Diversity and Cytogenomic Characterization of Wild Carrots in the Macaronesian Islands
Abstract
1. Introduction
2. Results
2.1. Reappraisal of Morphology, Ecology, and Conservation Status of Daucinae in Macaronesia
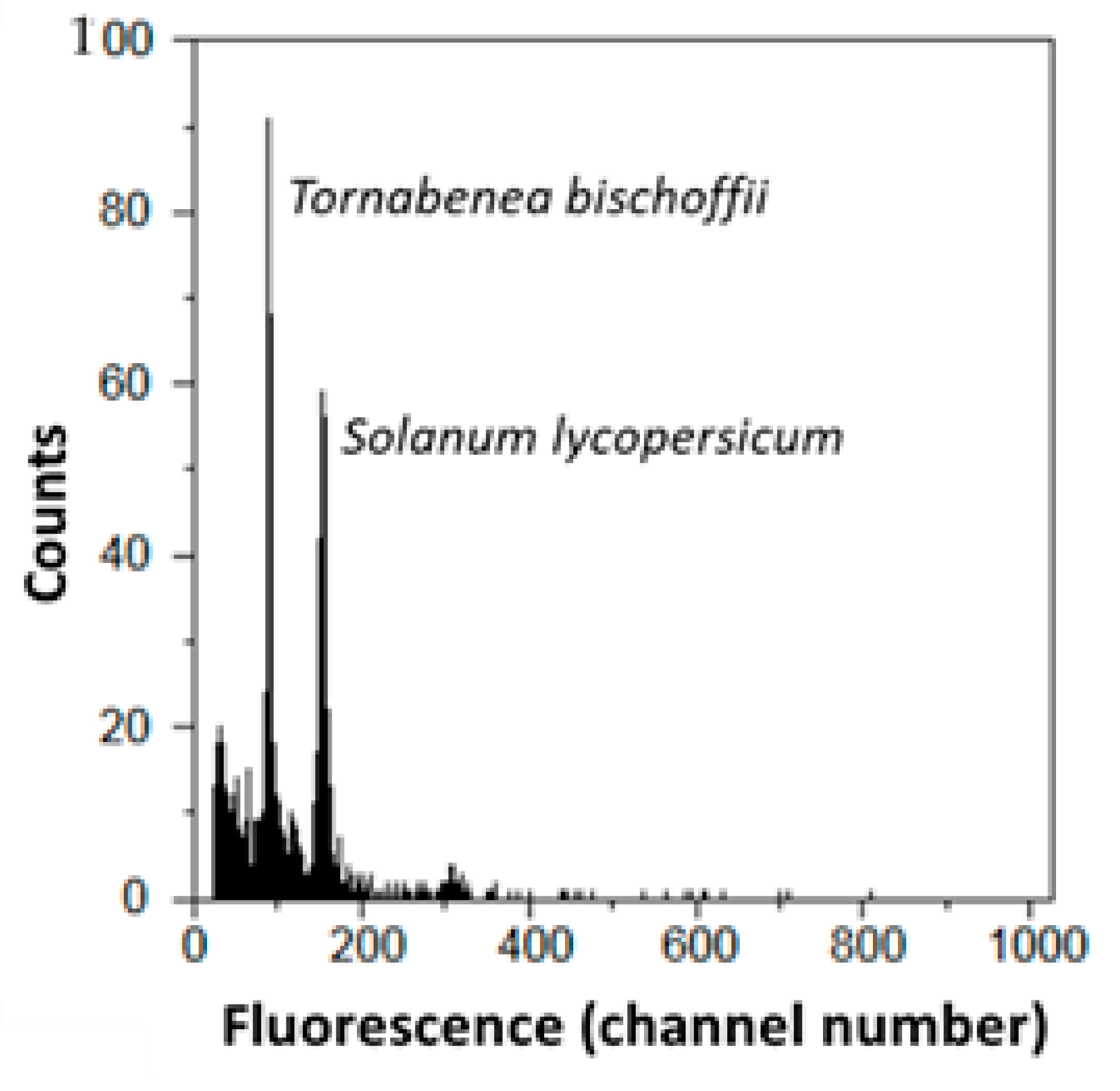
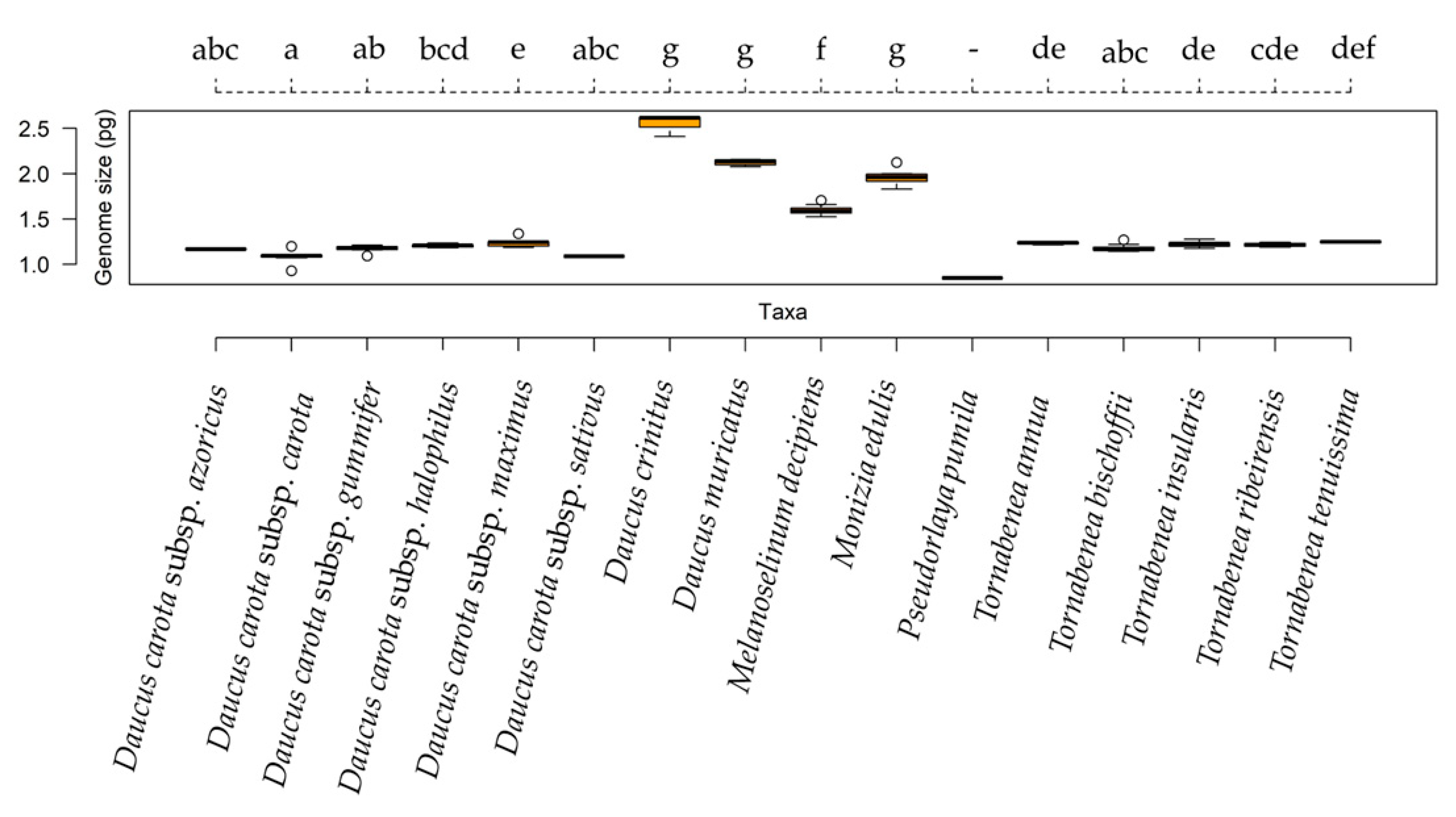
2.2. Cytogenomic Characterization
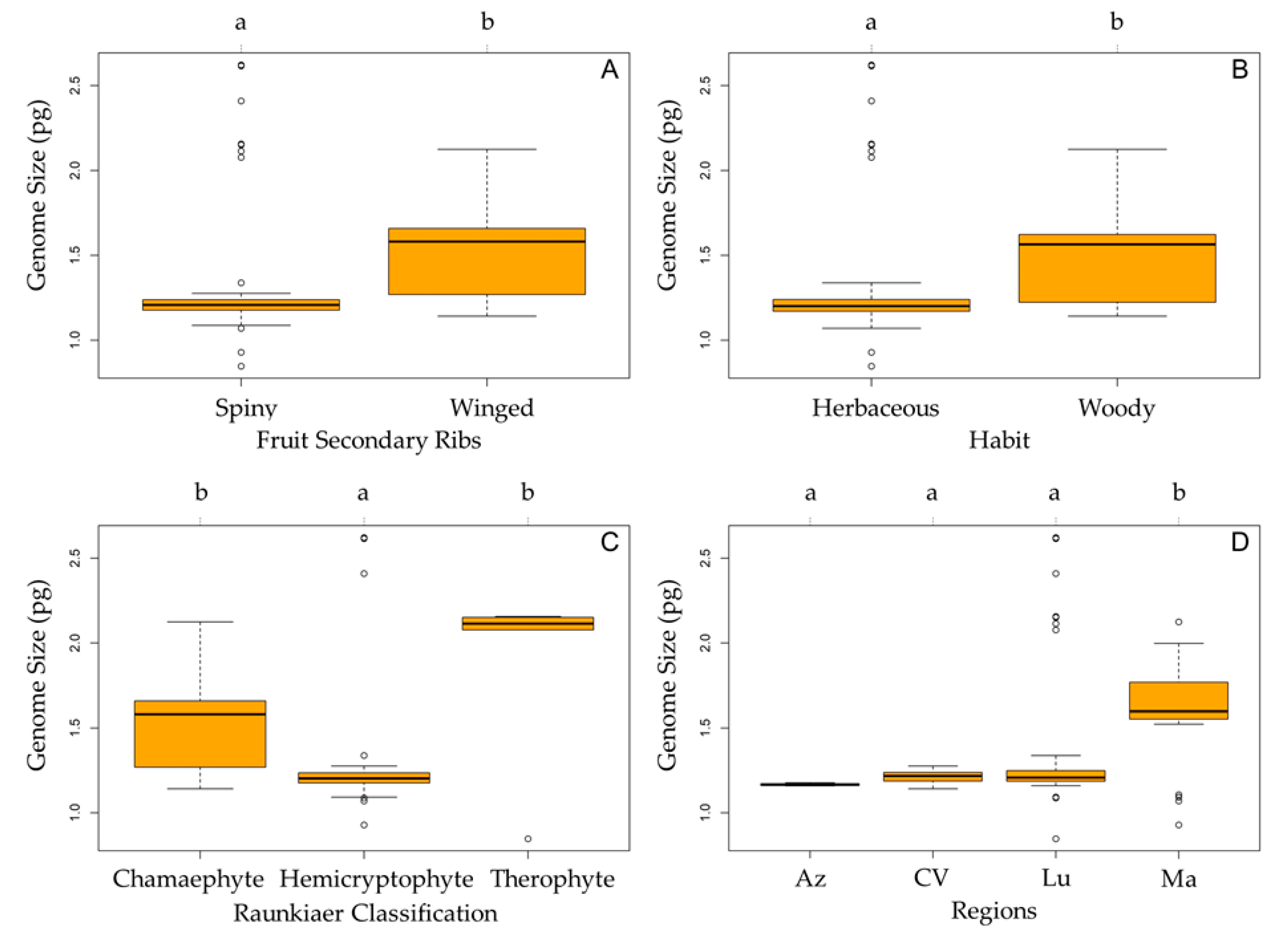
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Studied Macaronesian Endemics and Sampling
4.3. Data Collection
4.4. Cytogenomic Analysis
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pellicer, J.; Leitch, I.J. The Plant DNA C-values database (release 7.1): An updated online repository of plant genome size data for comparative studies. New Phytol. 2020, 226, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, D.; Loureiro, J.; Antoniadi, I.; Bainard, J.; Bureš, P.; Cápal, P.; Castro, M.; Castro, S.; Čertner, M.; Čertnerová, D.; et al. Best practices in plant cytometry. Cytometry 2021, 99, 311–317. [Google Scholar] [CrossRef]
- Laport, R.G.; Ng, J. Out of one, many: The biodiversity considerations of polyploidy. Am. J. Bot. 2017, 104, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Mota, L.; Castro, M.; Nobre, G.; Novoa, A.; Richardson, D.M.; Loureiro, J.; Castro, S. Genome size variation in Cactaceae and its relationship with invasiveness and seed traits. Biol. Invasions 2021, 23, 1–16. [Google Scholar] [CrossRef]
- Husband, B.C.; Baldwin, S.J.; Suda, J. The incidence of polyploidy in natural plant populations: Major patterns and evolutionary processes. In Plant Genome Diversity; Springer: Vienna, Austria, 2013; Volume 2, pp. 255–276. [Google Scholar]
- Wood, T.E.; Takebayashi, N.; Barker, M.S.; Mayrose, I.; Greenspoon, P.B.; Rieseberg, L.H. The frequency of polyploid speciation in vascular plants. Proc. Natl. Acad. Sci. USA 2009, 106, 13875–13879. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.; Schachtler, C.; Puppo, P.; Meimberg, H. Using a new RAD-sequencing approach to study the evolution of Micromeria in the Canary Islands. Mol. Phylogenet. Evol. 2018, 119, 160–169. [Google Scholar] [CrossRef]
- Suda, J.; Kyncl, T.; Freiová, R. Nuclear DNA amounts in Macaronesian angiosperms. Ann. Bot. 2003, 92, 153–164. [Google Scholar] [CrossRef]
- Suda, J.; Kyncl, T.; Jarolímová, V. Genome size variation in Macaronesian angiosperms: Forty percent of the Canarian endemic flora completed. Plant Syst. Evol. 2005, 252, 215–238. [Google Scholar] [CrossRef]
- Whitney, K.D.; Ahern, J.R.; Campbell, L.G.; Albert, L.P.; King, M.S. Patterns of hybridization in plants. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 175–182. [Google Scholar] [CrossRef]
- Kerbs, B.; Ressler, J.; Kelly, J.K.; Mort, M.E.; Santos-Guerra, A.; Gibson, M.J.; Caujapé-Castells, J.; Crawford, D.J.; Hiscock, S.J. The potential role of hybridization in diversification and speciation in an insular plant lineage: Insights from synthetic interspecific hybrids. AoB Plants 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Kapralov, M.V.; Filatov, D.A. Does large genome size limit speciation in endemic island floras. J. Bot. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007; pp. 46–74. [Google Scholar]
- García-Verdugo, C.; Sajeva, M.; La Mantia, T.; Harrouni, C.; Msanda, F.; Caujapé-Castells, J. Do island plant populations really have lower genetic variation than mainland populations? Effects of selection and distribution range on genetic diversity estimates. Mol. Ecol. 2015, 24, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Romeiras, M.M.; Vieira, A.; Silva, D.N.; Moura, M.; Santos-Guerra, A.; Batista, D.; Duarte, M.C.; Paulo, O. Evolutionary and Biogeographic Insights on the Macaronesian Beta-Patellifolia Species (Amaranthaceae) from a Time-Scaled Molecular Phylogeny. PLoS ONE 2016, 11, e0152456. [Google Scholar] [CrossRef]
- Rocha, V.; Duarte, M.C.; Catarino, S.; Duarte, I.; Romeiras, M.M. Cabo Verde’s Poaceae flora: A reservoir of crop wild relatives diversity for crop improvement. Front. Plant Sci. 2021, 12, 630217. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.C.F.; Maldaner, L.F.; Ottoni, P.M.N.; Molin, J.P. Carrot Yield Mapping: A Precision Agriculture Approach Based on Machine Learning. AI 2020, 1, 229–241. [Google Scholar] [CrossRef]
- Franco, J.A. Nova Flora de Portugal (Continente e Açores); Escolar Editora: Lisboa, Portugal, 1971; Volume I. [Google Scholar]
- Arbizu, C.I.; Ellison, S.L.; Senalik, D.; Simon, P.W.; Spooner, D.M. Genotyping-by-sequencing provides the discriminating power to investigate the subspecies of Daucus carota (Apiaceae). BMC Evol. Biol. 2016, 16, 234. [Google Scholar] [CrossRef]
- Frankiewicz, K.E.; Oskolski, A.; Banasiak, Ł.; Fernandes, F.; Reduron, J.P.; Reyes-Betancort, J.A.; Szczeparska, L.; Alsarraf, M.; Baczyński, J.; Spalik, K. Parallel evolution of arborescent carrots (Daucus) in Macaronesia. Am. J. Bot. 2020, 107, 394–412. [Google Scholar] [CrossRef]
- Hoffmann, G.F. Genera Plantarum Umbelliferarum Eorumque Characteres Naturales Secundum Numerum, Figuram, Situm Et Proportionem Omnium Fructificationis Partium: Accedunt Icones Et Analyses Æri Incisæ; Nabu Press: Moscow, Russia, 2012; Volume 1. [Google Scholar]
- Lowe, R.T. Novitiae florae maderensis: Or notes and gleanings of Maderan botany. Trans. Camb. Phylos. Foc. 1838, 6, 523. [Google Scholar]
- Chevalier, A. Les iles du Cap Vert. Géographie, biogéographie, agriculture. Flore de l'Archipel. J. Agr. Trab. Bot. Appl. 1935, 15, 733–1090. [Google Scholar] [CrossRef]
- Press, J.; Short, M. Flora of Madeira; HMSO: London, UK, 1994. [Google Scholar]
- Jardim, R.; Menezes de Sequeira, M. The vascular plants (Pteridophyta and Spermatophyta) of the Madeira and Selvagens Archipelagos. In A List of the Terrestrial Fungi, Flora and Fauna of Madeira and Selvagens Archipelagos. Funchal and Angra do Heroísmo; Borges, P.A.V., Aguiar, A.M.F., Carvalho, P., Jardim, R., Melo, I., Oliveira, P., Sérgio, C., Serrano, A.R.M., Vieira, P., Eds.; 2008; pp. 157–178. Available online: https://www.gbif.org/dataset/a43ec6d8-7b8a-4868-ad74-56b824c75698 (accessed on 25 July 2021).
- Lowe, R. A new species of Pericallis (Senecioneae, Asteraceae) endemic to Porto Santo (Madeira, Portugal). J. Bot. 1856, 8, 295. [Google Scholar]
- Lowe, R.T. Florulae Salvagicae Tentamen An Essay of the Flor of the Selvagens Islands; John Van Voorst: London, UK, 1869. [Google Scholar]
- Carvalho, J.A.; Fernandes, F.M.; Santos-Guerra, A. The vascular flora of Porto Santo: A catalogue of its islests. Bol. Mus. Munic. Funchal 2013, 63, 335. [Google Scholar]
- Hooker, W.J.; Vogel, T.; Webb, P.B.; Hooker, J.D.; Bentham, G. Niger Flora: Or, An Enumeration of the Plants of Western Tropical Africa; H. Bailliere: London, UK, 1849; Volume 1, pp. 131–132. [Google Scholar]
- Hooker, W.J. Hooker’s Journal of Botany and Kew Garden Miscellany; Reeve, Benham, and Reeve: London, UK, 1849; Volume 1, p. 370. [Google Scholar]
- Schmidt, J.A. Beiträge zur Flora der Cap Verdischen Inseln: Mit Berücksichtigung Aller bis Jetzt Daselbst Bekannten Wildwachsenden und Kultivirten Pflanzen. Nach Eigenen Untersuchungen und Mit Benutzung der Gewonnenen Resultate Anderer Reisenden Dargestellt; E. Mohr: Heidelberg, Germany, 1852. [Google Scholar]
- Lobin, W.; Zizka, G. Einteilung der Flora (Phanerogamae) der Kapverdischen Inseln nach ihrer Einwanderungsgeschichte. Cour. Forsch. Inst. Senckenberg 1987, 95, 127–153. [Google Scholar]
- Martins, E. Flora de Cabo Verde-Plantas Vasculares Apiaceae; IICT: Lisboa, Portugal; INIDA: Praia, Kaapverdië, 1996; Volume 67. [Google Scholar]
- Brochmann, C.R.Ø.H.; Lobin, W.; Kilian, N. The endemic vascular plants of the Cape Verde Islands, W. Africa. Sommerfeltia 1997, 24, 1–356. [Google Scholar] [CrossRef]
- Sánchez-Pinto, L.; Rodríguez, M.L.; Rodríguez, S.; Martín, K.; Cabrera, A.; Carmen Marrero, M. Pteridophyta, Spermatophyta. In Lista Preliminar de Especies Silvestres de Cabo Verde. Hongos, Plantas y Animales Terrestres; Arechavaleta Hernández, M., Pérez, N.Z., Gómez, M.M., Esquivel, J., Eds.; Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias: La Laguna, Spain, 2005; pp. 38–57. [Google Scholar]
- Downie, S.R.; Katz-Downie, D.S.; Spalik, K. A phylogeny of Apiaceae tribe Scandiceae: Evidence from nuclear ribosomal DNA internal transcribed spacer sequences. Am. J. Bot. 2000, 87, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Downie, S.; Plunkett, G.; Watson, M.; Spalik, K.; Katz-Downie, D.; Valiejo-Roman, C.; Terentieva, E.; Troitsky, A.; Lee, B.-Y.; Lahham, J. Tribes and clades within Apiaceae subfamily Apioideae: The contribution of molecular data. Edinb. J. Bot. 2001, 58, 301–330. [Google Scholar] [CrossRef]
- Spalik, K.; Downie, S.R. Intercontinental disjunctions in Cryptotaenia (Apiaceae, Oenantheae): An appraisal using molecular data. J. Biogeogr. 2007, 34, 2039–2054. [Google Scholar] [CrossRef]
- Iorizzo, M.; Senalik, D.A.; Ellison, S.L.; Grzebelus, D.; Cavagnaro, P.F.; Allender, C.; Brunet, J.; Spooner, D.M.; Van Deynze, A.; Simon, P.W. Genetic structure and domestication of carrot (Daucus carota subsp. sativus) (Apiaceae). Am. J. Bot. 2013, 100, 930–938. [Google Scholar] [CrossRef]
- Spooner, D.; Rojas, P.; Bonierbale, M.; Mueller, L.A.; Srivastav, M.; Senalik, D.; Simon, P. Molecular phylogeny of Daucus (Apiaceae). Syst. Bot. 2013, 38, 850–857. [Google Scholar] [CrossRef]
- Banasiak, Ł.; Wojewódzka, A.; Baczyński, J.; Reduron, J.-P.; Piwczyński, M.; Kurzyna-Młynik, R.; Gutaker, R.; Czarnocka-Cieciura, A.; Kosmala-Grzechnik, S.; Spalik, K. Phylogeny of Apiaceae subtribe Daucinae and the taxonomic delineation of its genera. Taxon 2016, 65, 563–585. [Google Scholar] [CrossRef]
- Wojewódzka, A.; Baczyński, J.; Banasiak, Ł.; Downie, S.R.; Czarnocka-Cieciura, A.; Gierek, M.; Frankiewicz, K.; Spalik, K. Evolutionary shifts in fruit dispersal syndromes in Apiaceae tribe Scandiceae. Plant Syst. Evol. 2019, 305, 401–414. [Google Scholar] [CrossRef]
- Martínez-Flores, F.; Crespo, M.B.; Simon, P.W.; Ruess, H.; Reitsma, K.; Geoffriau, E.; Spooner, D.M. Subspecies Variation of Daucus carota Coastal (“Gummifer”) Morphotypes (Apiaceae) Using Genotyping-by-Sequencing. Syst. Bot. 2020, 45, 688–702. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Pena, A.R.; Menezes, T.; Vasconcelos, R.; Monteiro, F.; Paulo, O.S.; Moura, M. Shortcomings of phylogenetic studies on recent radiated insular groups: A meta-analysis using Cabo Verde biodiversity. Int. J. Mol. Sci. 2019, 20, 2782. [Google Scholar] [CrossRef] [PubMed]
- Góis-Marques, C.A.; de Nascimento, L.; Fernández-Palacios, J.M.; Madeira, J.; Menezes de Sequeira, M. Tracing insular woodiness in giant Daucus (sl) fruit fossils from the Early Pleistocene of Madeira Island (Portugal). Taxon 2019, 68, 1314–1320. [Google Scholar] [CrossRef]
- Gregory, T.R. The C-value enigma in plants and animals: A review of parallels and an appeal for partnership. Ann. Bot. 2005, 95, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Hessen, D.O.; Persson, J. Genome size as a determinant of growth and life-history traits in crustaceans. Biol. J. Linn. Soc. 2009, 98, 393–399. [Google Scholar] [CrossRef]
- Brilhante, M.; Roxo, G.; Catarino, S.; Santos, P.; Reyes-Batancort, J.A.; Caujapé-Castels, J.; Sequeira, M.M.; Talhinhas, P.; Romeiras, M.M. Diversification of Aeonium species across Macaronesian Archipelagos: Correlations between genome-size variation and their conservation status. Front. Ecol. Evol. 2021, 9, 607338. [Google Scholar] [CrossRef]
- Nowicka, A.; Sliwinska, E.; Grzebelus, D.; Baranski, R.; Simon, P.W.; Nothnagel, T.; Grzebelus, E. Nuclear DNA content variation within the genus Daucus (Apiaceae) determined by flow cytometry. Sci. Hortic. 2016, 209, 132–138. [Google Scholar] [CrossRef]
- Guerra, M. Chromosome numbers in plant cytotaxonomy: Concepts and implications. Cytogenet. Genome. Res. 2008, 120, 339–350. [Google Scholar] [CrossRef]
- Dias, E.F.; Kilian, N.; Silva, L.; Schaefer, H.; Carine, M.; Rudall, P.J.; Santos-Guerra, A.; Moura, M. Phylogeography of the Macaronesian lettuce species Lactuca watsoniana and L. palmensis (Asteraceae). Biochem. Genet. 2018, 56, 315–340. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiaer; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Chumová, Z.; Krejčíková, J.; Mandáková, T.; Suda, J.; Trávníček, P. Evolutionary and taxonomic implications of variation in nuclear genome size: Lesson from the grass genus Anthoxanthum (Poaceae). PLoS ONE 2015, 10, e0133748. [Google Scholar] [CrossRef]
- Rong, J.; Janson, S.; Umehara, M.; Ono, M.; Vrieling, K. Historical and contemporary gene dispersal in wild carrot (Daucus carota ssp. carota) populations. Ann. Bot. 2010, 106, 285–296. [Google Scholar] [CrossRef]
- Schmidt, K.; Lobin, W. Tornabenea ribeirensis (Apiaceaeb a new species from São Nicolau, Cape Verde Islands (West Africa). Feddes Repert. 1999, 110, 7–11. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Paulo, O.S.; Duarte, M.C.; Pina-Martins, F.; Cotrim, M.H.; Carine, M.A.; Pais, M.S. Origin and diversification of the genus Echium (Boraginaceae) in the Cape Verde archipelago. Taxon 2011, 60, 1375–1385. [Google Scholar] [CrossRef]
- Gillespie, R.G. Oceanic islands: Models of diversity. Encycl. Biodivers. 2007, 1–13. [Google Scholar]
- Caceres, M.; De Pace, C.; Mugnozza, G.S.; Kotsonis, P.; Ceccarelli, M.; Cionini, P. Genome size variations within Dasypyrum villosum: Correlations with chromosomal traits, environmental factors and plant phenotypic characteristics and behaviour in reproduction. Theor. Appl. Genet. 1998, 96, 559–567. [Google Scholar] [CrossRef]
- Nürk, N.M.; Atchison, G.W.; Hughes, C.E. Island woodiness underpins accelerated disparification in plant radiations. New Phytol. 2019, 224, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Carine, M.A.; Santos-Guerra, A.; Guma, I.R.; Reyes-Betancort, J.A. Endemism and evolution of the Macaronesian Flora. In Beyond Cladistics; Williams, D.M.K.S., Ed.; University of California Press: Oakland, CA, USA, 2010; pp. 101–124. [Google Scholar]
- Beaulieu, J.M.; Smith, S.A.; Leitch, I.J. On the tempo of genome size evolution in Angiosperms. J. Bot. 2010, 2010, 989152. [Google Scholar] [CrossRef]
- Monteiro, F.; Frese, L.; Castro, S.; Duarte, M.C.; Paulo, O.S.; Loureiro, J.; Romeiras, M.M. Genetic and genomic tools to asssist sugar beet improvement: The value of the crop wild relatives. Front. Plant Sci. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Flores, F. Sistemática del Género Daucus L. (Apiaceae): Implicaciones Taxonómicas y Filogenéticas. Ph.D. Thesis, Universitat d’Alacant-Universidad de Alicante, San Vicente del Raspeig, Spain, 2016. [Google Scholar]
- Silva, L.; Pinto, N.; Press, B.; Rumsay, F.; Carine, M.; Henderson, S.; Sjögren, E. Lista das plantas vasculares (Pteridophyta e Spermatophyta). In A List of the Terrestrial Fauna (Mollusca and Arthropoda) and Flora (Bryophyta, Pteridophyta and Spermatophyta) from the Azores; Borges, P.A.V., Cunha, R., Gabriel, R., Martins, A.F., Silva, L., Vieira, V., Eds.; Direcção Regional do Ambiente and Universidade dos Açores: Ponta Delgada, Portugal, 2005; pp. 131–155. [Google Scholar]
- Menezes de Sequeira, M.; Espírito Santo, D.; Aguiar, C.; Capelo, J.; Honrado, J.J. Checklist da Flora de Portugal Continental, Açores e Madeira; Associaçã,o Lusitana de Fitossociologia: Lisboa, Portugal, 2011. [Google Scholar]
- Franco, J.d.A.; Rocha-Afonso, M. Nova Flora de Portugal (Continente e Açores); Escolar Editora: Lisboa, Portugal, 1984; Volume II. [Google Scholar]
- Castroviejo, S. Flora ibérica: Plantas vasculares de la Península Ibérica e Islas Baleares. In Flora Ibérica; Real Jardin Botanico Consejo Superior de Investigaciones Cie: Madrid, Spain, 2003; Volume 10, pp. 1–784. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Rivas-Martinez, S.; Lousã, M.; Costa, J.C.; Duarte, M.C. Geobotanical survey of Cabo Verde Islands (West Africa). Int. J. Geobot. Res. 2017, 17, 1–103. [Google Scholar] [CrossRef]
- Dalgaard, V. Checklist of chromosome numbers counted in Madeiran flowering plants, with notes on polyploidy, life form, endemism and evolution. Nord. J. Bot. 1994, 14, 241–255. [Google Scholar] [CrossRef]
- Bramwell, D.; Murray, B. A preliminary report on the cytology of some Cape Verde islands plants. Cuad. Bot. Canaria 1972, 14/15, 27–29. [Google Scholar]
- Borgen, L. Chromosome numbers of Macaronesian flowering plants. II. Nord. J. Bot. 1974, 21, 195–210. [Google Scholar]
- Zizka, G. Chromosomenzahlungen bei einigen kapverdischen Pflanzen. Cour. Forsch.-Inst. Senckenberg 1986, 81, 181–182. [Google Scholar]
- Grosso, A.; Rodrigues, L.; Gomes, I.; Martins, E.; Teixeira, G. Preliminary data on microcharacters and chromosome number in Tornabenea species (Apiaceae) from Cape Verde Islands. Plant Biosyst. 2008, 142, 87–93. [Google Scholar] [CrossRef][Green Version]
- Spooner, D.M. The carrot genome. In Daucus: Taxonomy, Phylogeny, Distribution; Simon, P., Iorizzo, M., Grzebelus, D., Baranski, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 9–26. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2020-1594. Available online: https://www.iucnredlist.org (accessed on 25 March 2021).
- Corvelo, R.A.F. Estatuto de Conservação das Plantas Vasculares Endémicas dos Açores Segundo os Critérios da IUCN: Implicações ao Nível do Ordenamento do Território e do Planeamento Ambiental. Ph.D. Thesis, Universidade dos Açores, Ponta Delgada, Portugal, 2010. [Google Scholar]
- Romeiras, M.M.; Catarino, S.; Gomes, I.; Fernandes, C.; Costa, J.C.; Caujapé-Castells, J.; Duarte, M.C. IUCN Red List assessment of the Cape Verde endemic flora: Towards a global strategy for plant conservation in Macaronesia. Bot. J. Linn. Soc. 2016, 180, 413–425. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.; Rodriguez, E.; Doležel, J.; Santos, C. Two new nuclear isolation buffers for plant DNA flow cytometry: A test with 37 species. Ann. Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef]
- Doležel, J.; Sgorbati, S.; Lucretti, S. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 1992, 85, 625–631. [Google Scholar] [CrossRef]
- Carvalho, R.; Silva, E.; Loureiro, J.; Ramos, A.P.; Talhinhas, P. Caracterisation of Puccinia hemerocallidis causing the first outbreak of daylily rust in Europe. Rev. Ciênc. Agr. 2018, 41, 110–115. [Google Scholar] [CrossRef]
- Guilengue, N.; Alves, S.; Talhinhas, P.; Neves-Martins, J. Genetic and genomic diversity in a tarwi (Lupinus mutabilis Sweet) germplasm collection and adaptability to Mediterranean climate conditions. Agronomy 2020, 10, 21. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 30 March 2021).
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Series B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Pearson, GA, USA, 2010. [Google Scholar]
- Siegel, S.; Castellan, N.J. Non Parametric Statistics for the Behavioural Sciences; MacGraw Hill Inc.: New York, NY, USA, 1988; pp. 213–214. [Google Scholar]
- Conover, W.J.; Iman, R.L. On Multiple-Comparison Procedures; Technical Report; Los Alamos Scientific Laboratory: Los Alamos, NM, USA, 1979.
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). R Package. 2014. Available online: http://CRAN.R-project.org/package=PMCMR (accessed on 25 July 2021).
- Ávila, S.P.; Cordeiro, R.; Madeira, P.; Silva, L.; Medeiros, A.; Rebelo, A.C.; Melo, C.; Neto, A.I.; Haroun, R.; Monteiro, A.; et al. Global change impacts on large-scale biogeographic patterns of marine organisms on Atlantic oceanic islands. Mar. Pollut. Bull. 2018, 126, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Parelho, C.; Rodrigues, A.; do Carmo Barreto, M.; Cruz, J.V.; Rasche, F.; Silva, L.; Garcia, P. Bioaccumulation and potential ecotoxicological effects of trace metals along a management intensity gradient in volcanic pasturelands. Chemosphere 2021, 273, 128601. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.G.; Kim, J.H. Central limit theorem: The cornerstone of modern statistics. Korean J. Anesthesiol. 2017, 70, 144–156. [Google Scholar] [CrossRef]

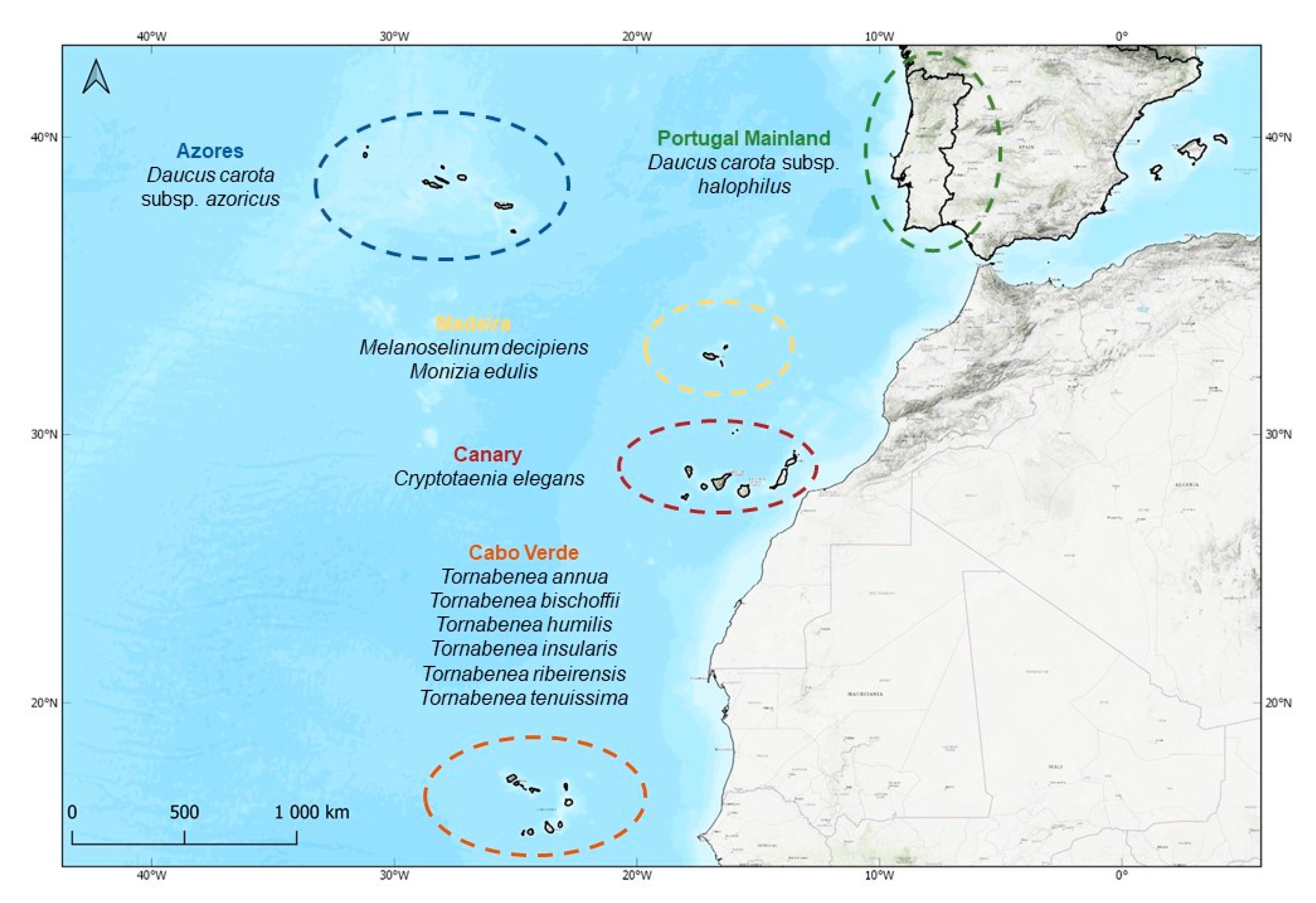
| Taxa | Geographical Distribution | Native Status in Macaronesia | Conservation Status | Ecology |
|---|---|---|---|---|
| Daucus carota L. subsp. azoricus Franco | Azores | Endemic | LC | Sea rocks, cliffs, pastures, uncultivated or cultivated lands, roadsides; from 0–800 m |
| Daucus carota L. subsp. carota | Europa, Asia, Siberia, N. Africa | Native | NE | Ruderal |
| Daucus carota subsp. gummifer (Syme) Hook.f. | Europe | - | NE | Coastal cliffs and dunes and uncultivated lands, on sandy soil |
| Daucus carota L. subsp. halophilus (Brot.) A. Pujadas | Mainland Portugal | - | DD | Coastal cliffs and coastal upland areas |
| Daucus carota L. subsp. maximus (Desf.) Ball | Mediterranean; Asia | Native | NE | Ruderal |
| Daucus carota L. subsp. sativus (Hoffm.) Arcang. | Widely cultivated | - | NE | Cultivated |
| Daucus crinitus Desf. | Africa; Europe | - | LC | Grassland, shrubland |
| Daucus muricatus (L.) L. | Africa; Europe | Naturalized | LC | Grassland, shrubland |
| Melanoselinum decipiens (Schrad. & J.C.Wendl.) Hoffm. | Madeira | Endemic | NE | Shady rocks and banks in laurel forests |
| Monizia edulis Lowe | Madeira | Endemic | CR | Mainly found on fissures in cliffs |
| Pseudorlaya pumila (L.) Grande | Mediterranean; Canaries | Native | NE | Marine sands, mainly primary dune |
| Tornabenea annua Bég. | Cabo Verde | Endemic | EN | Southern hygrophyte restricted to montane areas on Santiago |
| Tornabenea bischoffii J.A. Schmidt | Cabo Verde | Endemic | EN | Northern hygrophyte restricted to montane areas on Santo Antão |
| Tornabenea insularis (Parl. Ex Webb) Parl. Ex Webb | Cabo Verde | Endemic | EN | Sub-humid montane areas above 600 m of S. Vicente, S. Nicolau, and Brava |
| Tornabenea ribeirensis Schmidt & Lobin | Cabo Verde | Endemic | CR | Restricted to shady and seasonally damp bottoms of valleys of very few Ribeiras in the North of S. Nicolau |
| Tornabenea tenuissima (A. Chev.) A. Hansen & Sunding | Cabo Verde | Endemic | CR | Restricted to sub-humid montane areas; mainly above 1200 m on Fogo |
| Taxa | Habit | Leaves | Inflorescence | Bracts | Bracteoles | Fruits | Secondary Ribs |
|---|---|---|---|---|---|---|---|
| Daucus carota subsp. azoricus | Annual or biennial, up to 70 cm. Herbaceous | Hispid, 2–3 pinnate. | Large terminal umbel, up to 9 cm diameter | 6–11 pinnatisect. Filiform lobes. | 7–9 simple. Linear lobes. | 2–4 mm, cylindrical. | Spiny |
| Daucus carota subsp. carota | Perennial, up to 110 cm. Herbaceous. | Basal, 1–3 (4) pinnate oblong to lanceolate, while uper leaves, 1–3 pinnate are linnear to lanceolate. | (1.5) 3–7 (11) cm diameter, becoming strongly contracted in fruit. | 7–9 pinnatisect. Sublinear or filiform lobes. | 6–9 simple. Linear lobes. | 1.8–3.2 × 1–1.8 mm, ellipticals, purplish or light brown. | Spiny |
| Daucus carota subsp. gummifer | Perennial, up to 50 cm. Herbaceous. | Basal, (1) 2–3 (4) pinnate. Upper leaves similar to basal ones. | (1.5) 3–6 (10) cm diameter, convex to sub–hemispherical, slightly contracted in fruit. | 7–10 shorter than the rays. Linear to lanceolate lobes. | 7–9 with a trifid apex. Lanceolate. | 1.8–3.0 × 1.3–2.5 mm, oblong to ovoid, brown. | Spiny |
| Daucus carota subsp. halophilus | Perennial, up to 25 cm. Herbaceous. | Basal leaves 1–2 (3) pinnate. Upper leaves similar to basal ones, 1–2 pinnate. | (3) 4–12 cm diameter hemispherical and slightly contracted in fruit. | 8–10 pinnatisect. Ovoid lobes. | 7–8 simple, trifid apex. Ovoid to widely lanceolate. | 2–3.5 × 1.5–2.5 mm, ovoid to elliptical, purplish, or brown. | Spiny |
| Daucus carota subsp. maximus | Perennial up to 220 cm. Herbaceous. | Basal, (1) 2–3 pinnate ovate to oblong. Upper leaves similar to basal ones 1–2 pinnate. | 12–23 cm diameter, becoming strongly contracted in fruit. | 10–13 pinnatisect. Linear or filiform lobes. | 6–10 simple. Short and linear lobes. | 1.5–2.5 × 1–2 mm, ellipsoid–oblong, sometimes subspherical. | Spiny |
| Daucus carota subsp. sativus | Perennial, up to 78 cm. Herbaceous. | Basal, 3–4 pinnate, largely petiolate. Upper leaves 2 (3) pinnate. | 5–10 cm diameter, slightly convex. | (8) 10–13 pinnatisect. Long and linear lobes. | 7–9 simple. Linear to lanceolate lobes. | 3–3.5 × 1.2–2.0 mm, oblong, brown. | Spiny |
| Daucus crinitus | Perennial, up to 115 cm. | Basal, 3–4 pinnate, sessile or subsessile segments. Upper leaves similar to basal, 1–3 pinnate. | Convex, does not contract in fruit. | 5–10 simple, pinnatisect to trifid. Linear to lanceolate lobes. | 5–9 simple. Lobes linear to lanceolate. | 4–7 (9) mm, elliptical and sometimes oblong. | Spiny |
| Daucus muricatus | Annual up to 105 cm. Herbaceous. | Basal leaves (2) 3–4 pinnate, hispid. Upper leaves similar but slighty smaller. | Long peduncle. Slightly convex. Sterile central flower absent | (4) 6–10 pinnatisect. Linear or setaceous lobes. | 4–9 simple. Linear lobes. | 5–8 (10) m, elliptical. | Spiny |
| Melanoselinum decipiens | Tall rosetted perennial monocarpic, up to 3 m. Woody. | Large triangular, up to 60 cm. | 50–90 cm diameter, terminal above leaf crown. | 10–20, 20–30 mm. Leafy. | As long as the pedicels. | 12–14 mm, oblong, pubescent, blackish. | Winged |
| Monizia edulis | Long lived perennial, up to 1 m. Woody. | Yellowish–green, glossy, triangular in out line. | Paniculate, 20–25 rays in each umbel. | Lanceolate or linear, puberulent, fringed at margin. | Lanceolate or linear, puberulent, fringed at margin. | 10–14 × 5–7 mm, oblong to ellipsoid, pubescent, pale coloured when ripe. | Winged |
| Pseudorlaya pumila | Annual up to 30 cm. Herbaceous. | 2–3 pinnate, hispid. | 3–7 unequal rays. | 2–5 linear to pinnatisect. | 3–5 similar to the bracts but smaller. | (5.5) 7.5–12 × 3.5–10 mm, having the spines in the dorsal and lateral ribs different sizes. | Spiny |
| Tornabenea annua | Annual or biennial, up to 80 cm. Herbaceous. | Up to 35 cm, 2–3 pinnate, 3–6 pairs of pinnae. | More or less flat, up to 7.5 cm diameter. | 7–8, entire rarely somewhat bi or trifid. Inconspicuous. | 7–8. Inconspicuous. | 3.5 mm, strongly compressed dorsally. | Spiny |
| Tornabenea bischoffii | Stout perennial up to 1.5 m. Woody. | 35 - 50 cm, 2 (3) pinnate, 7 pairs of pinnae. | Hemispherical, nearly spherical when fruiting, up to 9 cm diameter. | 10–15, up to 3 cm long. Pinnately divided. | Trifid, bifid, or entire. | Up to 2 mm long, only slightly compressed dorsally. | Winged |
| Tornabenea insularis | Stout perennial up to 90 cm. Woody. | Up to 30 cm, sub coriaceous to delicate, 1–2 pinnate, 3–6 pairs of pinnae. | Flat to hemispherical, up to 9 cm diameter. | 4–13, up to 2.8 cm long, pinnately divided. In fruiting slightly deflexed. | 7–9 narrow, trifid, bifid or entire. | 2 mm, long elliptical in dorsoventral view. | Spiny |
| Tornabenea ribeirensis | Annual or biennial, up to 80 cm. Herbaceous. | Thin light green, lamina deltoid to obovate in outline, 3–4 pairs of pinnae. | Umbels with up to 25 rays, upward direct bristles, spread, and slightly constricted when fruiting. | 3–10 cm long, mainly undivided, rarely one bi or trifid. | Inconspicuous. 2–3 mm long. Undivided. | 2.5 mm, compressed dorsally. | Winged |
| Tornabenea tenuissima | Stout perennial up to 1 m high. Woody. | Up to 40 cm, 2–3 pinnate, up to 6 (7) pairs of pinnae. Segments very narrow, filiform. | Hemispherical, nearly spherical when fruiting, up to 9 cm diameter. | Up to 10, 2 cm long. Pinnately divided, segments slender and narrow. | Trifid, bifid, or rarely entire. | Reddish brown, up to 3.9 mm, compressed dorsally. | Spiny |
| Taxa | 2C-Values ± SD (pg) | Group | Sample CV (%) | Previous 2C-Value (pg) | Chromossome Number | Origin |
|---|---|---|---|---|---|---|
| Daucus carota subsp. azoricus | 1.167 ± 0.024 | abc | 4.470 | 1.064 | 18 | Az |
| Daucus carota subsp. carota | 1.093 ± 0.085 | a | 4.298 | 0.989 | 18 | Lu |
| Daucus carota subsp. gummifer | 1.173 ± 0.036 | ab | 4.821 | 1.016 | 18 | Lu |
| Daucus carota subsp. halophilus | 1.205 ± 0.028 | bcd | 4.624 | 1.154 | 18 | Lu |
| Daucus carota subsp. maximus | 1.244 ± 0.062 | e | 4.897 | 0.920 | 18 | Lu |
| Daucus carota subsp. sativus | 1.087 ± 0.021 | abc | 4.984 | 0.960 | 18 | Lu |
| Daucus crinitus | 2.544 ± 0.102 | g | 3.655 | 2.403 | 22 | Lu |
| Daucus muricatus | 2.135 ± 0.040 | g | 2.314 | 2.036 | 20 | Lu |
| Melanoselinum decipiens | 1.591 ± 0.046 | f | 3.431 | - | 22 | Ma |
| Monizia edulis | 1.940 ± 0.081 | g | 2.310 | - | 22 | Ma |
| Pseudorlaya pumila | 0.847 ± 0.031 | - | 5.990 | - | 26 | Lu |
| Tornabenea annua | 1.235 ± 0.018 | de | 3.781 | - | 18 | CV |
| Tornabenea bischoffii | 1.259 ± 0.211 | abc | 3.309 | - | 22 | CV |
| Tornabenea insularis | 1.223 ± 0.031 | de | 3.668 | - | 18 | CV |
| Tornabenea ribeirensis | 1.214 ± 0.026 | cde | 4.016 | - | - | CV |
| Tornabenea tenuissima | 1.248 ± 0.012 | def | 5.370 | - | 16 | CV |
| General Linear Model | AIC | R2 |
|---|---|---|
| Taxon | −387.044 | 0.9851364 |
| Region + Raunkiaer classification + habit | 63.587 | 0.3459922 |
| Full | 63.587 | 0.3459922 |
| Region + Raunkiaer classification | 63.845 | 0.3325822 |
| Raunkiaer classification | 76.666 | 0.2203145 |
| Region | 79.530 | 0.2159600 |
| Fruit secondary ribs | 89.827 | 0.1188375 |
| Habit | 98.585 | 0.0548866 |
| Null | 103.543 | - |
| Habitat | 105.587 | 0.0004328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roxo, G.; Moura, M.; Talhinhas, P.; Costa, J.C.; Silva, L.; Vasconcelos, R.; de Sequeira, M.M.; Romeiras, M.M. Diversity and Cytogenomic Characterization of Wild Carrots in the Macaronesian Islands. Plants 2021, 10, 1954. https://doi.org/10.3390/plants10091954
Roxo G, Moura M, Talhinhas P, Costa JC, Silva L, Vasconcelos R, de Sequeira MM, Romeiras MM. Diversity and Cytogenomic Characterization of Wild Carrots in the Macaronesian Islands. Plants. 2021; 10(9):1954. https://doi.org/10.3390/plants10091954
Chicago/Turabian StyleRoxo, Guilherme, Mónica Moura, Pedro Talhinhas, José Carlos Costa, Luís Silva, Raquel Vasconcelos, Miguel Menezes de Sequeira, and Maria Manuel Romeiras. 2021. "Diversity and Cytogenomic Characterization of Wild Carrots in the Macaronesian Islands" Plants 10, no. 9: 1954. https://doi.org/10.3390/plants10091954
APA StyleRoxo, G., Moura, M., Talhinhas, P., Costa, J. C., Silva, L., Vasconcelos, R., de Sequeira, M. M., & Romeiras, M. M. (2021). Diversity and Cytogenomic Characterization of Wild Carrots in the Macaronesian Islands. Plants, 10(9), 1954. https://doi.org/10.3390/plants10091954










