Guard-Cell-Specific Expression of Phototropin2 C-Terminal Fragment Enhances Leaf Transpiration
Abstract
1. Introduction
2. Results
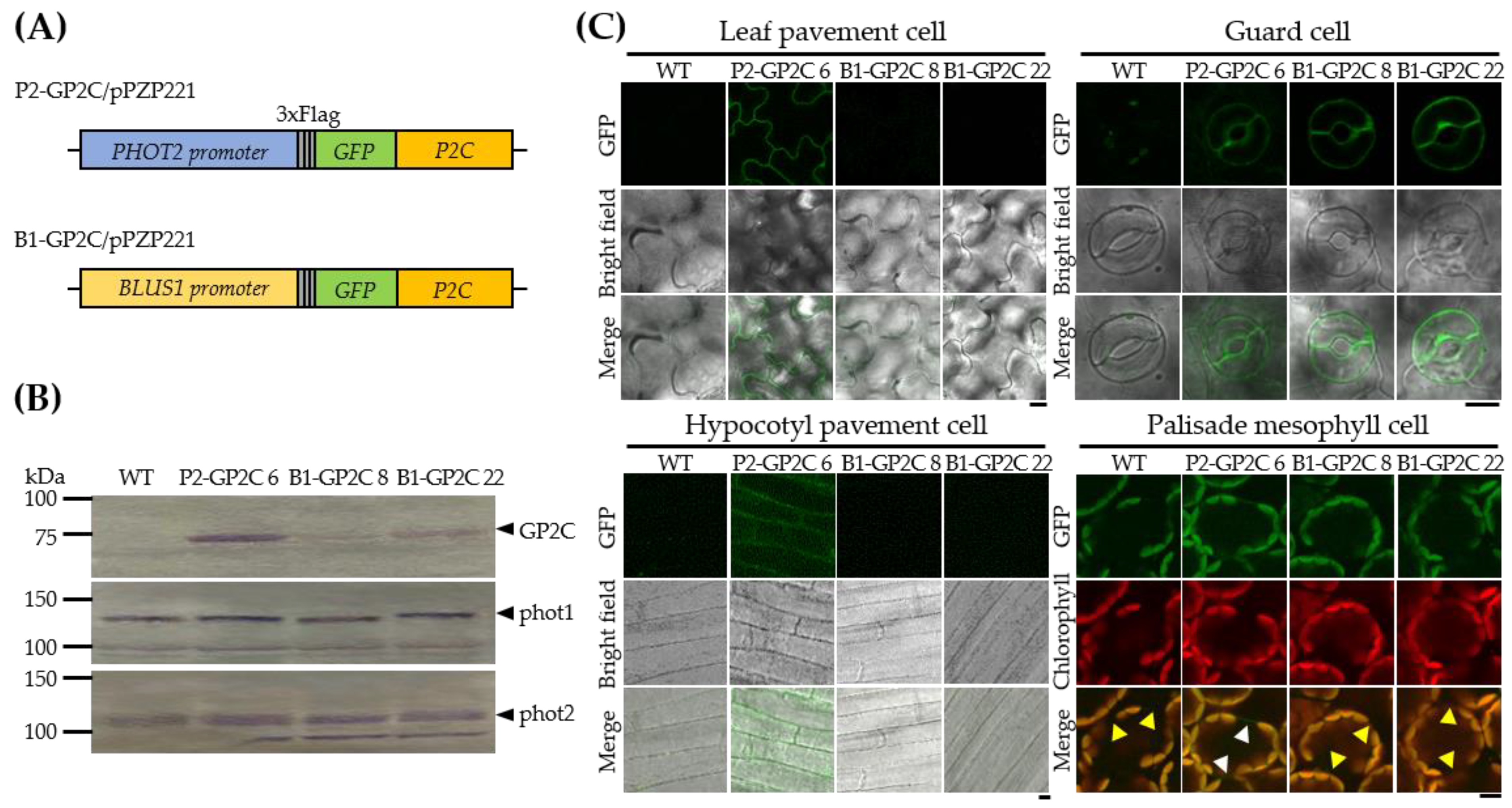
2.1. B1-GP2C Plants Express P2C in a Guard-Cell-Specific Manner
2.2. B1-GP2C Plants Show Normal Chloroplast Photorelocation Movement
2.3. B1-GP2C Plants Show Normal Hypocotyl Growth and Phototropic Response
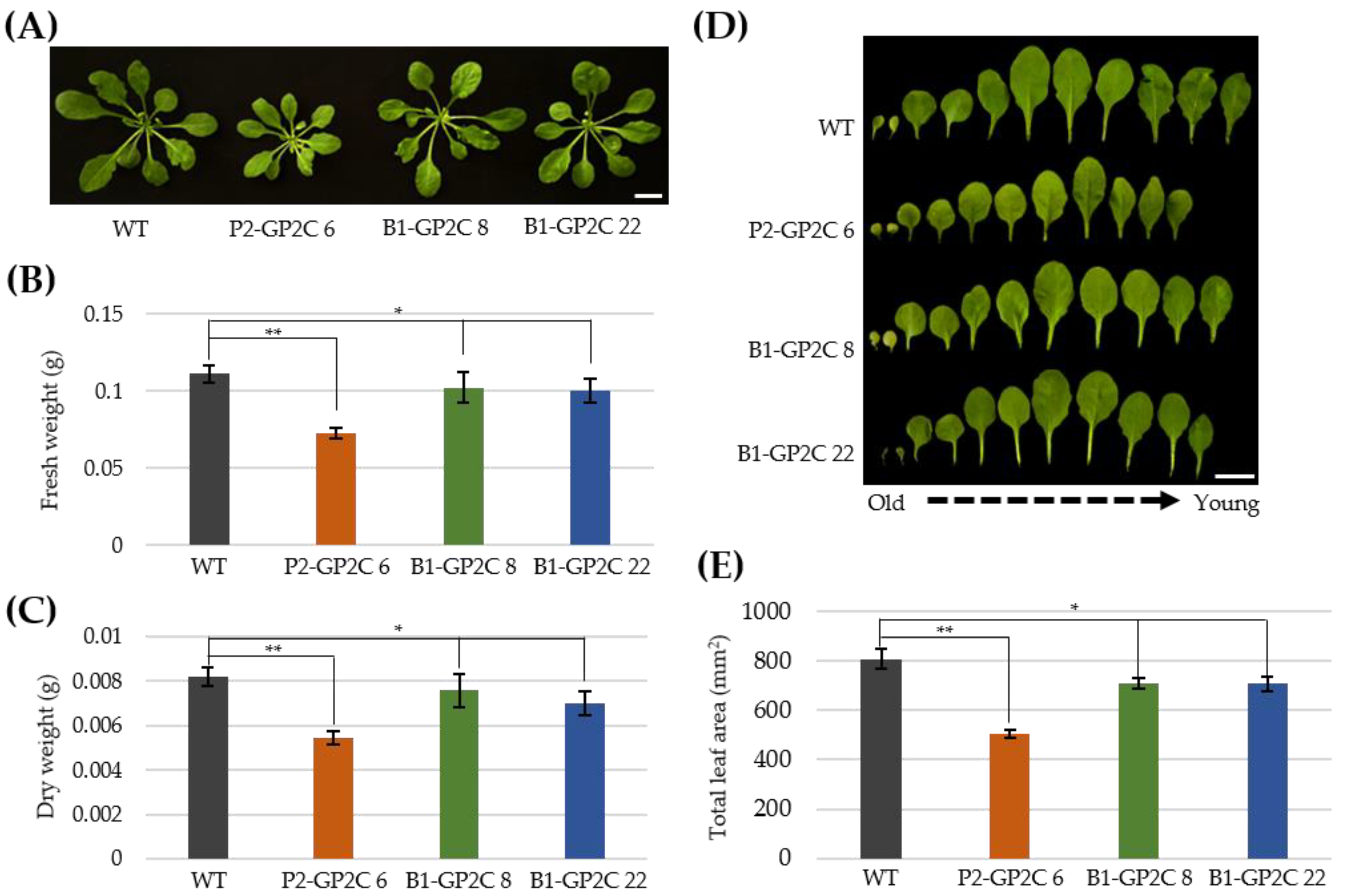
2.4. B1-GP2C Plants Show Normal Growth Phenotypes
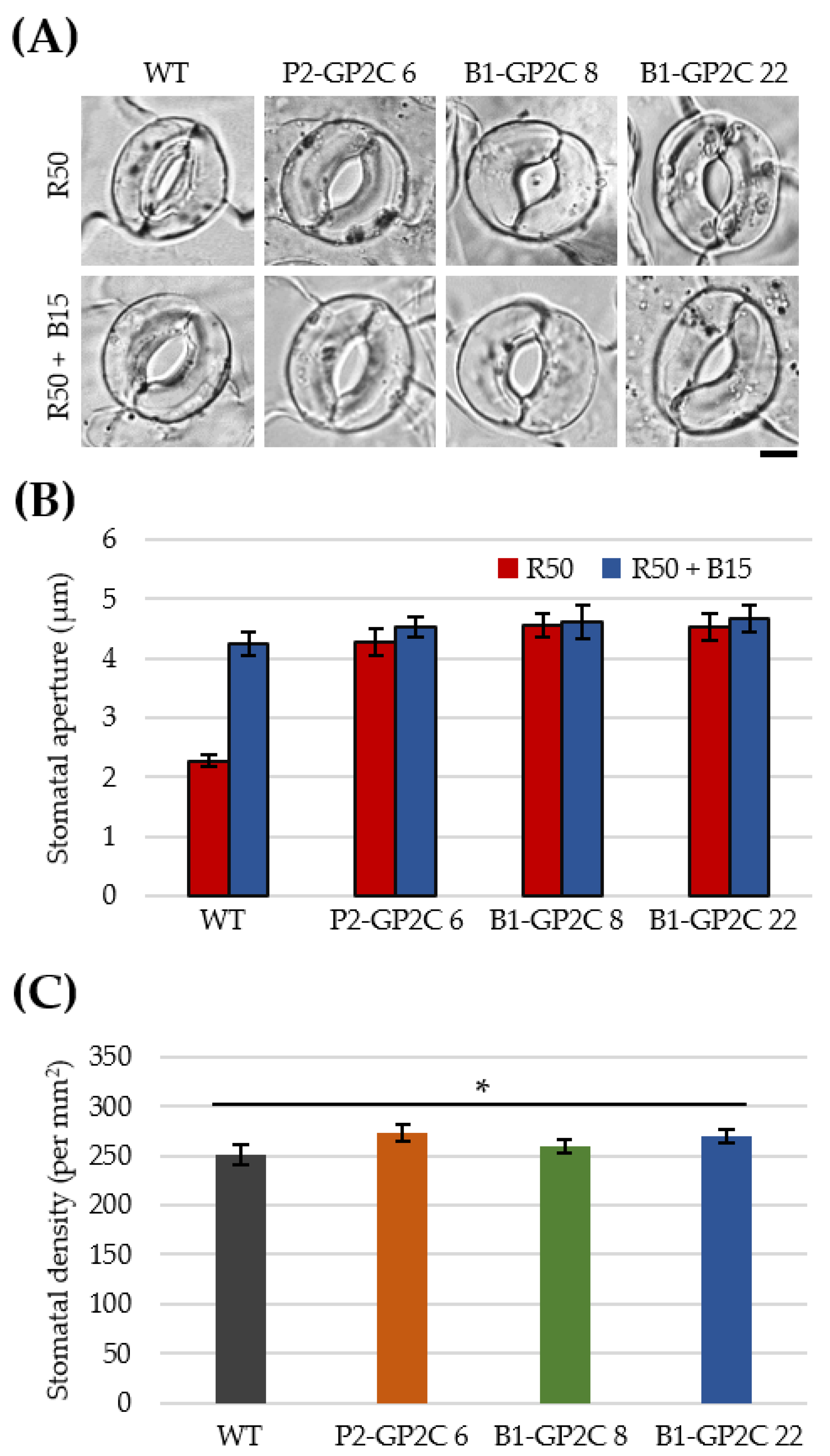
2.5. Both P2-GP2C and B1-GP2C Plants Show Constitutive Stomatal Opening Regardless of Blue Light
2.6. B1-GP2C Plants Show Enhanced Transpiration
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Binary Vector Construction and Agrobacterium-Mediated Transformation
4.3. Protein Extraction and Western Blot Analysis
4.4. Confocal Laser Scanning Microscopy and Image Analysis
4.5. Chloroplast Photorelocation Movement
4.6. Hypocotyl Growth and Phototropism
4.7. Vegetative Growth Phenotype
4.8. Stomatal Opening
4.9. Leaf Transpiration
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Briggs, W.R.; Christie, J.M. Phototropins 1 and 2: Versatile Plant Blue-Light Receptors. Trends Plant Sci. 2002, 7, 204–210. [Google Scholar] [CrossRef]
- Huala, E.; Oeller, P.W.; Liscum, E.; Han, I.-S.; Larsen, E.; Briggs, W.R. Arabidopsis NPH1: A Protein Kinase with a Putative Redox-Sensing Domain. Science 1997, 278, 2120–2123. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Sakai, T.; Suetsugu, N.; Oikawa, K.; Ishiguro, S.; Kato, T.; Tabata, S.; Okada, K.; Wada, M. Arabidopsis NPL1: A Phototropin Homolog Controlling the Chloroplast High-Light Avoidance Response. Science 2001, 291, 2138–2141. [Google Scholar] [CrossRef]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast Avoidance Movement Reduces Photodamage in Plants. Nature 2002, 420, 829. [Google Scholar] [CrossRef]
- Sakai, T.; Kagawa, T.; Kasahara, M.; Swartz, T.E.; Christie, J.M.; Briggs, W.R.; Wada, M.; Okada, K. Arabidopsis Nph1 and Npl1: Blue Light Receptors That Mediate Both Phototropism and Chloroplast Relocation. Proc. Natl. Acad. Sci. USA 2001, 98, 6969–6974. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Briggs, W.R. Cellular and Subcellular Localization of Phototropin 1. Plant Cell 2002, 14, 1723–1735. [Google Scholar] [CrossRef]
- Talbott, L.D.; Shmayevich, I.J.; Chung, Y.; Hammad, J.W.; Zeiger, E. Blue Light and Phytochrome-Mediated Stomatal Opening in the Npq1 and Phot1 Phot2 Mutants of Arabidopsis. Plant Physiol. 2003, 133, 1522–1529. [Google Scholar] [CrossRef]
- Wada, M.; Kagawa, T.; Sato, Y. Chloroplast Movement. Annu. Rev. Plant Biol. 2003, 54, 455–468. [Google Scholar] [CrossRef]
- Gotoh, E.; Suetsugu, N.; Yamori, W.; Ishishita, K.; Kiyabu, R.; Fukuda, M.; Higa, T.; Shirouchi, B.; Wada, M. Chloroplast Accumulation Response Enhances Leaf Photosynthesis and Plant Biomass Production. Plant Physiol. 2018, 178, 1358–1369. [Google Scholar] [CrossRef]
- Suetsugu, N.; Kagawa, T.; Wada, M. An Auxilin-like J-Domain Protein, JAC1, Regulates Phototropin-Mediated Chloroplast Movement in Arabidopsis. Plant Physiol. 2005, 139, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Jarillo, J.A.; Gabrys, H.; Capel, J.; Alonso, J.M.; Ecker, J.R.; Cashmore, A.R. Phototropin-Related NPL1 Controls Chloroplast Relocation Induced by Blue Light. Nature 2001, 410, 952–954. [Google Scholar] [CrossRef]
- Esmon, C.A.; Tinsley, A.G.; Ljung, K.; Sandberg, G.; Hearne, L.B.; Liscum, E. A Gradient of Auxin and Auxin-Dependent Transcription Precedes Tropic Growth Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 236–241. [Google Scholar] [CrossRef]
- Fankhauser, C.; Christie, J.M. Plant Phototropic Growth. Curr. Biol. 2015, 25, R384–R389. [Google Scholar] [CrossRef]
- Briggs, W.R.; Beck, C.F.; Cashmore, A.R.; Christie, J.M.; Hughes, J.; Jarillo, J.A.; Kagawa, T.; Kanegae, H.; Liscum, E.; Nagatani, A. The Phototropin Family of Photoreceptors. Plant Cell 2001, 13, 993–997. [Google Scholar] [CrossRef]
- Inoue, S.; Kinoshita, T.; Matsumoto, M.; Nakayama, K.I.; Doi, M.; Shimazaki, K. Blue Light-Induced Autophosphorylation of Phototropin Is a Primary Step for Signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 5626–5631. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K. Phot1 and Phot2 Mediate Blue Light Regulation of Stomatal Opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Inoue, S.; Kinoshita, T.; Shimazaki, K. Possible Involvement of Phototropins in Leaf Movement of Kidney Bean in Response to Blue Light. Plant Physiol. 2005, 138, 1994–2004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light Regulation of Stomatal Movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed]
- Takemiya, A.; Kinoshita, T.; Asanuma, M.; Shimazaki, K. Protein Phosphatase 1 Positively Regulates Stomatal Opening in Response to Blue Light in Vicia Faba. Proc. Natl. Acad. Sci. USA 2006, 103, 13549–13554. [Google Scholar] [CrossRef]
- Takemiya, A.; Yamauchi, S.; Yano, T.; Ariyoshi, C.; Shimazaki, K. Identification of a Regulatory Subunit of Protein Phosphatase 1 Which Mediates Blue Light Signaling for Stomatal Opening. Plant Cell Physiol. 2013, 54, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Takemiya, A.; Sakamoto, T.; Kurata, T.; Tsutsumi, T.; Kinoshita, T.; Shimazaki, K. The Plasma Membrane H+-ATPase AHA1 Plays a Major Role in Stomatal Opening in Response to Blue Light. Plant Physiol. 2016, 171, 2731–2743. [Google Scholar] [CrossRef]
- Takemiya, A.; Sugiyama, N.; Fujimoto, H.; Tsutsumi, T.; Yamauchi, S.; Hiyama, A.; Tada, Y.; Christie, J.M.; Shimazaki, K. Phosphorylation of BLUS1 Kinase by Phototropins Is a Primary Step in Stomatal Opening. Nat. Commun. 2013, 4, 2094. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-W.; Rothfels, C.J.; Melkonian, M.; Villarreal, J.C.; Stevenson, D.W.; Graham, S.W.; Wong, G.K.-S.; Mathews, S.; Pryer, K.M. The Origin and Evolution of Phototropins. Front. Plant Sci. 2015, 6, 637. [Google Scholar] [CrossRef] [PubMed]
- Herrou, J.; Crosson, S. Function, Structure and Mechanism of Bacterial Photosensory LOV Proteins. Nat. Rev. Microbiol. 2011, 9, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.; Christie, J.M.; Knieb, E.; Lempert, U.; Briggs, W.R. Photochemical and Mutational Analysis of the FMN-Binding Domains of the Plant Blue Light Receptor, Phototropin. Biochemistry 2000, 39, 9401–9410. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Tseng, T.-S.; Kaiserli, E.; Sullivan, S.; Christie, J.M.; Briggs, W.R. Physiological Roles of the Light, Oxygen, or Voltage Domains of Phototropin 1 and Phototropin 2 in Arabidopsis. Plant Physiol. 2007, 143, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.M.; Swartz, T.E.; Bogomolni, R.A.; Briggs, W.R. Phototropin LOV Domains Exhibit Distinct Roles in Regulating Photoreceptor Function. Plant J. 2002, 32, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.M.; Christie, J.M.; Gardner, K.H. Disruption of the LOV-Jα Helix Interaction Activates Phototropin Kinase Activity. Biochemistry 2004, 43, 16184–16192. [Google Scholar] [CrossRef]
- Christie, J.M. Phototropin Blue-Light Receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef]
- Demarsy, E.; Schepens, I.; Okajima, K.; Hersch, M.; Bergmann, S.; Christie, J.; Shimazaki, K.; Tokutomi, S.; Fankhauser, C. Phytochrome Kinase Substrate 4 Is Phosphorylated by the Phototropin 1 Photoreceptor. EMBO J. 2012, 31, 3457–3467. [Google Scholar] [CrossRef]
- Inoue, S.; Matsushita, T.; Tomokiyo, Y.; Matsumoto, M.; Nakayama, K.I.; Kinoshita, T.; Shimazaki, K. Functional Analyses of the Activation Loop of Phototropin2 in Arabidopsis. Plant Physiol. 2011, 156, 117–128. [Google Scholar] [CrossRef]
- Kong, S.-G.; Suzuki, T.; Tamura, K.; Mochizuki, N.; Hara-Nishimura, I.; Nagatani, A. Blue Light-Induced Association of Phototropin 2 with the Golgi Apparatus. Plant J. 2006, 45, 994–1005. [Google Scholar] [CrossRef]
- Kong, S.-G.; Suetsugu, N.; Kikuchi, S.; Nakai, M.; Nagatani, A.; Wada, M. Both Phototropin 1 and 2 Localize on the Chloroplast Outer Membrane with Distinct Localization Activity. Plant Cell Physiol. 2013, 54, 80–92. [Google Scholar] [CrossRef]
- Ishishita, K.; Higa, T.; Tanaka, H.; Inoue, S.; Chung, A.; Ushijima, T.; Matsushita, T.; Kinoshita, T.; Nakai, M.; Wada, M. Phototropin2 Contributes to the Chloroplast Avoidance Response at the Chloroplast-Plasma Membrane Interface. Plant Physiol. 2020, 183, 304–316. [Google Scholar] [CrossRef]
- Kong, S.-G.; Kinoshita, T.; Shimazaki, K.; Mochizuki, N.; Suzuki, T.; Nagatani, A. The C-Terminal Kinase Fragment of Arabidopsis Phototropin 2 Triggers Constitutive Phototropin Responses. Plant J. 2007, 51, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-G.; Kagawa, T.; Wada, M.; Nagatani, A. A C-Terminal Membrane Association Domain of Phototropin 2 Is Necessary for Chloroplast Movement. Plant Cell Physiol. 2013, 54, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Kong, S.-G. Analysis of Chloroplast Movement and Relocation in Arabidopsis. In Chloroplast Research in Arabidopsis; Springer: Berlin/Heidelberg, Germany, 2011; pp. 87–102. [Google Scholar]
- Onodera, A.; Kong, S.-G.; Doi, M.; Shimazaki, K.-I.; Christie, J.; Mochizuki, N.; Nagatani, A. Phototropin from Chlamydomonas Reinhardtii Is Functional in Arabidopsis Thaliana. Plant Cell Physiol. 2005, 46, 367–374. [Google Scholar] [CrossRef]
- Christie, J.M.; Yang, H.; Richter, G.L.; Sullivan, S.; Thomson, C.E.; Lin, J.; Titapiwatanakun, B.; Ennis, M.; Kaiserli, E.; Lee, O.R. Phot1 Inhibition of ABCB19 Primes Lateral Auxin Fluxes in the Shoot Apex Required for Phototropism. PLoS Biol. 2011, 9, e1001076. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, P.; Maloney, G.S.; Muday, G.K. Localized Induction of the ATP-Binding Cassette B19 Auxin Transporter Enhances Adventitious Root Formation in Arabidopsis. Plant Physiol. 2013, 162, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Jiang, Y.; Wang, X.; Huang, S.; Yuan, M.; Guo, Y. AP3M Harbors Actin Filament Binding Activity That Is Crucial for Vacuole Morphology and Stomatal Closure in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 18132–18141. [Google Scholar] [CrossRef] [PubMed]
- Farber, M.; Attia, Z.; Weiss, D. Cytokinin Activity Increases Stomatal Density and Transpiration Rate in Tomato. J. Exp. Bot. 2016, 67, 6351–6362. [Google Scholar] [CrossRef]
- Lake, J.A.; Woodward, F.I. Response of Stomatal Numbers to CO2 and Humidity: Control by Transpiration Rate and Abscisic Acid. New Phytol. 2008, 179, 397–404. [Google Scholar] [CrossRef]
- Orsini, F.; Alnayef, M.; Bona, S.; Maggio, A.; Gianquinto, G. Low Stomatal Density and Reduced Transpiration Facilitate Strawberry Adaptation to Salinity. Environ. Exp. Bot. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Gypser, S.; Halke, C.; Koning, L.; Freese, D.; Lebzien, S. Transpiration and Biomass Production of the Bioenergy Crop Giant Knotweed Igniscum under Various Supplies of Water and Nutrients. J. Hydrol. Hydromech 2014, 62, 316–323. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Primo-Millo, E.; Forner-Giner, M.Á. An Integrated View of Whole-Tree Hydraulic Architecture. Does Stomatal or Hydraulic Conductance Determine Whole Tree Transpiration? PLoS ONE 2016, 11, e0155246. [Google Scholar]
- Wang, Y.; Sun, Y.; Niu, G.; Deng, C.; Wang, Y.; Gardea-Torresdey, J. Growth, Gas Exchange, and Mineral Nutrients of Ornamental Grasses Irrigated with Saline Water. HortScience 2019, 54, 1840–1846. [Google Scholar] [CrossRef]
- Han, K.-T.; Ruan, L.-W. Effects of Indoor Plants on Air Quality: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 16019–16051. [Google Scholar] [CrossRef]
- Pamonpol, K.; Areerob, T.; Prueksakorn, K. Indoor Air Quality Improvement by Simple Ventilated Practice and Sansevieria Trifasciata. Atmosphere 2020, 11, 271. [Google Scholar] [CrossRef]
- Wolverton, B.C.; Johnson, A.; Bounds, K. Interior Landscape Plants for Indoor Air Pollution Abatement. NASA Tech. Rep. Serv. 1989, 15, 1–30. [Google Scholar]
- Darrall, N.M. The Effect of Air Pollutants on Physiological Processes in Plants. Plant Cell Environ. 1989, 12, 1–30. [Google Scholar] [CrossRef]
- Jones, H.G. Stomatal Control of Photosynthesis and Transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified Method for Agrobacterium-Mediated Transformation of Arabidopsis Thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, J.; Bong, H.; Wada, M.; Kong, S.-G. ACTIN2 Functions in Chloroplast Photorelocation Movement in Arabidopsis Thaliana. J. Plant Biol. 2020, 63, 379–389. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riu, Y.-S.; Song, H.-G.; Kim, H.-S.; Kong, S.-G. Guard-Cell-Specific Expression of Phototropin2 C-Terminal Fragment Enhances Leaf Transpiration. Plants 2022, 11, 65. https://doi.org/10.3390/plants11010065
Riu Y-S, Song H-G, Kim H-S, Kong S-G. Guard-Cell-Specific Expression of Phototropin2 C-Terminal Fragment Enhances Leaf Transpiration. Plants. 2022; 11(1):65. https://doi.org/10.3390/plants11010065
Chicago/Turabian StyleRiu, Young-Sun, Hyun-Geun Song, Hwi-Su Kim, and Sam-Geun Kong. 2022. "Guard-Cell-Specific Expression of Phototropin2 C-Terminal Fragment Enhances Leaf Transpiration" Plants 11, no. 1: 65. https://doi.org/10.3390/plants11010065
APA StyleRiu, Y.-S., Song, H.-G., Kim, H.-S., & Kong, S.-G. (2022). Guard-Cell-Specific Expression of Phototropin2 C-Terminal Fragment Enhances Leaf Transpiration. Plants, 11(1), 65. https://doi.org/10.3390/plants11010065






