguado, E.; Cebrián, G.; Iglesias, J.; Romero, J.; Martínez, C.; Garrido, D.; Rebolloso, M.; Valenzuela, J.; Jamilena, M. Effect of ethylene-insensitive mutation etr2b on postharvest chilling injury in zucchini fruit. Agriculture 2020, 10, 532. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Serrano, M.; Pretel, M.T.; Botella, M.A.; Amorós, A. Physicochemical changes during date ripening related to ethylene production. Food Sci. Technol. Int. 2001, 7, 31–36. [Google Scholar] [CrossRef]
- Jiang, F.; Lopez, A.; Jeon, S.; De Freitas, S.T.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.T.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. 2019, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Li, C.; Ge, Y.; Li, X.; Cheng, Y.; Hou, J.; Li, J. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. LWT 2020, 127, 109431. [Google Scholar] [CrossRef]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B. Post-harvest application of methyl jasmonate, 1-methylcyclopropene and salicylic acid elevates health-promoting compounds in cold-stored ‘kinnow’ mandarin (Citrus nobilis Lour x C. deliciosa Tenora) Fruit. Int. J. Fruit Sci. 2021, 21, 147–157. [Google Scholar] [CrossRef]
- Boonyaritthongchai, P.; Supapvanich, S. Effects of methyl jasmonate on physicochemical qualities and internal browning of ‘queen’ pineapple fruit during cold storage. Hortic. Environ. Biotechnol. 2017, 58, 479–487. [Google Scholar] [CrossRef]
- Liu, G.; Li, B.; Li, X.; Wei, Y.; Liu, D.; Shi, H. Comparative physiological analysis of methyl jasmonate in the delay of postharvest physiological deterioration and cell oxidative damage in Cassava. Biomolecules 2019, 9, 451. [Google Scholar] [CrossRef] [Green Version]
- Peach, K.; Tracy, M.V. Modern Methods of Plant Analysis; Springer: Heidelberg/Berlin, Germany, 1955; Volumes III and IV, pp. 258–261. [Google Scholar]
- Wild, A. Russell’s Soil Conditions and Plant Growth; Longman Scientific and Technical: Harlow, UK, 1988. [Google Scholar]
- Cottenie, A.; Verlo, M.; Kjekens, L.; Camerlynch, R. Chemical Analysis of Plant and Soil; Laboratory of Analytical Agrochemistry, State University: Gent, Belgium, 1982; pp. 80–284. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods; New Age International Publishers (P.) Ltd.: New Delhi, India, 1996. [Google Scholar]
- A.O.A.C. Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1980. [Google Scholar]
- Malick, C.P.; Singh, M.B. Estimation of total phenols. In Plant Enzymology and Histo Enzymology; Kalyani Publishers: New Delhi, India, 1980; p. 286. [Google Scholar]
- Maxwell, D.P.; Bateman, D.F. Changes in the activities of some oxidases in extracts of Rhizoctonia-infected bean hypocotyls in relation to lesion maturation. Phytopathology 1967, 57, 132–136. [Google Scholar]
- Galeazzi, M.A.M.; Sgarbieri, V.C.; Constantinides, S.M. Isolation, purification and physicochemical characterization of polyphenoloxidases (PPO) from a dwarf variety of banana (Musa cavendishii, L). J. Food Sci. 1981, 46, 150–155. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 23 December 2021).

| Treatment | Nitrogen (%) | Phosphorus (%) | Potassium (%) | ||||
|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 1.27 ± 0.02 g | 1.28 ± 0.01 g | 0.20 ± 0.01 g | 0.21 ± 0.01 g | 1.40 ± 0.01 f | 1.41 ± 0.02 f | |
| Methyl Jasmonate (ppm) | 10 | 1.27 ± 0.04 g | 1.28 ± 0.01 g | 0.23 ± 0.01 f | 0.24 ± 0.01 f | 1.41 ± 0.01 ef | 1.43 ± 0.01 f |
| 20 | 1.32 ± 0.02 fg | 1.33 ± 0.01 f | 0.24 ± 0.01 f | 0.25 ± 0.02 f | 1.42 ± 0.01 ef | 1.43 ± 0.02 f | |
| 50 | 1.33 ± 0.02 f | 1.35 ± 0.02 f | 0.28 ± 0.02 e | 0.29 ± 0.03 e | 1.43 ± 0.02 e | 1.48 ± 0.02 e | |
| Melatonin (ppm) | 10 | 1.42 ± 0.03 e | 1.44 ± 0.02 e | 0.39 ± 0.01 d | 0.40 ± 0.03 d | 1.54 ± 0.02 d | 1.56 ± 0.03 d |
| 20 | 1.46 ± 0.01 de | 1.47 ± 0.01 d | 0.40 ± 0.01 cd | 0.41 ± 0.02 cd | 1.55 ± 0.01 d | 1.58 ± 0.02 d | |
| 50 | 1.48 ± 0.01 d | 1.49 ± 0.04 d | 0.41 ± 0.02 c | 0.43 ± 0.02 c | 1.61 ± 0.01 c | 1.62 ± 0.01 c | |
| Methyl Jasmonate + Melatonin (ppm) | 10 | 1.55 ± 0.02 c | 1.56 ± 0.03 c | 0.44 ± 0.01 b | 0.46 ± 0.01 b | 1.68 ± 0.02 b | 1.68 ± 0.03 b |
| 20 | 1.64 ± 0.01 b | 1.67 ± 0.02 b | 0.47 ± 0.02 a | 0.49 ± 0.03 a | 1.69 ± 0.03 b | 1.71 ± 0.02 b | |
| 50 | 1.80 ± 0.03 a | 1.82 ± 0.03 a | 0.48 ± 0.02 a | 0.49 ± 0.03 a | 1.73 ± 0.03 a | 1.74 ± 0.03 a | |
| Treatment | Yield/Palm (kg) | Bunch Weight (kg) | Fruit Length (cm) | Fruit Diameter (cm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| Control | 96.4 ± 1.85 h | 96.6 ± 1.93 i | 9.47 ± 0.45 g | 9.57 ± 0.881 g | 2.99 ± 0.41 g | 3.00 ± 0.62 g | 2.52 ± 0.35 h | 2.53 ± 0.21 h | |
| Methyl Jasmonate (ppm) | 10 | 100.4 ± 2.86 g | 100.5 ± 2.11 h | 9.73 ± 0.81 fg | 9.80 ± 1.01 fg | 3.01 ± 0.33 fg | 3.05 ± 0.81 fg | 2.53 ± 0.84 gh | 2.55 ± 0.47 h |
| 20 | 104.9 ± 1.81 f | 105.4 ± 1.89 g | 10.4 ± 0.74 ef | 10.5 ± 0.96 e | 3.04 ± 0.72 fg | 3.07 ± 0.56 fg | 2.55 ± 0.32 gh | 2.57 ± 0.73 gh | |
| 50 | 105.5 ± 2.42 f | 105.6 ± 1.74 g | 10.7 ± 0.70 e | 10.8 ± 0.93 e | 3.05 ± 0.33 fg | 3.08 ± 0.61 fg | 2.57 ± 0.44 f | 2.59 ± 0.55 g | |
| Melatonin (ppm) | 10 | 115.1 ± 1.78 e | 115.7 ± 2.01 f | 11.8 ± 0.91 d | 12.4 ± 1.13 d | 3.23 ± 0.64 e | 3.25 ± 0.47 e | 2.64 ± 0.62 e | 2.65 ± 0.51 f |
| 20 | 119.1 ± 1.65 d | 119.3 ± 1.68 e | 12.2 ± 1.01 cd | 12.5 ± 1.12 d | 3.30 ± 0.44 d | 3.34 ± 0.63 d | 2.67 ± 0.94 e | 2.68 ± 0.32 e | |
| 50 | 121.3 ± 2.31 d | 123.0 ± 1.77 d | 12.7 ± 1.00 c | 13.0 ± 1.22 cd | 3.38 ± 0.95 c | 3.40 ± 0.70 c | 2.74 ± 0.32 d | 2.75 ± 0.19 d | |
| Methyl Jasmonate + Melatonin (ppm) | 10 | 128.3 ± 2.57 c | 129.6 ± 2.10 c | 12.9 ± 0.98 c | 13.5 ± 0.99 bc | 3.45 ± 0.55 b | 3.48 ± 0.45 b | 2.78 ± 0.61 c | 2.79 ± 0.75 c |
| 20 | 132.9 ± 2.30 b | 134.7 ± 2.11 b | 13.7 ± 1.02 b | 13.9 ± 1.30 b | 3.48 ± 0.43 b | 3.49 ± 0.69 b | 2.82 ± 0.49 b | 2.84 ± 0.84 b | |
| 50 | 145.5 ± 1.95 a | 146.3 ± 2.05 a | 14.3 ± 1.10a | 14.6 ± 1.15 a | 3.54 ± 0.87 a | 3.59 ± 0.83 a | 2.85 ± 0.63 a | 2.88 ± 0.90 a | |
| Treatment | Days after Harvest | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight Loss (%) | Fruit Decay (%) | ||||||||
| 14 | 28 | 14 | 28 | 14 | 28 | 14 | 28 | ||
| 2020 | 2021 | 2020 | 2021 | ||||||
| Control | 1.40 ± 0.31 a | 3.03 ± 0.51 a | 1.83 ± 0.44 a | 3.30 ± 0.60 a | 6.0 ± 1.0 a | 15.0 ± 1.1 a | 6.3 ± 1.0 a | 15.7 ± 1.2 a | |
| Methyl Jasmonate (ppm) | 10 | 1.04 ± 0.22 b | 2.27 ± 0.34 b | 0.93 ± 0.12 b | 1.67 ± 0.47 b | 3.0 ± 0.9 b | 8.67 ± 0.9 b | 4.0 ± 0.7 b | 8.67 ± 0.9 b |
| 20 | 0.48 ± 0.08 c | 1.10 ± 0.37 c | 0.41 ± 0.09 c | 0.83 ± 0.14 c | 2.7 ± 0.8 bc | 7.67 ± 0.6 c | 3.7 ± 0.5 bc | 8.33 ± 0.8 bc | |
| 50 | 0.16 ± 0.03 d | 0.49 ± 0.09 d | 0.22 ± 0.05 d | 0.60 ± 0.11 d | 2.7 ± 0.7 bc | 6.67 ± 0.6 d | 3.0 ± 0.5 d | 8.00 ± 0.4 c | |
| Melatonin (ppm) | 10 | 0.23 ± 0.06 bc | 0.49 ± 0.07 d | 0.20 ± 0.05 d | 0.49 ± 0.09 de | 2.3 ± 0.6 c | 6.33 ± 0.7 de | 3.0 ± 0.6 d | 7.33 ± 0.7 d |
| 20 | 0.25 ± 0.05 bc | 0.42 ± 0.06 de | 0.18 ± 0.04 de | 0.42 ± 0.09 e | 2.3 ± 0.6 c | 6.33 ± 0.5 de | 2.7 ± 0.7 de | 6.67 ± 0.7 de | |
| 50 | 0.19 ± 0.08 d | 0.32 ± 0.11 f | 0.17 ± 0.05 de | 0.32 ± 0.14 f | 2.0 ± 0.5 cd | 4.67 ± 0.7 f | 2.7 ± 0.8 de | 5.00 ± 0.5 f | |
| Methyl Jasmonate + Melatonin (ppm) | 10 | 0.16 ± 0.07 de | 0.31 ± 0.13 f | 0.16 ± 0.07 e | 0.31 ± 0.15 f | 2.0 ± 0.7 cd | 4.00 ± 0.5 fg | 2.3 ± 0.7 e | 5.00 ± 0.4 f |
| 20 | 0.14 ± 0.05 e | 0.27 ± 0.08 g | 0.14 ± 0.03 e | 0.25 ± 0.09 g | 1.3 ± 0.5 e | 2.67 ± 0.6 h | 1.7 ± 0.3 f | 3.33 ± 0.6 g | |
| 50 | 0.10 ± 0.04 f | 0.16 ± 0.07 h | 0.11 ± 0.05 f | 0.14 ± 0.05 h | 1.0 ± 0.4 e | 2.00 ± 0.4 i | 1.0 ± 0.4 g | 2.00 ± 0.5 h | |
| Treatment | Days after Harvest | ||||
|---|---|---|---|---|---|
| 14 | 28 | 14 | 28 | ||
| 2020 | 2021 | ||||
| Control | 5.4 ± 0.20 d | 4.4 ± 0.11 e | 5.2 ± 0.19 d | 4.1 ± 0.17 f | |
| Methyl Jasmonate (ppm) | 10 | 5.8 ± 0.17 c | 5.0 ± 0.13 d | 5.6 ± 0.16 c | 5.0 ± 0.14 e |
| 20 | 5.9 ± 0.14 bc | 5.3 ± 0.16 cd | 5.7 ± 0.17 c | 5.1 ± 0.12 e | |
| 50 | 6.0 ± 0.19 b | 5.5 ± 0.14 c | 5.7 ± 0.14 c | 5.2 ± 0.17 de | |
| Melatonin (ppm) | 10 | 5.9 ± 0.13 bc | 5.3 ± 0.16 cd | 5.8 ± 0.13 bc | 5.3 ± 0.14 d |
| 20 | 6.0 ± 0.10 b | 5.5 ± 0.17 c | 5.8 ± 0.20 bc | 5.4 ± 0.15 cd | |
| 50 | 6.1 ± 0.15 ab | 5.7 ± 0.14 b | 5.9 ± 0.18 b | 5.5 ± 0.15 c | |
| Methyl Jasmonate + Melatonin (ppm) | 10 | 6.0 ± 0.16 b | 5.6 ± 0.16 bc | 5.9 ± 0.15 b | 5.6 ± 0.18 bc |
| 20 | 6.1 ± 0.18 ab | 5.9 ± 0.19 ab | 6.0 ± 0.17 ab | 5.7 ± 0.13 b | |
| 50 | 6.3 ± 0.20 a | 6.0 ± 0.20 a | 6.1 ± 0.21 a | 5.9 ± 0.20 a | |
| Treatment | Days after Harvest | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 0 | 14 | 28 | ||
| 2020 | 2021 | ||||||
| Control | 27.8 ± 0.3 h | 29.5 ± 1.0 f | 31.8 ± 0.6 e | 28.0 ± 0.5 i | 29.8 ± 0.7 f | 31.9 ± 0.7 e | |
| Methyl Jasmonate (ppm) | 10 | 28.7 ± 0.7 g | 30.7 ± 0.9 e | 32.8 ± 0.5 d | 28.9 ± 0.7 h | 30.8 ± 0.5 e | 32.8 ± 0.9 d |
| 20 | 30.4 ± 1.0 f | 32.2 ± 1.1 d | 34.2 ± 0.9 c | 30.6 ± 0.8 g | 32.3 ± 0.6 d | 34.2 ± 0.7 c | |
| 50 | 31.7 ± 0.9 e | 33.5 ± 0.9 c | 35.1 ± 0.7 abc | 31.8 ± 0.6 f | 33.6 ± 0.6 c | 35.2 ± 0.9 bc | |
| Melatonin (ppm) | 10 | 32.3 ± 0.6 d | 33.6 ± 0.7 bc | 34.5 ± 0.8 bc | 32.3 ± 0.8 e | 33.7 ± 0.7 c | 34.6 ± 1.0 c |
| 20 | 32.8 ± 0.8 cd | 33.7 ± 0.5 bc | 34.6 ± 0.9 bc | 32.8 ± 0.9 de | 33.8 ± 1.1 c | 34.7 ± 0.8 c | |
| 50 | 33.1 ± 0.7 c | 34.2 ± 0.5 bc | 35.1 ± 1.0 bc | 33.2 ± 1.1 cd | 34.3 ± 1.0 bc | 35.2 ± 0.8 bc | |
| Methyl Jasmonate + Melatonin (ppm) | 10 | 33.4 ± 1.0 bc | 34.6 ± 0.6 bc | 35.5 ± 0.7 b | 33.5 ± 1.0 bc | 34.6 ± 0.9 bc | 35.6 ± 0.6 b |
| 20 | 33.8 ± 1.1 b | 34.7 ± 0.8 b | 35.3 ± 0.8 bc | 33.9 ± 0.7 b | 34.8 ± 0.8 b | 35.4 ± 0.9 bc | |
| 50 | 34.4 ± 0.9 a | 35.3 ± 0.9 a | 36.0 ± 0.9 a | 34.6 ± 1.0 a | 35.4 ± 1.0 a | 36.0 ± 0.8 a | |
| Treatment | Days after Harvest | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 0 | 14 | 28 | ||
| 2020 | 2021 | ||||||
| Control | 24.4 ± 0.4 g | 26.4 ± 0.8 g | 25.5 ± 0.4 h | 24.4 ± 0.6 g | 26.5 ± 0.7 g | 25.4 ± 0.6 h | |
| Methyl Jasmonate (ppm) | 10 | 25.7 ± 0.8 f | 27.3 ± 0.7 f | 26.4 ± 0.8 g | 25.7 ± 0.8 f | 27.3 ± 0.8 f | 26.5 ± 0.5 g |
| 20 | 25.8 ± 0.7 f | 27.9 ± 0.6 f | 27.0 ± 0.5 g | 25.8 ± 0.9 f | 27.9 ± 0.5 ef | 27.1 ± 0.7 g | |
| 50 | 27.0 ± 0.9 e | 29.0 ± 0.9 e | 28.3 ± 0.6 f | 27.0 ± 0.7 e | 28.7 ± 0.5 e | 28.0 ± 0.6 f | |
| Melatonin (ppm) | 10 | 28.7 ± 0.6 d | 30.6 ± 0.4 d | 29.7 ± 0.6 e | 28.7 ± 0.7 d | 30.7 ± 0.7 d | 29.7 ± 0.5 e |
| 20 | 29.8 ± 1.0 c | 31.4 ± 0.8 c | 30.2 ± 1.0 de | 29.8 ± 0.9 c | 31.4 ± 1.0 cd | 30.3 ± 0.9 de | |
| 50 | 30.2 ± 1.0 bc | 32.1 ± 1.0 bc | 30.8 ± 0.9 cd | 30.2 ± 0.8 bc | 32.1 ± 0.7 bc | 30.8 ± 1.0 cd | |
| Methyl Jasmonate + Melatonin (ppm) | 10 | 30.5 ± 0.8 bc | 32.4 ± 0.9 b | 31.4 ± 1.0 bc | 30.5 ± 0.7 bc | 32.4 ± 0.6 b | 31.5 ± 1.0 bc |
| 20 | 30.9 ± 0.9 ab | 32.7 ± 1.1 b | 31.9 ± 1.0 ab | 30.9 ± 1.0 b | 32.7 ± 0.9 ab | 31.9 ± 0.9 ab | |
| 50 | 31.6 ± 1.1 a | 33.4 ± 1.0 a | 32.4 ± 0.9 a | 31.7 ± 1.0 a | 33.5 ± 0.9 a | 32.5 ± 1.1 a | |
| Treatment | Days after Harvest | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 0 | 14 | 28 | ||
| 2020 | 2021 | ||||||
| Control | 0.33 ± 0.07 a | 0.32 ± 0.06 a | 0.31 ± 0.06 a | 0.34 ± 0.05 a | 0.33 ± 0.08 a | 0.31 ± 0.07 a | |
| Methyl Jasmonate (ppm) | 10 | 0.30 ± 0.03 b | 0.29 ± 0.05 b | 0.28 ± 0.07 b | 0.32 ± 0.06 b | 0.31 ± 0.05 b | 0.29 ± 0.06 b |
| 20 | 0.28 ± 0.03 c | 0.26 ± 0.07 c | 0.26 ± 0.05 c | 0.30 ± 0.05 c | 0.29 ± 0.05 c | 0.27 ± 0.05 c | |
| 50 | 0.26 ± 0.05 c | 0.25 ± 0.06 c | 0.24 ± 0.05 d | 0.28 ± 0.07 d | 0.27 ± 0.06 d | 0.24 ± 0.07 d | |
| Melatonin (ppm) | 10 | 0.23 ± 0.04 d | 0.21 ± 0.05 d | 0.20 ± 0.06 e | 0.25 ± 0.05 e | 0.24 ± 0.04 e | 0.21 ± 0.04 e |
| 20 | 0.22 ± 0.06 de | 0.20 ± 0.04 de | 0.19 ± 0.05 e | 0.24 ± 0.06 ef | 0.23 ± 0.04 ef | 0.20 ± 0.04 e | |
| 50 | 0.21 ± 0.05 ef | 0.20 ± 0.06 de | 0.18 ± 0.06 ef | 0.22 ± 0.04 fg | 0.21 ± 0.05 fg | 0.20 ± 0.06 e | |
| Methyl Jasmonate + Melatonin (ppm) | 10 | 0.20 ± 0.05 ef | 0.19 ± 0.05 ef | 0.17 ± 0.04 fg | 0.22 ± 0.05 fg | 0.20 ± 0.04 g | 0.19 ± 0.03 ef |
| 20 | 0.19 ± 0.06 fg | 0.18 ± 0.04 fg | 0.17 ± 0.05 fg | 0.21 ± 0.05 gh | 0.19 ± 0.06 gh | 0.18 ± 0.04 fg | |
| 50 | 0.18 ± 0.07 g | 0.17 ± 0.04 g | 0.16 ± 0.04 g | 0.19 ± 0.03 h | 0.18 ± 0.05 h | 0.17 ± 0.04 g | |
| RCC | N | P | K | YPP | BW | FL | FD | TSS | TS | TA | WL | FDC | FF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCC | 1 | |||||||||||||
| N | 0.93 *** | 1 | ||||||||||||
| P | 0.98 *** | 0.90 *** | 1 | |||||||||||
| K | 0.99 *** | 0.95 *** | 0.97 *** | 1 | ||||||||||
| YPP | 0.96 *** | 0.98 *** | 0.95 *** | 0.97 *** | 1 | |||||||||
| BW | 0.97 *** | 0.94 *** | 0.98 *** | 0.97 *** | 0.97 *** | 1 | ||||||||
| FL | 0.99 *** | 0.94 *** | 0.97 *** | 0.99 *** | 0.97 *** | 0.98 *** | 1 | |||||||
| FD | 0.98 *** | 0.96 *** | 0.95 *** | 0.99 *** | 0.98 *** | 0.97 *** | 0.99 *** | 1 | ||||||
| TSS | 0.75 *** | 0.74 *** | 0.81 *** | 0.77 *** | 0.80 *** | 0.82 *** | 0.76 *** | 0.77 *** | 1 | |||||
| TS | 0.97 *** | 0.90 *** | 0.98 *** | 0.96 *** | 0.94 *** | 0.96 *** | 0.97 *** | 0.95 *** | 0.83 *** | 1 | ||||
| TA | −0.93 *** | −0.86 *** | −0.96 *** | −0.91 *** | −0.91 *** | −0.95 *** | −0.93 *** | −0.91 *** | −0.87 *** | −0.97 *** | 1 | |||
| WL | −0.38 ** | −0.37 ** | −0.37 ** | −0.39 ** | −0.39 ** | −0.39 ** | −0.37 ** | −0.39 ** | −0.39 ** | −0.36 ** | 0.38 ** | 1 | ||
| FDC | −0.43 *** | −0.40 ** | −0.45 *** | −0.43 *** | −0.43 *** | −0.44 *** | −0.42 *** | −0.43 *** | −0.44 *** | −0.43 *** | 0.47 *** | 0.87 *** | 1 | |
| FF | 0.40 ** | 0.37 ** | 0.43 *** | 0.39** | 0.40 ** | 0.41 ** | 0.39 ** | 0.38 ** | 0.44 *** | 0.42 *** | −0.47 *** | −0.84 *** | −0.95 *** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekry, W.M.E.; Rashad, Y.M.; Alaraidh, I.A.; Mehany, T. Exogenous Application of Melatonin and Methyl Jasmonate as a Pre-Harvest Treatment Enhances Growth of Barhi Date Palm Trees, Prolongs Storability, and Maintains Quality of Their Fruits under Storage Conditions. Plants 2022, 11, 96. https://doi.org/10.3390/plants11010096
Fekry WME, Rashad YM, Alaraidh IA, Mehany T. Exogenous Application of Melatonin and Methyl Jasmonate as a Pre-Harvest Treatment Enhances Growth of Barhi Date Palm Trees, Prolongs Storability, and Maintains Quality of Their Fruits under Storage Conditions. Plants. 2022; 11(1):96. https://doi.org/10.3390/plants11010096
Chicago/Turabian StyleFekry, Waleed M. E., Younes M. Rashad, Ibrahim A. Alaraidh, and Taha Mehany. 2022. "Exogenous Application of Melatonin and Methyl Jasmonate as a Pre-Harvest Treatment Enhances Growth of Barhi Date Palm Trees, Prolongs Storability, and Maintains Quality of Their Fruits under Storage Conditions" Plants 11, no. 1: 96. https://doi.org/10.3390/plants11010096
APA StyleFekry, W. M. E., Rashad, Y. M., Alaraidh, I. A., & Mehany, T. (2022). Exogenous Application of Melatonin and Methyl Jasmonate as a Pre-Harvest Treatment Enhances Growth of Barhi Date Palm Trees, Prolongs Storability, and Maintains Quality of Their Fruits under Storage Conditions. Plants, 11(1), 96. https://doi.org/10.3390/plants11010096









