LC-MS Characterization and Biological Activities of Cuban Cultivars of Plectranthus neochilus Schltr
Abstract
1. Introduction
2. Results
2.1. Plant Quality Control Parameters
2.1.1. Fresh Leaves
2.1.2. Dry Leaves
2.2. Plant Extracts and Quality Control Determinations
2.2.1. Physical and Physicochemical Parameters of Plant and Extracts
2.2.2. Determination of the Phytochemical Profile of the Extracts
2.3. Antimicrobial Activity
2.4. Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Quality Control Drug Parameters Determinations
4.2.1. Macroscopic and EOY Determinations
4.2.2. Microscopic Determination
4.2.3. Total Ash Content and Total Extractable Matter
4.3. Plant Extracts Preparation and Quality Control Determinations
4.3.1. Determination of the Physical and Physicochemical Parameters of the Extracts
4.3.2. Determination of the Phytochemical Profile of the Extracts
4.4. Determination of the Antimicrobial Activity
4.4.1. Microorganisms
4.4.2. Strain Culture and Extract Dilution
4.4.3. Antibacterial and Antifungal Assays
4.4.4. Antileishmanial Assays
4.4.5. Antitrypanosomal Assays
4.4.6. Cytotoxicity Assays
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| AEP | Peak presence in alternative extract |
| BIOECO | Center for Biodiversity and Ecosystems |
| CPRG | Chlorophenol red-β-D-galactopyranoside |
| DMSO | Dimethyl sulfoxide |
| DLW | Dry leaves water maceration |
| DLE | Dry leaves ethanol maceration |
| EO | Essential oils |
| EOY | Essential oil yield |
| ESI | Electro Spray Ionization |
| FBMN | Feature-Based Molecular Networking |
| FCS | Fetal calf serum |
| FLD | Fresh leaves decoction |
| GNPS | Global Natural Products Social Molecular Networking |
| HFBS | Heat-inactivated fetal bovine serum |
| IC50 | Inhibitory concentration |
| LMPH | Laboratory for Microbiology, Parasitology, and Hygiene |
| MEM | Minimum Essential Medium Eagle |
| MHB | Mueller Hinton Broth |
| MoNA | MassBank of North America |
| NIST | National Institute of Standards and Technology |
| PDA | (Potato Dextrose Agar) |
| PBS | Phosphate buffered saline |
| PMM | Peritoneal macrophages from mice |
| Rt | Retention Time |
| SD | Standard deviation |
| SI | Selectivity Index |
| TSA | Tryptone Soy Agar |
| TIC | Total Ion Chromatogram |
| UPLC-DAD-MS/MS | Ultra-high performance liquid chromatography-Diode Array Detection-Mass/Mass WHO: World Health Organization |
References
- World Health Organization Access to Medicines: Making Market Forces Serve the Poor. Ten Years in Public Health 2007–2017. 2017. Available online: https://www.who.int/publications/10-year-review/chapter-medicines.pdf?ua=1 (accessed on 3 January 2022).
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Vasisht, K.; Sharma, N.; Karan, M. Current perspective in the international trade of medicinal plants material: An update. Curr. Pharm. Des. 2016, 22, 4288–4336. [Google Scholar] [CrossRef]
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Lima, G.P.P. Medicinal plants: Influence of environmental factors on the content of secondary metabolites. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 259–277. ISBN 978-3-319-68716-2. [Google Scholar]
- Yuan, Y.; Tang, X.; Jia, Z.; Li, C.; Ma, J.; Zhang, J. The effects of ecological factors on the main medicinal components of Dendrobium officinale under different cultivation modes. Forests 2020, 11, 94. [Google Scholar] [CrossRef]
- Caixeta, S.C.; Magalhães, L.G.; de Melo, N.I.; Wakabayashi, K.A.L.; Aguiar, G.P.; Aguiar, D.P.; Mantovani, A.L.L.; Alves, J.M.; Oliveira, P.F.; Tavares, D.C.; et al. Chemical composition and in vitro schistosomicidal activity of the essential oil of Plectranthus neochilus grown in southeast Brazil. Chem. Biodivers. 2011, 8, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- York, T.; De Wet, H.; van Vuuren, S.F. Plants used for treating respiratory infections in rural Maputaland, KwaZulu-Natal, South Africa. J. Ethnopharmacol. 2011, 135, 696–710. [Google Scholar] [CrossRef]
- Mota, L.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G.; Miguel, M.G.; Faleiro, M.L.; Ascensão, L. Volatile-oils composition, and bioactivity of the essential oils of Plectranthus barbatus, P. neochilus, and P. ornatus grown in Portugal. Chem. Biodivers. 2014, 11, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Matias, D.; Pereira, F.; Reis, C.P.; Simões, M.F.; Rijo, P. Antimicrobial screening of Plectranthus madagascariensis and P. neochilus extracts. Biomed. Biopharm. Res. 2015, 12, 127–138. [Google Scholar] [CrossRef]
- Borges, G.A.; Ferreira, J.F.; Elias, S.T.; Guerra, E.N.S.; Silveira, D.; Simeoni, L.A. Cytotoxic effect of Plectranthus neochilus extracts in head and neck carcinoma cell lines. Afr. J. Pharm. Pharmacol. 2016, 10, 157–163. [Google Scholar] [CrossRef][Green Version]
- de Souza, P.M.; de Sales, P.M.; Simeoni, L.A.; Silva, E.C.; Silveira, D.; de Oliveira Magalhães, P. Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Brazilian cerrado. Planta Med. 2012, 78, 393–399. [Google Scholar] [CrossRef]
- Ramborger, B.P.; Gomes Paz, M.E.; Kieling, K.M.C.; Sigal Carriço, M.R.; de Paula Gollino, G.; Costa, M.T.; Ribeiro, V.B.; Folmer, V.; Gasparotto Denardin, E.L.; de Jesus Soares, J.; et al. Toxicological parameters of aqueous residue after using Plectranthus neochilus for 2,4-D phytoremediation. Chemosphere 2021, 270, 128638. [Google Scholar] [CrossRef]
- Mendez, S.I.E.; Rifá, T.J. Dos especies de Plectranthus (Lamiaceae) de reciente introducción en Cuba. Bouteloua 2016, 26, 92–96. [Google Scholar]
- Rodríguez-Ferreiro, A.O.; Léon-Duharte, D.; Polanco-Durán, G.; Guisado-Bourzac, F.; Ochoa-Pacheco, A.; Escalona-Arranz, J.C. Ethnobotany of Plectranthus neochilus Schltr (Meprobamate) in Cuba. Boletín Latinoam. y del Caribe de Plantas Med. y Aromáticas 2020, 19, 236–246. [Google Scholar] [CrossRef]
- do Duarte, M.R.; Lopes, J.F. Stem and leaf anatomy of Plectranthus neochilus Schltr., Lamiaceae. Rev. Bras. Farmacogn. 2007, 17, 549–556. [Google Scholar] [CrossRef][Green Version]
- Crevelin, E.J.; Caixeta, S.C.; Dias, H.J.; Groppo, M.; Cunha, W.R.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial activity of the essential oil of Plectranthus neochilus against cariogenic bacteria. Evid.-Based Complement. Altern. Med. 2015, 2015, 102317. [Google Scholar] [CrossRef]
- Lawal, O.A.; Hutchings, A.H.; Oyedeji, O. Chemical composition of the leaf oil of Plectranthus neochilus Schltr. J. Essent. Oil Res. 2010, 22, 546–547. [Google Scholar] [CrossRef]
- Galbiatti, M.I.; Cassola, F.; Mesquita, A.T.; Pinheiro, G.P.; Mayer, J.L.S.; Sawaya, A.C.H.F. Plectranthus neochilus Schltr.: Anatomic and cytogenetic analyses and chemical characterization of its essential oil. S. Afr. J. Bot. 2021, 143, 97–106. [Google Scholar] [CrossRef]
- de Oliveira, G.G.; Carnevale Neto, F.; Demarque, D.P.; de Sousa Pereira-Junior, J.A.; Sampaio Peixoto Filho, R.C.; de Melo, S.J.; da Silva Almeida, J.R.G.; Lopes, J.L.C.; Lopes, N.P. Dereplication of flavonoid glycoconjugates from Adenocalymma imperatoris-maximilianii by untargeted tandem mass spectrometry-based molecular networking. Planta Med. 2017, 83, 636–646. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2008, 29, 1–16. [Google Scholar] [CrossRef]
- Grayer, R.J.; Eckert, M.R.; Lever, A.; Veitch, N.C.; Kite, G.C.; Paton, A.J. Distribution of exudate flavonoids in the genus Plectranthus. Biochem. Syst. Ecol. 2010, 38, 335–341. [Google Scholar] [CrossRef]
- Abdissa, N.; Frese, M.; Sewald, N. Antimicrobial abietane-type diterpenoids from Plectranthus punctatus. Molecules 2017, 22, 1919. [Google Scholar] [CrossRef]
- Cretton, S.; Saraux, N.; Monteillier, A.; Righi, D.; Marcourt, L.; Genta-Jouve, G.; Wolfender, J.L.; Cuendet, M.; Christen, P. Anti-inflammatory and antiproliferative diterpenoids from Plectranthus scutellarioides. Phytochemistry 2018, 154, 39–46. [Google Scholar] [CrossRef]
- Brito, E.; Gomes, E.; Falé, P.L.; Borges, C.; Pacheco, R.; Teixeira, V.; Machuqueiro, M.; Ascensão, L.; Serralheiro, M.L.M. Bioactivities of decoctions from Plectranthus species related to their traditional use on the treatment of digestive problems and alcohol intoxication. J. Ethnopharmacol. 2018, 220, 147–154. [Google Scholar] [CrossRef]
- Ablajan, K.; Abliz, Z.; Shang, X.-Y.; He, J.-M.; Zhang, R.-P.; Shi, J.-G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef]
- Garcia, C.; Teodósio, C.; Oliveira, C.; Oliveira, C.; Díaz-Lanza, A.; Reis, C.; Duarte, N.; Rijo, P. Naturally occurring Plectranthus-derived diterpenes with antitumoral activities. Curr. Pharm. Des. 2018, 24, 4207–4236. [Google Scholar] [CrossRef]
- Alasbahi, R.H.; Melzig, M.F. Plectranthus barbatus: A review of phytochemistry, ethnobotanical uses and pharmacology part 2. Planta Med. 2010, 76, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Ramborger, B.P.; Gomes Paz, M.E.; Gasparato Denardin, E.L.; de Jesus Soares, J.; Roehrs, R. A review of anatomical, physiological, biological characteristics and uses of Plectranthus neochilus. Ciência e Nat. 2020, 42, e12. [Google Scholar] [CrossRef]
- Matias, D.; Nicolai, M.; Fernandes, A.S.; Saraiva, N.; Almeida, J.; Saraiva, L.; Faustino, C.; Díaz-Lanza, A.M.; Reis, C.P.; Rijo, P. Comparison study of different extracts of Plectranthus madagascariensis, P. neochilus and the rare P. porcatus (Lamiaceae): Chemical characterization, antioxidant, antimicrobial and cytotoxic activities. Biomolecules 2019, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, I.A.; Lall, N. Plectranthus neochilus. In Underexplored Medicinal Plants from Sub-Saharan Africa; Lall, N., Ed.; Academic Press: London, UK, 2020; pp. 235–240. ISBN 978-0-12-816814-1. [Google Scholar]
- Galbiatti, M.I.; Pinheiro, G.P.; Antunes, E.R.M.; Hernandes, V.V.; Sawaya, A.C.H.F. Effect of environmental factors on Plectranthus neochilus volatile composition: A GC-MS-based metabolomics approach. Planta Med. Int. Open 2021, 8, e153–e160. [Google Scholar] [CrossRef]
- Llauradó Maury, G.; Méndez Rodríguez, D.; Hendrix, S.; Escalona Arranz, J.C.; Fung Boix, Y.; Pacheco, A.O.; García Díaz, J.; Morris Quevedo, H.J.; Ferrer Dubois, A.; Isaac Aleman, E.; et al. Antioxidants in plants: A valorization potential emphasizing the need for the conservation of plant biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Milaneze-Gutierre, M.A.; Famelli, M.C.; Capel, L.S.; Romagnolo, M.B. Caracterização morfológica dos tricomas foliares e caulinares de duas espécies de Lamiaceae conhecidas popularmente como “falso-boldo”. Acta Sci. Biol. Sci. 2007, 29, 125–130. [Google Scholar] [CrossRef]
- Mizutani, M.; Kanaoka, M.M. Environmental sensing and morphological plasticity in plants. Semin. Cell Dev. Biol. 2018, 83, 69–77. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Toma, M.; Ntungwe, E.; Kowalczyk, T.; Skała, E.; Wieczfinska, J.; Śliwiński, T.; Rijo, P. Insight the biological activities of selected abietane diterpenes isolated from Plectranthus spp. Biomolecules 2020, 10, 194. [Google Scholar] [CrossRef]
- Śliwiński, T.; Sitarek, P.; Skała, E.; Isca, V.M.S.; Synowiec, E.; Kowalczyk, T.; Bijak, M.; Rijo, P. Diterpenoids from Plectranthus spp. as potential chemotherapeutic agents via apoptosis. Pharmaceuticals 2020, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, G.P.; Lima, K.A.; Severiano, M.E.; Groppo, M.; Ambrósio, S.R.; Crevelin, E.J. Antifungal activity of the essential oils of Plectranthus neochilus (Lamiaceae) and Tagetes erecta (Asteraceae) cultivated in Brazil. Int. J. Complement. Altern. Med. 2018, 11, 00343. [Google Scholar] [CrossRef][Green Version]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- García Díaz, J.; EscalonaArranz, J.C.; da Gama Jaén Batista, D.; Monzote Fidalgo, L.; de La Vega Acosta, J.; de Macedo, M.B.; Cos, P. Antileishmanial potentialities of Croton linearis leaf essential oil. Nat. Prod. Commun. 2018, 13, 629–634. [Google Scholar] [CrossRef]
- García-Diaz, J.; Escalona-Arranz, J.C.; Rojas-Vargas, J.A.; Machado-García, R. Gordillo-Pérez, M. Escalona-Caparros, A. Pharmacognostic and phythochemical studies of Croton linearis Jacq. leaves. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 512–518. [Google Scholar]
- Theobald, W.L.; Krahulik, J.L.; Rollins, R.C. Trichome description and classification. In Anatomy of the Dicotyledons, Volume I; Metcalfe, C.R., Chalk, L., Eds.; Clarendon Press: Oxford, UK, 1979; pp. 40–53. [Google Scholar]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials World Health Organization Geneva; World Health Organization: Geneva, Switzerland, 1998; ISBN 9241545100. [Google Scholar]
- Zeng, G.; Tan, M.; Peng, X.; Luo, Q. Standardization and quality control of herbal extracts and products. In Traditional Herbal Medicine Research Methods: Identification, Analysis, Bioassay, and Pharmaceutical and Clinical Studies; Liu, W.J.H., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 377–428. ISBN 978-0-470-14936-2. [Google Scholar]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Van der Gheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
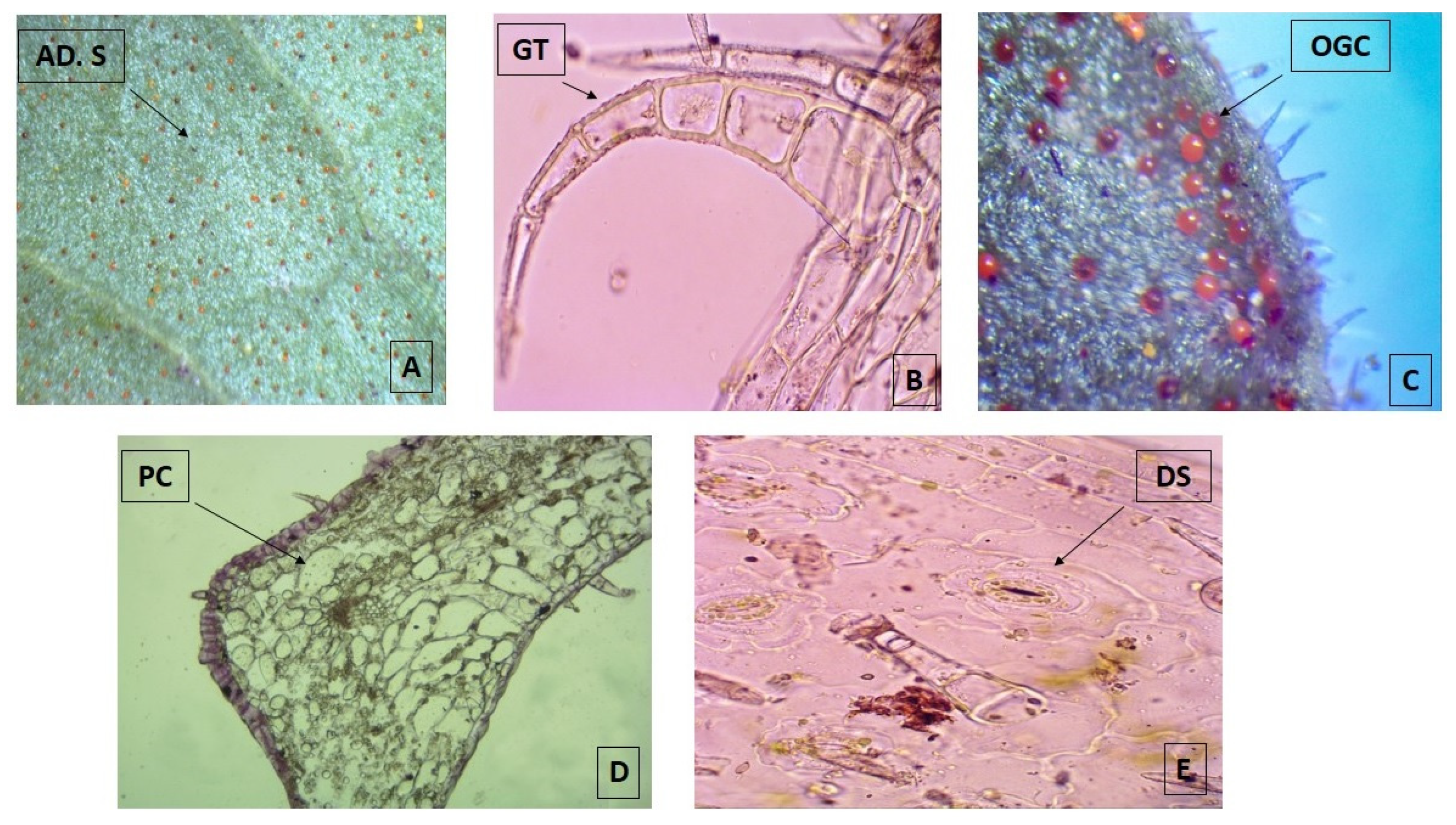

| Parameter | Batch 1 (February) | Batch 2 (May) | Batch 3 (August) | Batch 4 (November) | LDL (95%) | UDL (95%) |
|---|---|---|---|---|---|---|
| Total ash content (%) | 8.1 a ± 1.4 | 8.4 a ± 2.0 | 9.7 a ± 1.3 | 8.5 a ± 1.7 | 4.8 | 12.5 |
| Ethanol total soluble substances (%) | 18.5 b ± 2.0 | 20.2 b ± 2.7 | 17.5 b ± 1.9 | 20.2 b ± 0.4 | 14.5 | 23.7 |
| Water total soluble substances (%) | 22.6 c ± 1.3 | 24.1 c ± 1.4 | 22.1 c ± 1.1 | 23.5 c ± 1.6 | 19.8 | 26.3 |
| Parameter | Fresh Leaves Decoction (FLD) | Dry Leaves Water Maceration (DLW) | Dry Leaves Ethanol Maceration (DLE) |
|---|---|---|---|
| Organoleptic characteristics | Color: light green Smell: characteristic of the plant Texture: slightly dense | Color: light brown Smell: characteristic of the plant Texture: slightly turbid | Color: dark green Smell: characteristic of the plant Texture: transparent |
| Total extractable substances (%) | 15.99 b ± 0.01 | 20.19 c ± 0.01 | 10.67 a ± 0.01 |
| pH | 5.27 c ± 0.01 | 4.85 b ± 0.02 | 4.12 a ± 0.01 |
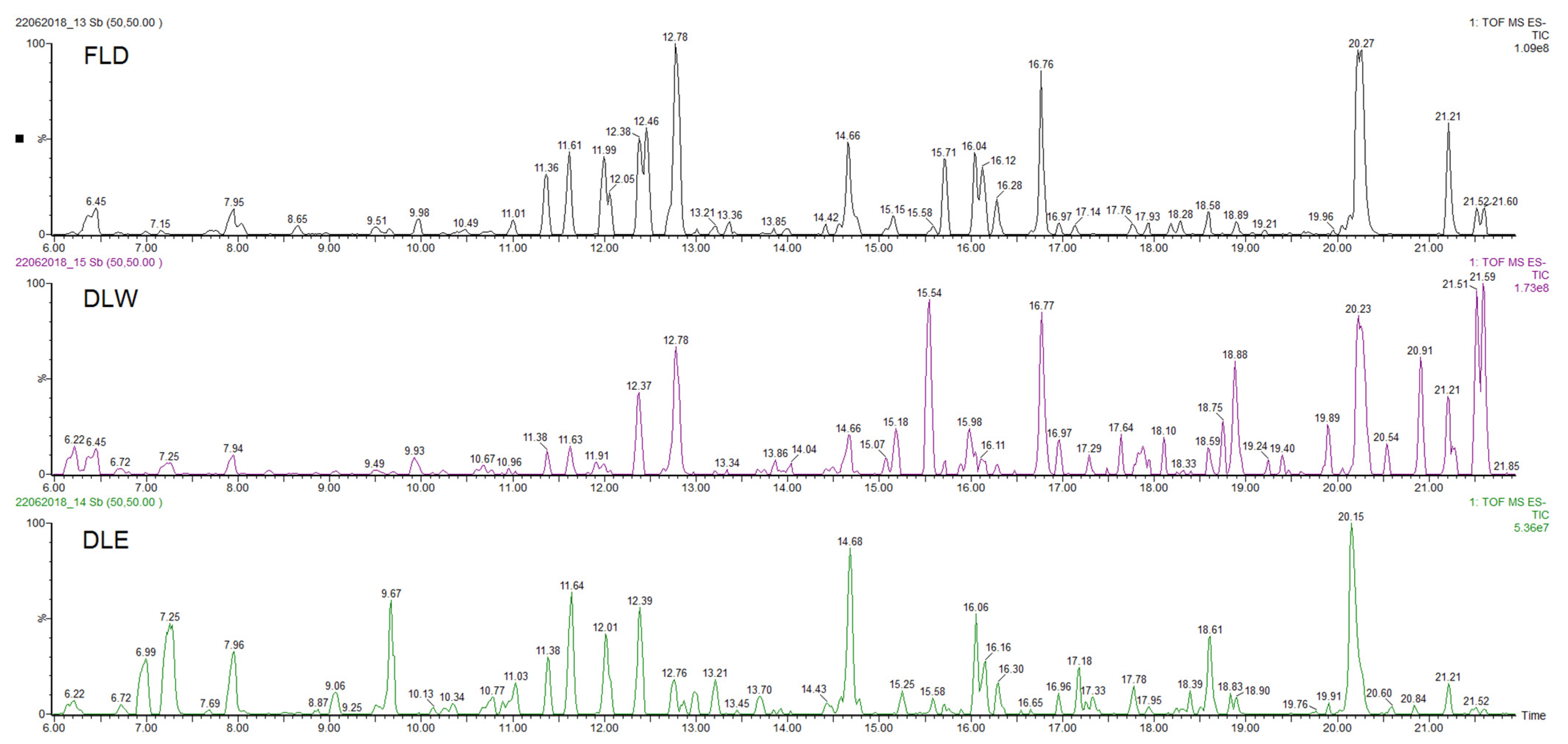
| Compound | Rt (min) | Accurate Mass [M − H]− (m/z) | Error (ppm) | MS/MS Ions (Rel. Intensity, %) | Molecular Formula | Tentative Identification | AEP |
|---|---|---|---|---|---|---|---|
| 1 | 6.45 | 387.1647 | −1.3 | 207(17), 163(8) | C18H28O9 | 12-Hydroxyjasmonic acid glucoside | DLW |
| 2 | 7.95 | 593.1553 | 1.5 | 503(12), 473(37), 413(5), 383(11), 353(19) | C27H30O15 | Vicenin-2 | DLW, DLE |
| 3 | 11.36 | 491.0858 | −1.0 | 475(51), 315(59), 299(64) | C22H20O13 | 4′-Methoxy-quercetin-3-O-glucuronide | DLW, DLE |
| 4 | 11.61 | 461.0721 | −0.9 | 285(57), 255(22) | C21H18O12 | Luteolin-O-glucuronide | DLW, DLE |
| 5 | 11.99 | 491.0829 | 0.4 | 315(69), 299(33) | C22H20O13 | 7-Methoxy-quercetin-3-O-glucuronide | DLW, DLE * |
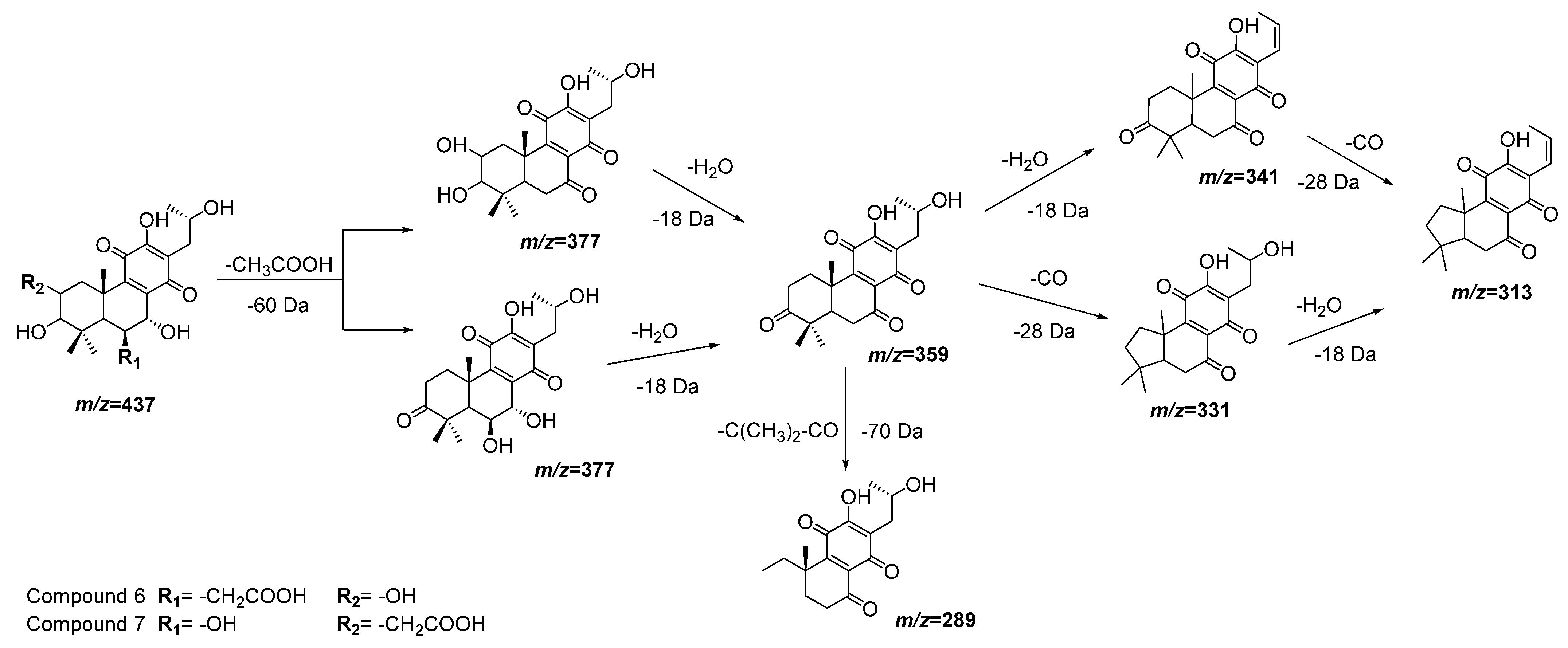
| 6 | 12.05 | 437.1805 | 0.7 | 377(100), 359(86), 341(22), 331(30), 315(62) | C22H30O9 | 3,6,7,12,16-Pentahydroxy-2-acetyl-5,8,12-abietatrien-11,14-dione | DLW *, DLE * |
| 7 | 12.38 | 437.1816 | −0.2 | 377(38), 359(41), 289(71) | C22H30O9 | 2,3,7,12,16-Pentahydroxy-6-acetyl-5,8,12-abietatrien-11,14-dion | DLW, DLE |
| 8 | 12.46 | 467.2131 | 0.4 | 437(18), 421(36), 289(100) | C20H36O12 | 2-(8-(Hydroxymethoxy)oct-1-en-3-yloxy)-hexoside-pentose | None |
| 9 | 12.79 | 359.0778 | 1.6 | 197(25), 179(23), 161(48), 135(7) | C18H15O8 | Rosmarinic acid | DLW, DLE |
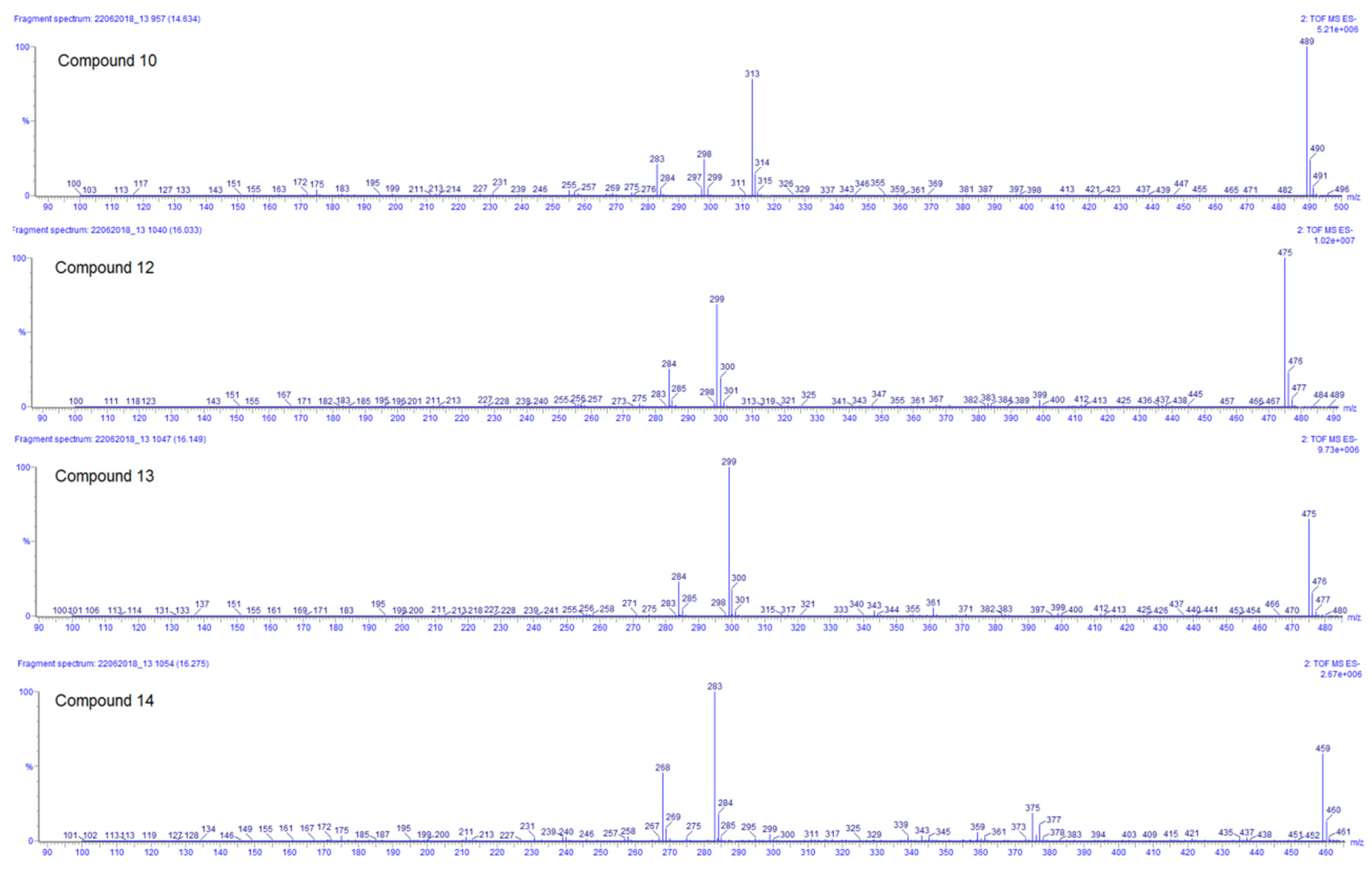
| 10 | 14.66 | 489.1032 | −1.4 | 313(57), 298(19), 283(18) | C23H22O12 | 3′,4′-Dimethoxy-luteolin-7-glucuronide | DLW, DLE |
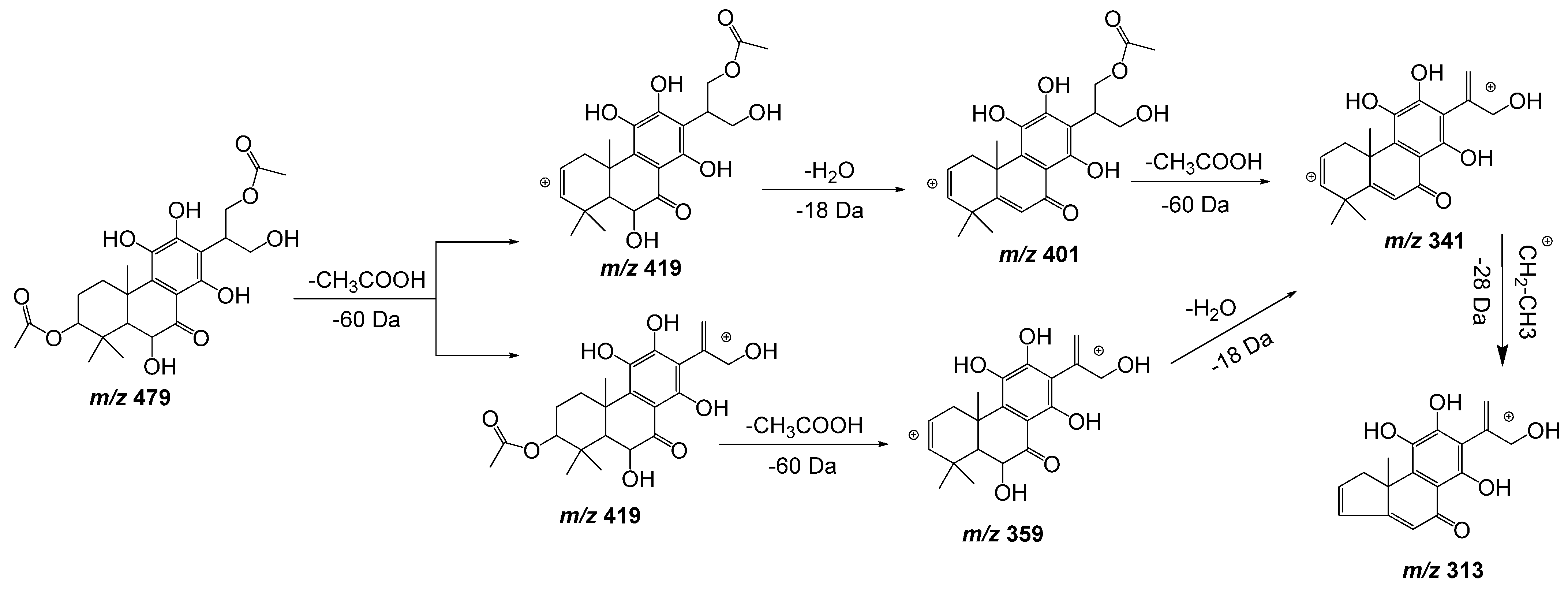
| 11 | 15.71 | 479.1918 | −1.5 | 419(86), 401(62), 359(41), 341(24), 313(21) | C24H32O10 | 6,11,12,14,16-Pentahydroxy-3,17diacetyl-8,11,13-abietatrien-7-one | DLW *, DLE * |
| 12 | 16.04 | 475.0871 | −1.3 | 299(73), 284(31) | C22H20O12 | Methoxy-kaempferol-7-glucuronide | DLW *, DLE |
| 13 | 16.12 | 475.0874 | −0.6 | 299(100), 284(39) | C22H20O12 | Methoxy-kaempferol-3-glucuronide | DLW * |
| 14 | 16.28 | 459.0930 | −0.4 | 283(100), 268(51) | C22H20O11 | Methoxy-apigenin-5-glucuronide | DLW, DLE |
| 15 | 16.76 | 511.2578 | 1.0 | 493(27), 467(76), 305(9) | C26H40O10 | Hexosyl-6β-hydroxicarnosol | DLW |
| 16 | 18.58 | 435.1661 | 1.1 | 375(42), 357(19),327(9) | C22H28O9 | 3,6,11,12,14-Pentahydroxy-2-acetyl-5,7,11,13-abietatetraen-7-one | DLW, DLE |
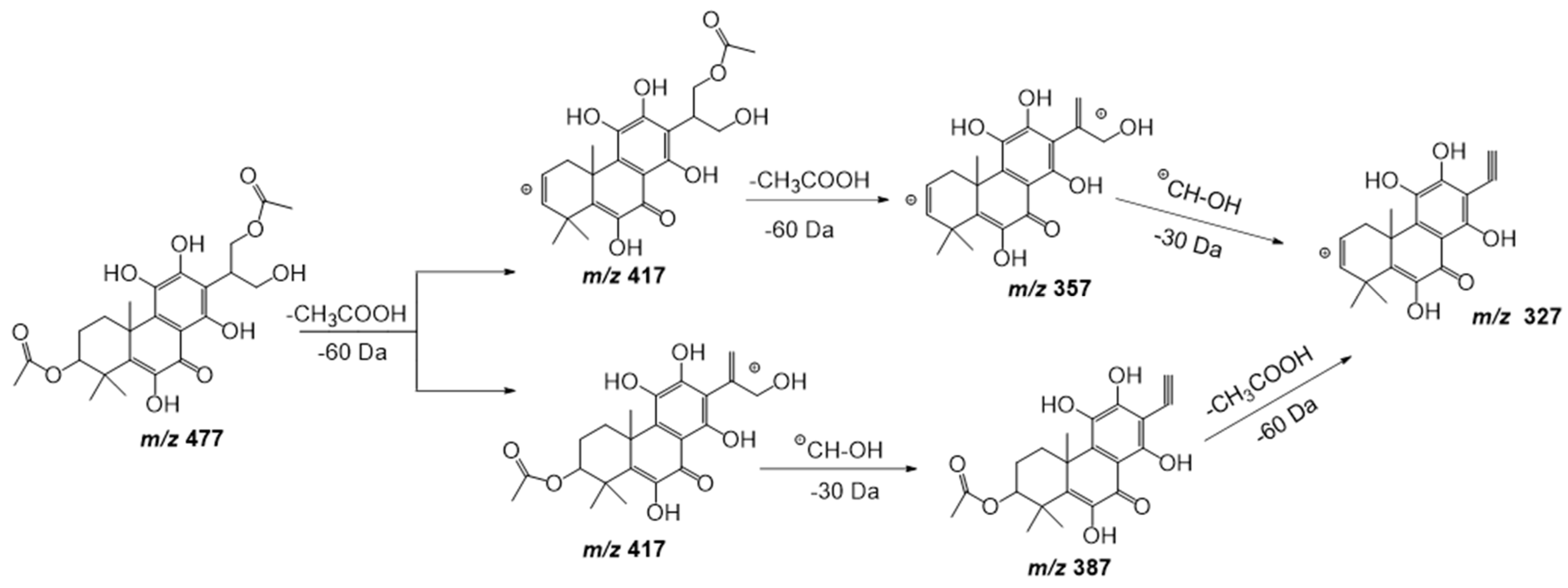
| 17 | 20.27 | 477.1798 | −1.0 | 417(100), 387(17), 357(23), 327(11) | C24H30O10 | 6,11,12,14,16-Pentahydroxy-3,17-diacetyl-5,8,11,13-abietatetraen-7-one | DLW |
| 18 | 21.21 | 419.1721 | −1.4 | 359(51), 341(6) | C22H28O8 | 3,6,12-Trihydroxy-2-acetyl-8,12-abietadien-7,11,14-trione | DLW, DLE |
| Extract | IC50 ± SD (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | A. fumigatus | T. cruzi | T. b. brucei | T. b. rhodesiense | L. infantum | L. amazonensis | |
| FLD | >128 | >128 | 70.7 ± 2.4 | >128 | >128 | >128 | 64.4 ± 1.2 | >128 | >128 |
| DLW | >128 | >128 | >128 | >128 | >128 | >128 | >128 | 60.1 ± 3.2 | >128 |
| DLE | 56.6 ± 1.8 | >128 | >128 | >128 | 16.6 ± 0.6 | 16.6 ± 1.0 | 16.1 ± 0.5 | 64.9 ± 2.1 | >128 |
| Doxycycline | 0.04 ± 0.0 | 0.6 ± 0.0 | - | - | - | - | - | - | - |
| Flucytosine | - | - | 0.7 ± 0.0 | - | - | - | - | - | - |
| Terbinafine | - | - | - | 0.3 ± 0.0 | - | - | - | - | - |
| Benznidazol | - | - | - | - | 1.8 ± 0.0 | - | - | - | - |
| Suramine | - | - | - | - | - | 0.02 ± 0.0 | 0.03 ± 0.0 | - | - |
| Miltefosine | - | - | - | - | - | - | - | 10.8 ± 1.3 | |
| Glucantime | - | - | - | - | - | - | - | - | 12.3 ± 2.9 |
| Extract | IC50 ± SD (µg/mL) | SI | ||
|---|---|---|---|---|
| MRC-5 | RAW 264.7 | THP-1 | ||
| FLD | >256 | >256 | >256 | ≈4 (C. albicans and T. brucei rhodesiense) |
| DLW | >256 | >256 | >256 | ≈4 (L. infantum) |
| DLE | <16 | <16 | <32 | ≈1 |
| Tamoxifen | 8.3 ± 1.1 | 10.9 ± 2.3 | 10.3 ± 2.1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Ferreiro, A.O.; Ochoa-Pacheco, A.; Méndez-Rodriguez, D.; Ortiz-Beatón, E.; Font-Salmo, O.; Guisado-Bourzac, F.; Molina-Bertrán, S.; Monzote, L.; Cos, P.; Foubert, K.; et al. LC-MS Characterization and Biological Activities of Cuban Cultivars of Plectranthus neochilus Schltr. Plants 2022, 11, 134. https://doi.org/10.3390/plants11010134
Rodríguez-Ferreiro AO, Ochoa-Pacheco A, Méndez-Rodriguez D, Ortiz-Beatón E, Font-Salmo O, Guisado-Bourzac F, Molina-Bertrán S, Monzote L, Cos P, Foubert K, et al. LC-MS Characterization and Biological Activities of Cuban Cultivars of Plectranthus neochilus Schltr. Plants. 2022; 11(1):134. https://doi.org/10.3390/plants11010134
Chicago/Turabian StyleRodríguez-Ferreiro, Annarli O., Ania Ochoa-Pacheco, Daniel Méndez-Rodriguez, Emilia Ortiz-Beatón, Oneida Font-Salmo, Frenkel Guisado-Bourzac, Silvia Molina-Bertrán, Lianet Monzote, Paul Cos, Kenn Foubert, and et al. 2022. "LC-MS Characterization and Biological Activities of Cuban Cultivars of Plectranthus neochilus Schltr" Plants 11, no. 1: 134. https://doi.org/10.3390/plants11010134
APA StyleRodríguez-Ferreiro, A. O., Ochoa-Pacheco, A., Méndez-Rodriguez, D., Ortiz-Beatón, E., Font-Salmo, O., Guisado-Bourzac, F., Molina-Bertrán, S., Monzote, L., Cos, P., Foubert, K., Pieters, L., Perez-Novo, C., Vanden Berghe, W., Escalona-Arranz, J. C., & Setzer, W. N. (2022). LC-MS Characterization and Biological Activities of Cuban Cultivars of Plectranthus neochilus Schltr. Plants, 11(1), 134. https://doi.org/10.3390/plants11010134








