A Concise Review of Dendrocalamus asper and Related Bamboos: Germplasm Conservation, Propagation and Molecular Biology
Abstract
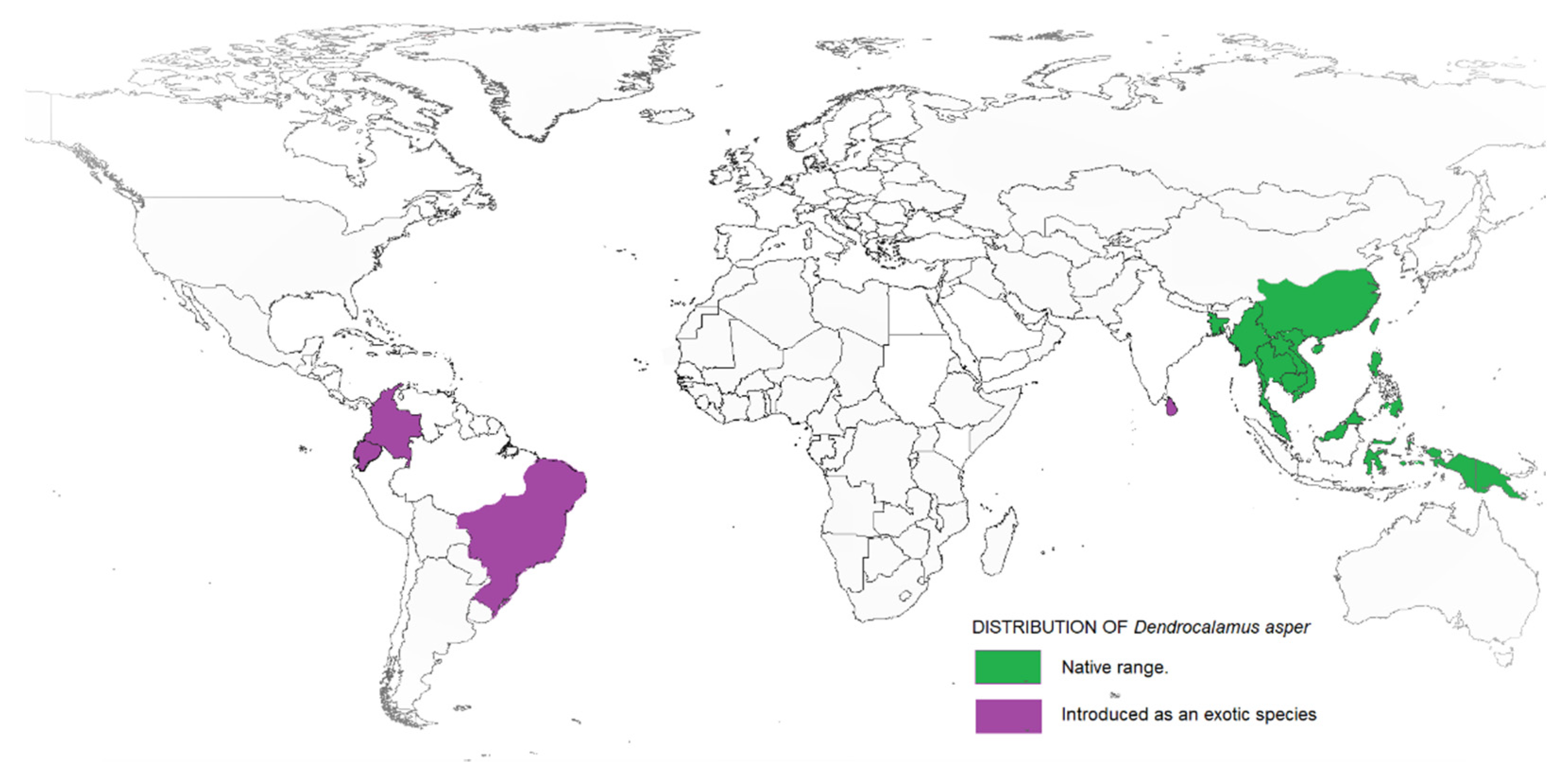
1. Introduction
2. Dendrocalamus asper
3. Commercialization of D. asper in the Industry
3.1. Nutritional Composition
3.2. Renewable Construction
3.3. Renewable Energy
4. Bamboo Propagation and Diseases
4.1. Traditional Propagation of Bamboos and Tissue Culture
4.2. Bamboo Diseases
5. Regeneration of D. asper
5.1. Explant Surface Sterilization
5.2. Organogenesis
5.3. Somatic Embryogenesis
5.4. Rooting
5.5. Hardening and Acclimatization
6. Bamboo Dormancy and Bud Breaking
6.1. Seed Dormancy
6.2. Bud Position on the Bamboo Plants
6.3. Season Collection of Explants
7. Molecular Approaches for the Characterization of Bamboo Populations and Genomes
7.1. Identification and Phylogenetic Relationships of Bamboos
7.2. Clonal Fidelity
8. DNA Barcoding in Bamboos
9. Bamboo Genomics
10. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanaka, E.; Tanaka, C.; Mori, N.; Kuwahara, Y.; Tsuda, M. Phenylpropanoid amides of serotonin accumulate in witches’ broom diseased bamboo. Phytochemistry. 2003, 64, 965–969. [Google Scholar] [CrossRef]
- Yeasmin, L.; Ali, M.N.; Gantait, S.; Chakraborty, S. Bamboo: An overview on its genetic diversity and characterization. 3 Biotech 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Canavan, S.; Richardson, D.M.; Visser, V.; Roux, J.J.; Vorontsova, M.S.; Wilson, J.R. The global distribution of bamboos: Assessing correlates of introduction and invasion. AoB Plants 2016, 9, 78. [Google Scholar] [CrossRef]
- Clark, L.G.; Londoño, X.; Ruiz-Sanchez, E. Bamboo taxonomy and habitat. In Bamboo, Tropical Forestry; Liese, W., Kohl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 10, pp. 1–30. [Google Scholar] [CrossRef]
- Mera, F.A.T.; Xu, C. Plantation management and bamboo resource economics in China. Cienc. Tecnol. 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Hoogendoorn, J.C.; Benton, A. 13 Bamboo and rattan production and the implications of globalization. In Forests and Globalization: Challenges and Opportunities for Sustainable Development; Nikolakis, W., Innes, J., Eds.; Routledge: London, UK, 2014; pp. 166–184. [Google Scholar]
- Liese, W.; Kohl, M. Bamboo: The Plant and Its Uses (Tropical Forestry); Springer: Basel, Switzerland, 2015; p. 356. [Google Scholar]
- Bansal, A.K.; Zoolagud, S.S. Bamboo composites: Material of the future. J. Bamboo Ratt. 2002, 1, 119–130. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by Chinese bamboo forests and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Grand View Research. Bamboos Market Size Worth $98.3 Billion by 2025|CAGR: 5.0%. 2020. Available online: https://www.grandviewresearch.com/press-release/global-bamboos-market (accessed on 20 August 2020).
- MTIB, Malaysian Timber Industry Board. Sabah Bamboo Industry Has Huge Potential, Sabah News, Daily Express. 2020. Available online: http://www.dailyexpress.com.my/news/155947/sabah-bamboo-industry-has-huge-potential (accessed on 8 October 2020).
- Konzen, E.R.; Peron, R.; Ito, M.A.; Brondani, G.E.; Tsai, S.M. Molecular identification of bamboo genera and species based on RAPD-RFLP markers. Silva Fenn. 2017, 51, 1–16. [Google Scholar] [CrossRef]
- Kelchner, S.A.; Group, B.P. Higher level phylogenetic relationships within the bamboos (Poaceae: Bambusoideae) based on five plastid markers. Mol. Phylogenet. Evol. 2013, 67, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Barthwal, S.; Ginwal, H.S. Genetic diversity in bamboos: Conservation and improvement for productivity. In Bamboos in India, 3rd ed.; Shailendra, K., Singh, Y.P., Dinesh, K., Manisha, T., Santan, B., Eds.; ENVIS Centre on Forestry: Dehradun, India, 2016; Volume 3, pp. 131–146. [Google Scholar]
- Narin, C.; Kanokporn, P. Effect of thickness on qualities of dried sweet bamboo shoots (Dendrocalamus asper Backer) products. J. Food Health Bioenv Sci. 2021, 12, 1–8. [Google Scholar]
- Banerjee, M.; Gantait, S.; Pramanik, B.R. A two step method for accelerated mass propagation of Dendrocalamus asper and their evaluation in field. Physiol. Mol. Biol. Plants 2011, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Kumar, S.M.; Seca, G.; Maheran, A.A.; Aini, A.S.N. Mass Propagation of Dendrocalamus asper By Branch Cutting. J. Trop. For. Sci. 2018, 30, 82–88. [Google Scholar] [CrossRef]
- Benton, A. Priority Species of Bamboo. In Bamboo (Tropical Forestry); Liese, M., Kohl, M., Eds.; Springer: Cham, Switzerland, 2015; Volume 10, pp. 31–41. [Google Scholar] [CrossRef]
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Micropropagation of Dendrocalamus asper {Schult. & Schult. F.} Backer ex k. Heyne): An exotic edible bamboo. J. Plant Biochem. Biotechnol. 2012, 21, 220–228. [Google Scholar] [CrossRef]
- Sowmya, C.; Jagadish, M.R.; Syam, V. Cultivation prospects of Dendrocalamus asper backer for edible shoots in semiarid and humid tropics of peninsular India. Int. J. Plant Anim. Environ. Sci. 2015, 5, 95–101. [Google Scholar]
- Partey, S.T.; Sarfo, D.A.; Frith, O.; Kwaku, M.; Thevathasan, N.V. Potentials of Bamboo- Based Agroforestry for sustainable development in Sub—Saharan Africa: A review. Agric. Res. J. 2017, 6, 22–32. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Ranaei, F.; Ahmad, Z. Application of Bamboo Plants in Nine Aspects. Sci. World J. 2020, 2020, 7284203. [Google Scholar] [CrossRef] [PubMed]
- Mudoi, K.D.; Saikia, S.P.; Goswami, A.; Gogoi, A. Micropropagation of important bamboos: A review. Afr. J. Biotechnol. 2013, 12, 2770–2785. [Google Scholar] [CrossRef]
- Verma, P.; Mishra, N. A Review In Vitro Regeneration of Bamboo Plants by Plant Tissue Culture Techniques. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 6, 58–67. [Google Scholar]
- Sandhu, M.; Wani, S.H.; Jiménez, V.M. In vitro propagation of bamboo species through axillary shoot proliferation: A review. Plant Cell Tissue Organ Cult. 2018, 132, 27–53. [Google Scholar] [CrossRef]
- Yuan, J.; Yue, J.; Gu, X.; Lin, C.; Lin, C. Flowering of Woody Bamboo in Tissue Culture Systems. Front. Plant Sci. 2017, 8, 1589. [Google Scholar] [CrossRef]
- Singh, S.R.; Singh, R.; Kalia, S.; Dalal, S.; Dhawan, A.K.; Kalia, R.K. Limitations, progress and prospects of application of biotechnological tools in improvement of bamboo—A plant with extraordinary qualities. Physiol. Mol. Biol. Plants 2013, 19, 21–41. [Google Scholar] [CrossRef]
- Jiang, K.; Zhou, M. Recent advances in bamboo molecular biology. J. Trop. Subtrop. Bot. 2014, 22, 632–642. [Google Scholar] [CrossRef]
- Bhandari, S.; Tyagi, K.; Singh, B.; Goutam, U. Role of Molecular Markers to Study Genetic Diversity in Bamboo: A Review. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 86–97. [Google Scholar]
- Nabilah, M.K.; Wilson, T.L.Y.; Julius, K.; Kenneth, F.R. A Glance at Molecular Identification of Bamboo (Poaceae: Bambusoideae). Borneo Int. J. Biotechnol. 2020, 1, 19–33. [Google Scholar] [CrossRef]
- Goyal, A.K.; Kar, P.; Sen, A. Advancement of bamboo taxonomy in the era of molecular biology: A review. In Biology of Useful Plants and Microbes; Sen, A., Ed.; Narosa Publication House: New Delhi, India, 2013; pp. 197–208. [Google Scholar]
- Sijimol, K.; Dev, S.A.; Muralidharan, E.M.; Sreekumar, V.B. DNA barcoding: An emerging tool for precise identification and certification of planting stock in taxonomically challenging bamboo species. J. Bamboo Ratt. 2014, 13, 29–33. [Google Scholar]
- Thapa, P.; Amita, B.; Priyanka, S.; Kiran, D. Advances in Bamboo Biotechnology: Present Status and Future Perspective. In Biotechnologies of Crop Improvement; Springer: New York, NY, USA, 2018; Volume 1, pp. 243–265. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Yrjälä, K.; Vinod, K.K.; Sharma, A.; Cho, J.; Satheesh, V.; Zhou, M. Genetics and genomics of moso bamboo (Phyllostachys edulis): Current status, future challenges, and biotechnological opportunities toward a sustainable bamboo industry. Food Energy Secur. 2020, 9, 1–36. [Google Scholar] [CrossRef]
- Choudhury, D.; Sahu, J.K.; Sharma, G.D. Value addition to bamboo shoots: A review. J. Food Sci. Technol. 2012, 49, 407–414. [Google Scholar] [CrossRef]
- Lu, B.; Wu, X.; Tie, X.; Zhang, Y. Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem. Toxicol. 2005, 43, 783–792. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Pohl, F.; Lin, P.K.T. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef]
- Galeotti, F.; Barile, E.; Curir, P.; Dolci, M.; lanzotti, v. Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem. Lett. 2008, 1, 44–48. [Google Scholar] [CrossRef]
- Matilla, P.; Hellstrom, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Kong, C.K.; Tan, Y.N.; Chye, F.Y.; Sit, N.W. Nutritional compositions, biological activities, and phytochemical contents of the edible bamboo shoot, Dendrocalamus asper, from Malaysia. Int. Food Res. J. 2020, 27, 546–556. [Google Scholar]
- Pandey, A.K.; Ojha, V. Precooking, processing of bamboo shoots for removal of anti-nutrients. J. Food Sci. Technol. 2012, 49, 407–414. [Google Scholar] [CrossRef]
- Choudhury, D.; Sahu, J.K.; Sharma, G.D. Bamboo shoot: Microbiology, biochemistry and technology of fermentation—A review. Indian J. Tradit. Knowl. 2012, 11, 242–249. [Google Scholar]
- Nirmala, C.; Sharma, M.L.; David, E. A comparative study of nutrient components of freshly harvested and canned bamboo shoots of D. giganteus Munro, bamboo science and culture. J. Am. Bamboo Soc. 2008, 21, 41–47. [Google Scholar]
- Bender, D.A. Micronutrients: Vitamins and minerals. In Harper’s Illustrated Biochemistry; Murray, R.K., Botham, K.M., Kennelly, P.J., Rodwell, V.W., Weil, P.A., Eds.; McGraw-Hill Medical: New York, NY, USA, 2012; pp. 525–542. [Google Scholar]
- Zhang, J.; Mohamad, F.H.; Wong, J.H.; Mohamad, H.; Ismail, A.H.; Yusoff, A.A.M.; Osman, H.; Wong, K.T.; Idris, Z.; Abdullah, J.M. The effects of 4-hydroxybenzoic acid identified from bamboo (Dendrocalamus asper) shoots on Kv1. 4 channel. Malays. J. Med. Sci. 2018, 25, 104–116. [Google Scholar] [CrossRef]
- Malanit, P.; Barbu, M.C.; Frühwald, A. The gluability and bonding quality of an Asian bamboo (Dendrocalamus asper) for the production of composite lumber. J. Trop. For. Sci. 2009, 21, 361–368. [Google Scholar]
- Vogtländer, J.; Van Der Lugt, P.; Brezet, H. The sustainability of bamboo products for local and Western European applications. LCAs and land-use. J. Clean. Prod. 2010, 18, 1260–1269. [Google Scholar] [CrossRef]
- Ogunbiyi, M.A.; Olawale, S.O.; Tudjegbe, O.E.; Akinola, S.R. Comparative analysis of the tensile strength of bamboo and reinforcement steel bars as structural member in building construction. Int. J. Sci. Technol. Res. 2015, 4, 551–553. [Google Scholar]
- Dixon, P.; Ahvenainen, P.; Aijazi, A.; Chen, S.; Lin, S.; Augusciak, P.; Borrega, M.; Svedström, K.; Gibson, L. Comparison of the structure and flexural properties of Moso, Guadua and Tre Gai bamboo. Constr. Build. Mater. 2015, 90, 11–17. [Google Scholar] [CrossRef]
- Amatosa, T.A.; Loretero, M.E. Analytical Behaviour in Mechanical Properties of Dendrocalamus asper Bamboo as Construction Building Materials in the Philippines. Glob. J. Eng. Technol. Rev. 2016, 1, 114–121. [Google Scholar] [CrossRef]
- Awalluddin, D.; Ariffin, M.M.; Osman, M.; Hussin, M.; Ismail, M.; Lee, H.; Lim, N.A.S. Mechanical properties of different bamboo species. MATEC Web Conf. 2017, 138, 1024. [Google Scholar] [CrossRef]
- Chin, K.L.; Ibrahim, S.; Hakeem, K.R.; H’ng, P.S.; Lee, S.H.; Lila, M.A.M. Bioenergy production from bamboo: Potential source from Malaysia’s perspective. BioResources 2012, 12, 6844–6867. [Google Scholar] [CrossRef]
- Rusch, F.; Lúcio, D.D.M.; De Campos, R.F. Potential of bamboo for energy purposes. Res. Soc. Dev. 2020, 9, e40973537. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Templeton, N.; Rollin, J.A.; Harvey, S.P.; Zhang, Y.P. Saccharification of a potential bioenergy crop, Phragmites australis (common reed), by lignocellulose fractionation followed by enzymatic hydrolysis at decreased cellulase loadings. Ind. Eng. Chem. Res. 2009, 48, 6441–6447. [Google Scholar] [CrossRef]
- Zhang, Y.H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef]
- Chin, K.L.; H’ng, P.S.; Chai, E.W.; Tey, B.T.; Chin, M.J.; Paridah, M.T.; Luqman, A.C.; Maminski, M. Fuel characteristics of solid biofuel derived from oil palm biomass and fast growing timber species in Malaysia. Bioenergy Res. 2013, 6, 75–82. [Google Scholar] [CrossRef]
- International Energy Outlook. Report Number: DOE/EIA-0383(2015). U.S. Department of Energy: Washington. Available online: http://www.eia.gov/forecasts/ieo (accessed on 18 November 2020).
- Hakeem, K.R.; Jawaid, M.; Alothman, O.Y. Agricultural biomass based potential materials. Agric. Biomass Based Potential Mater. 2015, 3, 1–505. [Google Scholar] [CrossRef]
- Sette, C.R., Jr.; de Castro, P.; Freitas, V.P.; Yamaji, F.M.; Almeida, R.d.A. Production and characterization of bamboo pellets. Biosci. J. 2016, 32, 922–930. [Google Scholar] [CrossRef]
- Freitas, P.; Silva, M.F.; Silva, R.T.; Coneglian, A.; Sette, C.R., Jr. Evaluation of briquettes from bamboo species produced under different temperatures. Int. J. Curr. Res. 2016, 8, 39260–39265. [Google Scholar]
- Santos, D.R.D.S.; Junior, C.R.S.; da Silva, M.F.; Yamaji, F.M.; Almeida, R.d.A. Potencial de espécies de Bambu como fonte energética Bamboo species potential as energy source. Sci. For. 2016, 44, 751–758. [Google Scholar] [CrossRef]
- Sritong, C.; Kunavongkrit, A.; Piumsombun, C. Bamboo: An Innovative Alternative Raw Material for Biomass Power Plants. Int. J. Innov. Manag. Technol. 2012, 3, 759–762. [Google Scholar] [CrossRef]
- Ayana, A.D.A.; Tadesse, Z.; Kebede, Y.; Ayana, D.A. Effect of Storage Media and Storage Time on Germination and Field Emergence of Oxytenanthera abyssinica Seeds. Int. J. Basic Appl. Sci. 2012, 1, 218–226. [Google Scholar]
- Singh, S.; Kumar, P.; Ansari, S.A. A simple method for large-scale propagation of Dendrocalamus asper. Sci. Hortic. 2004, 100, 251–255. [Google Scholar] [CrossRef]
- Seethalakshmi, K.; Jijeesh, C.M.; Unni, K.K. Traditional methods for bamboo propagation in nursery. In Proceedings of the International Conference on Improvement of Bamboo Productivity and Marketing for Sustainable Livelihood, New Delhi, Cane and Bamboo Technology Centre, Guwahati, India, 15–17 April 2008; pp. 28–36. [Google Scholar]
- Pasqualini, A.P.D.A.; Schneider, G.X.; Fraga, H.P.D.F.; Biasi, L.A.; Quoirin, M. In vitro establishment of Bambusa oldhamii Munro from fieldgrown matrices and molecular identification of endophytic bacteria. Pesqui. Agropecu. Trop. 2019, 49, e53673. [Google Scholar] [CrossRef]
- Embaye, K.; Weih, M.; Ledin, S.; Christersson, L. Biomass and nutrient distribution in a highland bamboo forest in southwest Ethiopia: Implications for management. For. Ecol. Manag. 2005, 204, 159–169. [Google Scholar] [CrossRef]
- Alexander, M.P.; Rao, T.C. In vitro culture of bamboo embryo. Curr. Sci. 1968, 37, 415. [Google Scholar]
- Nadha, H.K.; Salwan, R.; Kasana, R.C.; Anand, M.; Sood, A. Identification and elimination of bacterial contamination during in vitro propagation of Guadua angustifolia Kunth. Pharmacogn. Mag. 2012, 8, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Gielis, J.; Peeters, H.; Gillis, K.; Oprins, J.; Debergh, P.C. Tissue culture strategies for genetic improvement of bamboo. Acta Hortic. 2001, 552, 195–204. [Google Scholar] [CrossRef]
- Li, X.; Sun, C.; Zhou, B.; He, Y. Determination of Hemicellulose, Cellulose and Lignin in Moso Bamboo by Near Infrared Spectroscopy. Sci. Rep. 2015, 5, 17210. [Google Scholar] [CrossRef]
- Xu, M.Q.; Dai, Y.C.; Fan, S.H.; Jin, L.X.; Lu, Q.; Tian, G.Z.; Wang, L.F. Records of bamboo diseases and the taxonomy of their pathogens in China (II). For. Res. 2007, 20, 45–52. [Google Scholar]
- Morakotkarn, D.; Kawasaki, H.; Seki, T. Molecular diversity of bamboo-associated fungi isolated from Japan. FEMS Microbiol. Lett. 2007, 266, 10–19. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hattori, T.; Iwakiri, K.; Tamura, K.; Kuroda, A.; Sawada, Y. Current status of bamboo die back caused by the destructive disease ‘witches’-broom of bamboo’ in western Japan. Jpn. J. Conserv. Ecol. 2008, 13, 151–160. [Google Scholar] [CrossRef]
- Sharada, P.; Nagaveni, H.C.; Remadevi, O.K.; Jain, S.H. Toximetric studies on some major bamboo pathogens. Indian For. 2013, 139, 814–820. [Google Scholar]
- Zhang, X.; Yu, H.; Huang, H.; Liu, Y. Evaluation of biological pretreatment with white rot fungi for the enzymatic hydrolysis of bamboo culms. Int. Biodeter. Biodegr. 2007, 60, 159–164. [Google Scholar] [CrossRef]
- Xu, G.; Shi, Z.; Zhao, Y.; Deng, J.; Dong, M.; Liu, C.; Murugadoss, V.; Mai, X.; Guo, Z. Structural characterization of lignin and its carbohydrate complexes isolated from bamboo (Dendrocalamus sinicus). Int. J. Biol. Macromol. 2019, 126, 376–384. [Google Scholar] [CrossRef]
- Singh, L.; Ruprela, N.; Dafale, N.; Thul, S.T. Variation in Endophytic Bacterial Communities Associated with the Rhizomes of Tropical Bamboos. J. Sustain. For. 2021, 40, 1–13. [Google Scholar] [CrossRef]
- Shen, X.Y.; Cheng, Y.L.; Cai, C.J.; Fan, L.; Gao, J.; Hou, C.L. Diversity and antimicrobial activity of culturable endophytic fungi isolated from moso bamboo seeds. PLoS ONE 2014, 9, e95838. [Google Scholar] [CrossRef]
- Tang, X.; Goonasekara, I.D.; Jayawardena, R.; Jiang, H.B.; Li, J.; Hyde, K.; Kang, J. Arthrinium bambusicola (Fungi, Sordariomycetes), a new species from Schizostachyum brachycladum in northern Thailand. Biodivers. Data J. 2020, 23, e58755. [Google Scholar] [CrossRef]
- Geng, X.S.; Shu, J.P.; Peng, H.; Zhang, W. Fungal communities in twigs of three bamboo species based on high-throughput sequencing technology. Chin. J. Ecol. 2018, 37, 3493–3498. [Google Scholar] [CrossRef]
- Meng, M.; Lee, C.C. Function and structural organization of the replication protein of Bamboo mosaic virus. Front. Microbiol. 2017, 8, 522. [Google Scholar] [CrossRef]
- Chang, K.C.; Chang, L.T.; Huang, Y.W.; Lai, Y.C.; Lee, C.W.; Liao, J.T.; Lin, N.S.; Hsu, Y.H.; Hu, C.C. Transmission of bamboo mosaic virus in bamboos mediated by Insects in the order Diptera. Front. Microbiol. 2017, 8, 870. [Google Scholar] [CrossRef]
- Abe, S.; Neriya, Y.; Noguchi, K.; Hartono, S.; Sulandari, S.; Somowiyarjo, S.; Natsuaki, T. First report of the complete genomic sequences from Indonesian isolates of bamboo mosaic virus and detection of genomic recombination events. J. Gen. Plant Pathol. 2019, 85, 158–161. [Google Scholar] [CrossRef]
- Cheng, C.P. Host factors involved in the intracellular movement of Bamboo mosaic virus. Front. Microbiol. 2017, 8, 759. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; He, M.H.; Moffett, P.; Lin, N.S. Abscisic acid induces resistance against Bamboo mosaic virus through argonaute 2 and 3. Plant Physiol. 2017, 174, 339–355. [Google Scholar] [CrossRef]
- Lin, K.Y.; Hsu, Y.H.; Chen, H.C.; Lin, N.S. Transgenic resistance to Bamboo mosaic virus by expression of interfering satellite RNA. Mol. Plant Pathol. 2013, 14, 693–707. [Google Scholar] [CrossRef]
- Zhang, N.; Fang, W.; Shi, Y.; Liu, Q.; Yang, H.; Gui, R.; Lin, X. Somatic embryogenesis and organogenesis in Dendrocalamus hamiltonii. Plant Cell, Tissue Organ Cult. 2010, 103, 325–332. [Google Scholar] [CrossRef]
- Che, P.; Lall, S.; Nettleton, D.; Howell, S.H. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol. 2006, 141, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Arya, I.D.; Satsangi, R.; Arya, S. Rapid micropropagation of edible bamboo Dendrocalamus asper. J. Sustain. For. 2001, 14, 103–114. [Google Scholar] [CrossRef]
- Ali, A.H.; Nirmala, C.; Badal, T.; Sharma, M.L. In-vitro organogenesis and simultaneous formation of shoots and roots from callus in Dendrocalamus asper. In Proceedings of the 8th World Bamboo Congress, Bangkok, Thailand, 16–18 September 2009; pp. 31–40. [Google Scholar]
- Kumar, V.; Singh, S.; Banerjee, M. Albino Regenerants Proliferation of Dendrocalamus asper in vitro. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 5027–5033. [Google Scholar]
- Nadha, H.K. In Vitro Clonal Propagation of Some Important Woody Bamboos and Ascertaining Their Clonal Fidelity. Ph.D. Thesis, Department of Biotechnology and Environmental Sciences, Thapar University, Patiala, India, 3 March 2013. [Google Scholar]
- Ojha, A.; Verma, N.; Kumar, A. In vitro micropropagation of economically important edible bamboo (Dendrocalamus asper) through somatic embryos from root, leaves and nodal segments expiants. Res. Crop. 2009, 10, 430–436. [Google Scholar]
- Gusmiaty; Restu, M.; Larekeng, S.H.; Setiawan, E. The optimization of in vitro micropropagation of betung bamboo (Dendrocalamus asper backer) by medium concentrations and plant growth regulators. IOP Conf. Ser. Earth Environ. Sci. 2020, 575, 12024. [Google Scholar] [CrossRef]
- Suwannamek, A. In Vitro Culture of Dendrocalamus asper Backer. Master’s Thesis, Department of Horticulture, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand, 1992. [Google Scholar]
- Vongvijitra, R. Traditional vegetative propagation and tissue culture of some Thai bamboos. In Proceedings of the International Bamboo Workshop, Conchin, India, 14–18 November 1988; pp. 148–150. [Google Scholar]
- Arya, S.; Satsangi, R.; Arya, I.D. Large scale plant production of edible bamboo Dendrocalamus asper through somatic embryogenesis. Bamboo Sci. Cult. 2008, 21, 21–31. [Google Scholar]
- Shroti, K.; Upadhyay, R.; Niratkar, C.; Singh, M. Micropropagation of Dandrocalamus asper through Inter Nodal Segment. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 58–60. [Google Scholar]
- Ornellas, T.S.; Werner, D.; Holderbaum, D.F.; Scherer, R.F.; Guerra, M.P. Effects of Vitrofural, BAP and meta-Topolin in the in vitro culture of Dendrocalamus asper. Acta Hortic. 2015, 1155, 285–292. [Google Scholar] [CrossRef]
- Arya, S.; Satsangi, R.; Arya, I.D. Direct regeneration of shoots from immature inflorescences in Dendrocalamus asper (edible bamboo) leading to mass propagation. Bamboo Soc. 2008, 21, 14–20. [Google Scholar]
- Satsangi, R.; Kalia, S.; Arya, I.D.; Arya, S. Flowering in exotic bamboo Dendrocalamus asper in India. Indian For. 2001, 127, 1053–1057. [Google Scholar]
- Nadha, H.K.; Rahul, K.; Sharma, R.K.; Anand, M.; Sood, A. In vitro propagation of Dendrocalamus asper and testing the clonal fidelity using RAPD and ISSR markers. Int. J. Curr. Res. 2013, 5, 2060–2067. [Google Scholar]
- Arya, I.D.; Arya, S. In vitro culture establishment of exotic bamboo Dendrocalamus asper. Indian J. Exp. Biol. 1997, 35, 1252–1255. [Google Scholar]
- Arya, S.; Sharma, S.; Kaur, R.; Arya, I.D. Micropropagation of Dendrocalamus asper by shoot proliferation using seeds. Plant Cell Rep. 1999, 18, 879–882. [Google Scholar] [CrossRef]
- Semsuntud, N.; Ponoy, B.; Pattanaviboo, R.; Sathitviboo, R.; Nitiwatta-nachai, W.; Ramyangsi, S. Micropropagation of Dendrocalamus asper Backer. In Bamboo; Puangchit, L., Thaiutsi, B., Thamincha, S., Eds.; INBAR: Chiangmai, Thailand, 2000; pp. 81–93. [Google Scholar]
- Rathore, T.S.; Kabade, U.; Jagadish, M.R.; Somashekar, P.V.; Viswanath, S. Micropropagation and evaluation of growth performance of the selected industrially important bamboo species in southern India. In Proceedings of the 8th World Bamboo Congress, Bangkok, Thailand, 16–19 September 2009; pp. 41–55. [Google Scholar]
- Nirmala, C.; Ali, A.; Badal, T. De novo organogenesis in the form of rhizome in Dendrocalamus asper and D. membranaceus. Curr. Sci. 2011, 100, 468–470. [Google Scholar]
- Tuan, T.T.; Tu, H.L.T.; Giap, D.D.; Du, T.X. The increase in in vitro shoot multiplication rate of Dendrocalamus asper (Schult. f.) Back. ex Heyne. TAP CHI SINH HOC 2012, 34, 257–264. [Google Scholar] [CrossRef]
- Arya, I.D.; Arya, S. In vitro shoot proliferation and somatic embryogenesis: Means of rapid bamboo multiplication. In Proceedings of the 10th World Bamboo Congress, Propagation, Plantation and Management, Damnyang, Korea, 17–22 September 2015. [Google Scholar]
- Zang, Q.; Lui, Q.; Zhuge, F.; Wang, X.; Lin, X. In Vitro Regeneration Via Callus Induction in Dendrocalamus asper (Schult.) Backer. Propag. Ornam. Plants 2019, 19, 66–71. [Google Scholar]
- Singh, V.; Tyagi, A.; Chauhan, P.K.; Kumari, P.; Kaushal, S. Identification and prevention of bacterial contimination on explant used in plant tissue culture labs. Int. J. Pharm. Pharm. Sci. 2011, 3, 160–163. [Google Scholar]
- Oprins, J.; Grunewald, W.; Gillis, K.; Delaere, P.; Peeters, H.; Gielis, J. Micropropagation: A general method for commercial bamboo production. In Proceedings of the 7th World Bamboo Congress, New Delhi, India, 27 February–4 March 2004. [Google Scholar]
- Ray, S.S.; Ali, N. Biotic Contamination and Possible Ways of Sterilization: A Review with Reference to Bamboo Micropropagation. Braz. Arch. Biol. Technol. 2018, 60, 1–12. [Google Scholar] [CrossRef]
- Thakur, R.; Sood, A. An efficient method for explant sterilization for reduced contamination. Plant Cell Tissue Organ Cult. 2006, 84, 369–371. [Google Scholar] [CrossRef]
- Sen, M.K.; Hassan, M.; Nasrin, S.; Mostofa, M.A.H.; Dash, B.K. In vitro sterilization protocol for micropropagation of Achyranthes aspera L. node. Int. Res. J. Biotechnol. 2013, 4, 89–93. [Google Scholar]
- Venturieri, G.A.; Venturieri, A.R.; Leopoldo, G. Sterilization of culture media for orchids using a microwave oven. Vitr. Cell Dev. Biol. Plant 2013, 49, 137–144. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Guevara, E. Micropropagation of bamboo species through axillary shoot proliferation. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 465–476. [Google Scholar] [CrossRef]
- Ray, S.S.; Ali, M.N.; Mukherjee, S.; Chatterjee, G.; Banerjee, M. Elimination and molecular identification of endophytic bacterial contaminants during in vitro propagation of Bambusa balcooa. World J. Microbiol. Biotechnol. 2017, 33, 31. [Google Scholar] [CrossRef]
- Mehta, R.; Sharma, V.; Sood, A.; Sharma, M.; Sharma, R.K. Induction of somatic embryogenesis and analysis of genetic fidelity of in vitro-derived plantlets of Bambusa nutans Wall, using AFLP markers. Eur. J. For. Res. 2011, 130, 729–736. [Google Scholar] [CrossRef]
- Admas, A.; Kidane, B.; Admasu, M.; Misge, T. Develope Micro clonal -propagation protocol for Oxytenanthera abyssinica A. Rich. Munro to large scale micro-propagation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chaitali, M.; Meshram, M.P.; Baviskar, S.P.; Shanti, R.P. Identification of suitable surface sterilization method for bamboo (Dendrocalamus stocksii (Munro.)). J. Soils Crops 2021, 31, 99–101. [Google Scholar]
- Goyal, A.K.; Sen, A. In vitro regeneration of bamboos, the ‘Green Gold’: An overview. Indian J. Biotechnol. 2016, 15, 9–16. [Google Scholar]
- Sood, A.; Ahuja, P.S.; Sharma, M.; Sharma, O.P.; Godbole, S. In vitro protocols and field performance of elites of an important bamboo Dendrocalamus hamiltonii nees Et Arn. Ex Munro. Plant Cell Tissue Organ Cult. 2002, 71, 55–63. [Google Scholar] [CrossRef]
- Bag, N.; Chandra, S.; Palni, L.M.S.; Nandi, S.K. Micropropagation of Dev-ringal [Thamnocalamus spathiflorus (Trin.) Munro]—A temperate bamboo, and comparison between in vitro propagated plants and seedlings. Plant Sci. 2000, 156, 125–135. [Google Scholar] [CrossRef]
- Arshad, S.M.; Kumar, A.; Bhatnagar, S.K. Micropropagation of Bambusa wamin through shoot proliferation of mature nodal explants. J. Biol. Res. 2005, 3, 59–66. [Google Scholar]
- Srivastava, N.; Kamal, B.; Sharma, V.; Negi, Y.K.; Dobriyal, A.K.; Gupta, S.; Jadon, V.S. Standardization of Sterilization Protocol for Micropropagation of Aconitum heterophyllum—An Endangered Medicinal Herb. Acad. Areana 2010, 2, 37–42. [Google Scholar]
- Waikhom, S.D.; Louis, B. An effective protocol for micropropagation of edible bamboo species (Bambusa tulda and Melocanna baccifera) through nodal culture. Sci. World J. 2014, 8, 345794. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Priyanka, K.; Ashish, R. Refinement of protocol for rapid clonal regeneration of economical bamboo, Bambusa balcooa in the agroclimatic conditions of Bihar, India. Afr. J. Biotechnol. 2017, 16, 450–462. [Google Scholar] [CrossRef]
- Wei, Q.; Cao, J.; Qian, W.; Xu, M.; Li, Z.; Ding, Y. Establishment of an efficient micropropagation and callus regeneration system from the axillary buds of Bambusa ventricosa. Plant Cell Tissue Organ Cult. 2015, 122, 1–8. [Google Scholar] [CrossRef]
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Seasonal influences on in vitro bud break in Dendrocalamus hamiltonii Arn. ex Munro nodal explants and effect of culture microenvironment on large scale shoot multiplication and plantlet regeneration. Indian J. Plant Physiol. 2012, 17, 9–21. [Google Scholar]
- Lin, C.S.; Chang, W.C. Micropropagation of Bambusa edulis through nodal explants of field-grown culms and flowering of regenerated plantlets. Plant Cell Rep. 1998, 17, 617–620. [Google Scholar] [CrossRef]
- Ogita, S.; Kashiwagi, H.; Kato, Y. In vitro node culture of seedlings in bamboo plant, Phyllostachys meyeri McClure. Plant Biotechnol. 2008, 25, 381–385. [Google Scholar] [CrossRef]
- Silveira, A.A.D.C.; Lopes, F.J.F.; Sibov, S.T. Micropropagation of Bambusa oldhamii Munro in heterotrophic, mixotrophic and photomixotrophic systems. Plant Cell Tissue Organ Cult. 2020, 141, 1–12. [Google Scholar] [CrossRef]
- Chambers, S.M.; Heuch, J.H.R.; Pirrle, A. Micropropagation and in vitro flowering of the bamboo Dendrocalamus hamiltonii Munro. Plant Cell Tissue Organ Cult. 1991, 27, 45–48. [Google Scholar] [CrossRef]
- Kalpataru, D.M.; Siddhartha, P.S.; Mina, B. Effect of nodal positions, seasonal variations, shoot clump and growth regulators on micropropagation of commercially important bamboo, Bambusa nutans Wall. ex. Munro. Afr. J. Biotechnol. 2014, 13, 1961–1972. [Google Scholar] [CrossRef]
- Rather, M.M.; Thakur, A.; Panwar, M.; Sharma, S. In Vitro Sterilization Protocol for Micropropagation of Chimonobambusa jaunsarensis (Gamble) Bahadur and Naithani-A Rare and Endangered Hill Bamboo. Indian For. 2016, 142, 871–874. [Google Scholar]
- Sood, A.; Nadha, H.K.; Sood, S.; Walia, S.; Parkash, O. Large scale propagation of an exotic edible bamboo, Phyllostachys pubescens Mazel ex H. De Lehale (Moso Bamboo) using seeds. Indian J. Exp. Biol. 2014, 52, 755–758. [Google Scholar]
- Malini, N.; Anandakumar, C.R. Micropropagation of bamboo (Bambusa vulgaris) through nodal segment. Int. J. For. Crop Improv. 2013, 4, 36–39. [Google Scholar]
- Brar, J.; Anand, M.; Sood, A. In vitro seed germination of economically important edible bamboo Dendrocalamus membranaceus Munro. Indian J. Exp. Biol. 2013, 51, 88–96. [Google Scholar]
- Ali, H.; Nirmala, C.; Sharma, M.L. Control of in vitro contamination in Bamboos. Plant Cell Biotechnol. Mol. Biol. 2009, 10, 119–124. [Google Scholar]
- Ahmadi, E.; Nasr, S.M.H.; Jalilvand, H.; Savadkoohi, S.K. Contamination control of microbe Ziziphus spina [christti] seed in vitro culture. Trees Struct. Funct. 2012, 26, 1299–1304. [Google Scholar] [CrossRef]
- Ramanayake, S.M.S.D.; Yakandawala, K.; Deepika, P.K.D.N.; Ikbal, M.C.M. Studies on micropropagation of Dendrocalamus gigateus and Bambusa vulgaris var. striata. Popul. Environ. 1995, 1, 75–85. [Google Scholar]
- Ramanayake, S.M.S.D.; Yakandawala, K. Micropropagation of the giant bamboo (Dendrocalamus giganteus Munro) from nodal explants of field grown culms. Plant Sci. 1997, 129, 213–223. [Google Scholar] [CrossRef]
- Ramanayake, S.M.S.D.; Meemaduma, V.N.; Weerawardene, T.E. In vitro shoot proliferation and enhancement of rooting for the large-scale propagation of yellow bamboo (Bambusa vulgaris ’Striata’). Sci. Hortic. 2006, 110, 109–113. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Castillo, J.; Tavares, E.; Guevara, E.; Montiel, M. In vitro propagation of the neotropical giant bamboo, Guadua angustifolia Kunth, through axillary shoot proliferation. Plant Cell Tissue Organ Cult. 2006, 86, 389–395. [Google Scholar] [CrossRef]
- Pratibha, S.; Sarma, K.P. In vitro Propagation of Bambusa pallida on Commercial Scale in Assam, India. J. Environ. Res. Dev. 2014, 8, 895–902. [Google Scholar]
- Chowdhury, P.; Das, M.; Sikdar, S.R.; Pal, A. Influence of the physiological age and position of the nodal explants on micropropagation of field-grown Dendrocalamus strictus nees. Plant Cell Biotechnol. Mol. Biol. 2004, 5, 45–50. [Google Scholar]
- Bisht, P.; Pant, M.; Kant, A. In vitro propagation of Gigantochloa atroviolaceae Widjaja through nodal explants. J. Am. Sci. 2010, 6, 1019–1026. [Google Scholar]
- Anand, M.; Brar, J.; Sood, A. In Vitro Propagation of an Edible Bamboo Bambusa Bambos and Assessment of Clonal Fidelity through Molecular Markers. J. Med. Bioeng. 2013, 2, 257–261. [Google Scholar] [CrossRef]
- Mishra, Y.; Patel, P.K.; Yadav, S.; Shirin, F.; Ansari, S.A.A. micropropagation system for cloning of Bambusa tulda Roxb. Sci. Hortic. 2008, 115, 315–318. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Kelkar, S.M.; Watve, M.G.; Krishnamurthy, K.V. Characterization and control of endophytic bacterial contaminants in in vitro cultures of Piper spp., Taxus baccata subsp. wallichiana, and Withania somnifera. Can. J. Microbiol. 2007, 53, 63–74. [Google Scholar] [CrossRef]
- Orlikowska, T.; Zawadzka, M.; Zenkteler, E.; Sobiczewski, P. Influence of the biocides PPMTM and Vitrofural on bacteria isolated from contaminated plant tissue cultures and on plant microshoots grown on various media. J. Hortic. Sci. Biotechnol. 2012, 87, 223–230. [Google Scholar] [CrossRef]
- Wang, P.J.; Charles, A. Micropropagation Through Meristem Culture. In High-Tech and Micropropagation I. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 17, pp. 32–52. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Propagation by Tissue Culture, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008; p. 501. [Google Scholar] [CrossRef]
- Iliev, I.A. Factors affecting the axillary and adventitious shoot formation in woody plants in vitro. Acta Hortic. 2017, 1155, 15–28. [Google Scholar] [CrossRef]
- Ngomuo, M.; Mneney, E.; Ndakidemi, P. The Effects of Auxins and Cytokinin on Growth and Development of (Musa sp.) Var. “Yangambi” Explants in Tissue Culture. Am. J. Plant Sci. 2013, 4, 2174–2180. [Google Scholar] [CrossRef]
- Arya, S.; Rana, P.K.; Sharma, R.; Arya, I.D. Tissue culture technology for rapid multiplication of Dendrocalamus giganteus Munro. Indian For. 2006, 132, 345–357. [Google Scholar]
- Nogueira, J.S.; Gomes, H.T.; Scherwinski-Pereira, J.E. Micropropagation, plantlets production estimation and ISSR marker-based genetic fidelity analysis of Guadua magna and G. angustifólia. Pesqui. Agropecuária Trop. 2019, 49. [Google Scholar] [CrossRef]
- Shirin, F.; Rana, P.K. In vitro plantlet regeneration from nodal explants of field-grown culms in Bambusa glaucescens Wild. Plant Biotechnol. Rep. 2007, 1, 141–147. [Google Scholar] [CrossRef]
- Mudoi, K.D.; Borthakur, M. In vitro micropropagation of Bambusa balcooa Roxb through nodal explants from field-grown culms and scope for upscaling. Curr. Sci. 2009, 96, 962–966. [Google Scholar]
- Carimi, F.; Zottini, M.; Formentin, E.; Terzi, M.; Lo Schiavo, F. Cytokinins: New apoptotic inducers in plants. Planta 2003, 216, 413–421. [Google Scholar] [CrossRef]
- Chaturvedi, H.C.; Sharma, M.; Sharma, A.K. In vitro regeneration of Dendrocalamus strictus nees through nodal segments taken from field-grown culms. Plant Sci. 1993, 91, 97–101. [Google Scholar] [CrossRef]
- Das, P.; Rout, G.R. Analysis of current methods and approaches on the micropropagation of bamboo. Proc. Natl. Acad. Sci. USA Sect. B Biol. Sci. 1994, 64, 235–246. [Google Scholar]
- Saikat, G.; Nirmal, M. Tissue culture of Anthurium andreanum: A significant review and future prospective. Int. J. Bot. 2010, 6, 207–219. [Google Scholar] [CrossRef]
- Lin, C.S.; Kalpana, K.; Chang, W.C.; Lin, N.S. Improving multiple shoot proliferation in bamboo mosaic virus-free Bambusa oldhamii Munro propagation by liquid culture. HortScience 2007, 42, 1243–1246. [Google Scholar] [CrossRef]
- Singh, M.; Jaiswal, U.; Jaiswal, V.S. Thidiazuron-induced shoot multiplication and plant regeneration in bamboo (Dendrocalamus strictus nees). J. Plant Biochem. Biotechnol. 2001, 10, 133–137. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Mulgund, G.S.; Nataraja, K. Thidiazuron induced shoot regeneration of Costus speciosus (Koen.) Sm using thin rhizome sections. S. Afr. J. Bot. 2004, 70, 255–258. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Influence of growth regulators on physiology and senescence of cut stems of chrysanthemum (Chrysanthemum morifolium Ramat) Var. Thai Ching Queen. Int. J. Allied Pract Res. Rev. 2015, 2, 31–41. [Google Scholar]
- Aremu, A.O.; Bairu, M.W.; Doležal, K.; Finnie, J.F.; Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Ramanayake, S.M.S.D.; Wanniarachchi, W.A.V.R.; Tennakoon, T.M.A. Axillary shoot proliferation and in vitro flowering in an adult giant bamboo, Dendrocalamus giganteus Wall. Ex Munro. Vitr. Cell Dev. Biol. 2001, 37, 667–671. [Google Scholar] [CrossRef]
- Do Vale, P.A.A.; Júnior, J.B.D.O.; Costa, F.H.D.S.; Scherwinski-Pereira, J.E. Height and number of shoots on the survival and development of micropropagated bamboo plantlets during pre-acclimatization. Pesqui. Agropecu. Trop. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Saxena, S. In vitro propagation of the bamboo (Bambusa tulda Roxb.) through shoot proliferation. Plant Cell Rep. 1990, 9, 431–434. [Google Scholar] [CrossRef]
- Saxena, S.; Bhojwani, S.S. In vitro clonal multiplication of 4-year-old plants of the bamboo, Dendrocalamus longispathus kurz. Vitr. Cell Dev. Biol. Plant 1993, 29, 135–142. [Google Scholar] [CrossRef]
- Chongtham, N.; Bisht, M.S.; Premlata, T. In vitro Propagation of an Edible Bamboo Dendrocalamus latiflorus Munro Using Nodal Explants. In Proceedings of the 11th World Bamboo Congress, Xalapa, Veracruz, Mexico, 14–18 August 2018. [Google Scholar]
- Agnihotri, R.K.; Mishra, J.; Nandi, S.K. Improved in vitro shoot multiplication and rooting of Dendrocalamus hamiltonii Nees et Arn. Ex Munro: Production of genetically uniform plants and field evaluation. Acta Physiol. Plant. 2009, 31, 961–967. [Google Scholar] [CrossRef]
- Thorpe, T.A.; Harvy, I.S.; Kumar, P.P. Application of micropropagation to forestry. In Micropropagation: Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 311–336. [Google Scholar]
- Leva, A.; Petruccelli, R.; Rinaldi, L.M.R. Somaclonal Variation in Tissue Culture: A Case Study with Olive. Recent Adv. Plant Vitr. Cult. 2012, 123–150. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Huang, L.C.; Huang, B.L.; Chen, W.L. Tissue culture investigations of bamboo—IV. Organogenesis leading to adventitious shoots and plants in excised shoot apices. Environ. Exp. Bot. 1989, 29, 307–315. [Google Scholar] [CrossRef]
- Sood, A.; Palni, L.M.S.; Sharma, M.; Sharma, O.P. Improved methods of propagation of Maggar bamboo (Dendrocalamus hamiltonii Nees et. Arn. Ex Munro). In Biotechnology in India; Dwivedi, B.K., Ed.; Bioved Research Society: Allahabad, India, 1994; pp. 199–212. [Google Scholar]
- Mohanty, S.; Panda, M.K.; Subudhi, E.; Nayak, S. Plant regeneration from callus culture of Curcuma aromatica and in vitro detection of somaclonal variation through cytophotometric analysis. Biol. Plant. 2008, 52, 783–786. [Google Scholar] [CrossRef]
- Lin, S.; Liu, G.; Guo, T.; Zhang, L.; Wang, S.; Ding, Y. Shoot proliferation and callus regeneration from nodular buds of Drepanostachyum luodianense. J. For. Res. 2009, 30, 1997–2005. [Google Scholar] [CrossRef]
- Ye, S.; Cai, C.; Ren, H.; Wang, W.; Xiang, M.; Tang, X.; Zhu, C.; Yin, T.; Zhang, L.; Zhu, Q. An efficient plant regeneration and transformation system of Ma Bamboo (Dendrocalamus latiflorus munro) started from young shoot as explant. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Zang, Q.; Zhou, L.; Zhuge, F.; Yang, H.; Wang, X.; Lin, X. Callus induction and regeneration via shoot tips of Dendrocalamus hamiltonii. SpringerPlus 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Godbole, S.; Sood, A.; Thakur, R.; Sharma, M.; Ahuja, P.S. Somatic embryogenesis and its conversion into plantlets in a multipurpose bamboo, Dendrocalamus hamiltonii Nees et Arn. Ex Munro. Curr. Sci. 2002, 83, 885–889. [Google Scholar]
- Bag, N.; Palni, L.M.S.; Chandra, S.; Nandi, S.K. Somatic embryogenesis in ‘maggar’ bamboo (Dendrocalamus hamiltonii) and field performance of regenerated plants. Curr. Sci. 2012, 102, 1279–1287. [Google Scholar] [CrossRef]
- Islam, S.A.M.N.; Rahman, M.M. Micro-cloning in commercially important six bamboo species for mass propagation and at a large scale cultivation. Plant Tissue Cult. Biotechnol. 2005, 15, 103–111. [Google Scholar]
- Mishra, Y.; Patel, P.; Ansari, S.A. Acclimatization and Macroproliferation of Micropropagated Plants of Bambusa tulda Roxb. Asian J. Exp. Biol. Sci. 2011, 2, 498–501. [Google Scholar]
- Somashekar, P.V.; Rathore, T.S.; Shashidhar, K.S. Rapid and simplified method of micropropagation of Pseudoxytenanthera stocksii. In Forest Biotechnology in India; Ansari, S.A., Narayanan, C., Mandal, A.K., Eds.; Satish Serial Publishing House: Delhi, India, 2008; pp. 165–182. [Google Scholar]
- Rathore, T.S.; Rai, V.R. Micropropagation of Pseudoxytenanthera stocksii munro. Vitr. Cell. Dev. Biol. Plant. 2005, 41, 333–337. [Google Scholar] [CrossRef]
- Arya, S.; Kaur, B.; Arya, I.D. Micropropagation of economically important bamboo Dendrocalamus hamiltonii through axillary bus and seed culture. In Proceedings of the 8th World Bamboo Congress, Bangkok, Thailand, 16–19 September 2009; pp. 122–130. [Google Scholar]
- Suwal, M.M.; Lamichhane, J.; Gauchan, D.P. Regeneration Technique of Bamboo Species through Nodal Segments: A Review. Nepal J. Biotechnol. 2020, 8, 54–68. [Google Scholar] [CrossRef]
- Adarsh, K.; Gupta, B.B.; Negi, D.S. Vegetative propagation of Dendrocalamus strictus through macro-proliferation-II. Ind. For. 1991, 117, 621–623. [Google Scholar]
- Banik, R.L. A Manual for Vegetative Propagation of Bamboos, International Network for Bamboo and Rattan (INBAR) Technical Report No. 6; Bangladesh Forest Research Institute: Chattogram, Bangladesh, 1995; pp. 1–66. [Google Scholar]
- Adarsh, K.; Mohinder, P.; Shiv, K. Mass production of field planting stock of Dendrocalamus hamiltonii vegetatively through macro-proliferation. Ind. For. 1992, 118, 638–645. [Google Scholar]
- Considine, M.J.; Considine, J.A. On the language and physiology of dormancy and quiescence in plants. J. Exp. Bot. 2016, 67, 3189–3203. [Google Scholar] [CrossRef]
- Sharma, M.L.; Bala, N. Endogenous Levels of Plant Growth Substances in Seeds of Five Bamboo Species in Relation To Seed Viability. Indian J. Plant Physiol. 2006, 11, 358–363. [Google Scholar]
- Geetika, S.R.; Sharma, M.L. Effect of Pre-Sowing Invigouration Treatments on Performance of Ageing Dendrocalamus Hamiltonii Seeds. Int. J. Sci. Res. 2015, 4, 2277–8179. [Google Scholar]
- Chaiyarat, R. The effects of different treatments on seed germination and growth monastery Bamboo, Thyrsostachys siamensis. J. Bamboo Ratt. 2018, 17, 61–71. [Google Scholar]
- Airi, S.; Bhatt, I.D.; Bhatt, A.; Rawal, R.S.; Dhar, U. Variations in seed germination of Hippophae salicifolia with different presoaking treatments. J. For. Res. 2009, 20, 27–30. [Google Scholar] [CrossRef]
- Azad, M.S.; Zedan-Al-Musa, M.; Matin, M.A. Effects of pre-sowing treatments on seed germination of Melia azedarach. J. For. Res. 2010, 21, 193–196. [Google Scholar] [CrossRef]
- Azad, S.; Paul, N.K.; Matin, A. Do pre-sowing treatments affect seed germination in Albizia richardiana and Lagerstroemia speciosa? Front. Agric. China 2010, 4, 181–184. [Google Scholar] [CrossRef]
- Moghadam, A.K.; Mohammadi, K. Different priming treatments affected germination traits of safflower. Appl. Sci. Rep. 2013, 2, 22–25. [Google Scholar]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Karssen, C.M.; Zagorski, S.; Kepczynski, J.; Groot, S.P.C. Key role for endogenous gibberellins in the control of seed germination. Ann. Bot. 1989, 63, 71–80. [Google Scholar] [CrossRef]
- Devi, W.S.; Bengyella, L.; Sharma, G.J. In vitro seed germination and micropropagation of edible bamboo Dendrocalamus giganteus Munro using seeds. Biotechnology 2012, 11, 74–80. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Kumar, P.R.; Singh, A.K.; Bhatt, B.P. Effect of priming treatments on seed germination and seedling growth in bamboo [Dendrocalamus strictus (Roxb.) Nees]. Acta Ecol. Sin. 2020, 40, 128–133. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, M.L. Effect of pre-sowing invigouration treatments on performance of ageing Dendrocalamus strictus seeds. Int. J. Adv. Res. 2015, 3, 1521–1526. [Google Scholar]
- Li, J.; Gao, C.; Miao, Y.; Liu, Z.; Cui, K. Development of a highly efficient callus induction and plant regeneration system for Dendrocalamus sinicus using hypocotyls as explants. Plant Cell Tissue Organ Cult. 2021, 145, 117–125. [Google Scholar] [CrossRef]
- Raju, R.I.; Roy, S.K. Mass propagation of Bambusa bambos (L.) Voss through in vitro culture. Jahangirnagar Univ. J. Biol. Sci. 2017, 5, 15–26. [Google Scholar] [CrossRef]
- Pratibha, S.; Sarma, K.P. In vitro propagation of Bambusa tulda: An important plant for better environment. J. Environ. Res. Dev. 2013, 7, 1216–1223. [Google Scholar]
- Devi, W.S.; Sharma, G.J. In Vitro Propagation of Arundinaria callosa Munro—An Edible Bamboo from Nodal Explants of Mature Plants. Open Plant Sci. J. 2014, 3, 35–39. [Google Scholar] [CrossRef][Green Version]
- Hirimburegama, K.; Gamage, N. Propagation of Bambusa vulgaris (yellow bamboo) through nodal bud culture. J. Hortic. Sci. 1995, 70, 4469–4475. [Google Scholar] [CrossRef]
- Funada, R.; Kubo, T.; Tabuchi, M.; Sugiyama, T.; Fushitani, M. Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora Sieb. et Zucc. stems in relation to earlywood-latewood transition and cessation of tracheid production. Holzforschung 2001, 55, 128–134. [Google Scholar] [CrossRef]
- Das, M.; Pal, A. In vitro regeneration of Bambusa balcooa Roxb.: Factors affecting changes of morphogenetic competence in the axillary buds. Plant Cell Tissue Organ Cult. 2005, 81, 109–112. [Google Scholar] [CrossRef]
- Rangsiruji, A.; Binchai, S.; Pringsulaka, O. Species identification of economic bamboos in the genus dendrocalamus using SCAR and multiplex PCR. Songklanakarin J. Sci. Technol. 2018, 40, 640–647. [Google Scholar] [CrossRef]
- Das, M.; Bhattacharya, S.; Singh, P.; Filgueiras, T.S.; Pal, A. Bamboo Taxonomy and Diversity in the Era of Molecular Markers. Adv. Bot. Res. 2008, 47, 225–268. [Google Scholar] [CrossRef]
- Moges, A.D.; Admassu, B.; Belew, D.; Yesuf, M.; Njuguna, J.; Kyalo, M.; Ghimire, S.R. Development of microsatellite markers and analysis of genetic diversity and population structure of Colletotrichum gloeosporioides from Ethiopia. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Amom, T.; Tikendra, L.; Rahaman, H.; Potshangbam, A.; Nongdam, P. Evaluation of genetic relationship between 15 bamboo species of North-East India based on ISSR marker analysis. Mol. Biol. Res. Commun. 2018, 7, 7–15. [Google Scholar] [CrossRef]
- Desai, P.; Gajera, B.; Mankad, M.; Shah, S.; Patel, A.; Patil, G.; Narayanan, S.; Kumar, N. Comparative assessment of genetic diversity among Indian bamboo genotypes using RAPD and ISSR markers. Mol. Biol. Rep. 2015, 42, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Friar, E.; Kochert, G. A study of genetic variation and evolution of Phyllostachys (Bambusoideae: Poaceae) using nuclear restriction fragment length polymorphisms. Theor. Appl. Genet. 1994, 89, 265–270. [Google Scholar] [CrossRef]
- Nayak, S.; Rout, G.R.; Das, P. Evaluation of the genetic variability in bamboo using RAPD markers. Plant Soil Environ. 2003, 49, 24–28. [Google Scholar] [CrossRef]
- Das, M.; Bhattacharya, S.; Pal, A. Generation and characterization of SCARs by cloning and sequencing of RAPD products: A strategy for species-specific marker development in bamboo. Ann. Bot. 2005, 95, 835–841. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, L.; Peng, Z.; Sun, H.; Yue, X.; Lou, Y.; Dong, L.; Wang, L.; Gao, Z. Developing genome-wide microsatellite markers of bamboo and their applications on molecular marker assisted taxonomy for accessions in the genus Phyllostachys. Sci. Rep. 2015, 5, 8018. [Google Scholar] [CrossRef]
- Isagi, Y.; Oda, T.; Fukushima, K.; Lian, C. Predominance of a single clone of the most widely distributed bamboo species Phyllostachys edulis in East Asia. J. Plant Res. 2016, 129, 21–27. [Google Scholar] [CrossRef]
- Jiang, W.; Bai, T.; Dai, H.; Wei, Q.; Zhang, W.; Ding, Y. Microsatellite markers revealed moderate genetic diversity and population differentiation of moso bamboo (Phyllostachys edulis)—A primarily asexual reproduction species in China. Tree Genet. Genomes 2017, 13, 1–4. [Google Scholar] [CrossRef]
- Rossarolla, M.D.; Tomazetti, T.C.; Vieira, L.N.; Guerra, M.P.; Klabunde, G.H.; Scherer, R.F.; Pescador, R.; Nodari, R.O. Identification and characterization of SSR markers of Guadua chacoensis (Rojas) Londoño & PM Peterson and transferability to other bamboo species. 3 Biotech 2020, 10, 273. [Google Scholar] [CrossRef]
- Cai, K.; Zhu, L.; Zhang, K.; Li, L.; Zhao, Z.; Zeng, W.; Lin, X. Development and characterization of EST-SSR markers from RNA-Seq data in Phyllostachys violascens. Front. Plant Sci. 2019, 10, 50. [Google Scholar] [CrossRef]
- Kanok-Orn, S. The Study of Genetic Relationship and Molecular Markers Identification of SUT Dendrocalamus asper (Pai Tong Keaw). Master’s Thesis, Faculty of Science in Biotechnology, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2002. [Google Scholar]
- Rimbawanto, A. Genetic Diversity of Dendrocalamus asper in Java Revealed by Rapd Markers. Indones. J. For. Res. 2006, 3, 67–74. [Google Scholar] [CrossRef][Green Version]
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Evaluation of genetic fidelity of in vitro raised plants of Dendrocalamus asper (Schult. & Schult. F.) Backer ex K. Heyne using DNA-based markers. Acta Physiol. Plant 2013, 35, 419–430. [Google Scholar] [CrossRef]
- Gillis, K.; Gielis, J.; Peeters, H.; Dhooghe, E.; Oprins, J. Somatic embryogenesis from mature Bambusa balcooa Roxburgh as basis for mass production of elite forestry bamboos. Plant Cell Tissue Organ Cult. 2007, 91, 115–123. [Google Scholar] [CrossRef]
- Palombi, M.A.; Damiano, C. Comparison between RAPD and SSR molecular markers in detecting genetic variation in kiwifruit (Actinidia deliciosa A. Chev). Plant Cell Rep. 2002, 20, 1061–1066. [Google Scholar] [CrossRef]
- Das, M.; Pal, A. Clonal propagation and production of genetically uniform regenerants from axillary meristems of adult bamboo. J. Plant Biochem. Biotechnol. 2005, 14, 185–188. [Google Scholar] [CrossRef]
- Negi, D.; Saxena, S. Ascertaining clonal fidelity of tissue culture raised plants of Bambusa balcooa Roxb. using inter simple sequence repeat markers. New For. 2010, 40, 1–8. [Google Scholar] [CrossRef]
- Brar, J.; Shafi, A.; Sood, P.; Anand, M.; Sood, A. In-vitro propagation, biochemical studies and assessment of clonal fidelity through molecular markers in Bambusa balcooa. J. Trop. For. Sci. 2014, 26, 115–124. [Google Scholar]
- Lee, P.C.; Muniandi, S.K.; Shukor, N.A. In vitro regeneration of Bamboo species. Pertanika J. Sci. Technol. 2018, 4, 80–88. [Google Scholar]
- Goyal, A.K.; Pradhan, S.; Basistha, B.C.; Sen, A. Micropropagation and assessment of genetic fidelity of Dendrocalamus strictus (Roxb.) nees using RAPD and ISSR markers. 3 Biotech 2015, 5, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Negi, D.; Saxena, S. In vitro propagation of Bambusa nutans Wall. ex Munro through axillary shoot proliferation. Plant Biotechnol. Rep. 2011, 4, 35–43. [Google Scholar] [CrossRef]
- Nadha, H.K.; Kumar, R.; Sharma, R.K.; Anand, M.; Sood, A. Evaluation of clonal fidelity of in vitro raised plants of Guadua angustifolia Kunth using DNA-based markers. J. Med. Plant Res. 2011, 5, 5636–5641. [Google Scholar] [CrossRef]
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Ascertaining clonal fidelity of micropropagated plants of Dendrocalamus hamiltonii Nees et Arn. ex Munro using molecular markers. Vitr. Cell. Dev. Biol. Plant 2013, 49, 572–583. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Song, J.; Cao, Y.; Sun, Q.; Yao, H.; Wu, Q.; Chao, J.; Zhou, J.; Xue, W.; Duan, J. Application of the ITS2 region for barcoding medicinal plants of Selaginellaceae in Pteridophyta. PLoS ONE 2013, 8, e67818. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2014, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Bruni, I.; De Mattia, F.; Galimberti, A.; Galasso, G.; Banfi, E.; Casiraghi, M.; Labra, M. Identification of poisonous plants by DNA barcoding approach. Int. J. Leg. Med. 2010, 124, 595–603. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wong, K.L.; Shaw, P.C.; Wang, H.; Li, D.Z. Identification of the medicinal plants in Aconitum L. by DNA barcoding technique. Planta Med. 2010, 76, 1622–1628. [Google Scholar] [CrossRef]
- Valentini, A.; Pompanon, F.; Taberlet, P. DNA barcoding for ecologists. Trends Ecol. Evol. 2009, 24, 110–117. [Google Scholar] [CrossRef]
- Janzen, D.H.; Hajibabaei, M.; Burns, J.M.; Hallwachs, W.; Remigio, E.; Hebert, P.D. Wedding biodiversity inventory of a large and complex Lepidoptera fauna with DNA barcoding. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1835–1845. [Google Scholar] [CrossRef]
- Smith, M.A.; Fisher, B.L.; Hebert, P.D. DNA barcoding for effective biodiversity assessment of a hyperdiverse arthropod group: The ants of Madagascar. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1825–1834. [Google Scholar] [CrossRef]
- Cho, Y.; Mower, J.P.; Qiu, Y.L.; Palmer, J.D. Mitochondrial substitution rates are extraordinarily elevated and variable in a genus of flowering plants. Proc. Natl. Acad. Sci. USA 2004, 101, 17741–17746. [Google Scholar] [CrossRef]
- Fazekas, A.J.; Burgess, K.S.; Kesanakurti, P.R.; Graham, S.W.; Newmaster, S.G.; Husband, B.C.; Percy, D.M.; Hajibabaei, M.; Barrett, S.C. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLoS ONE 2008, 3, e2802. [Google Scholar] [CrossRef]
- Liu, J.I.E.; Moeller, M.; Gao, L.M.; Zhang, D.Q.; Li, D.Z. DNA barcoding for the discrimination of Eurasian yews (Taxus L., Taxaceae) and the discovery of cryptic species. Mol. Ecol. Res. 2011, 11, 89–100. [Google Scholar] [CrossRef]
- Maloukh, L.; Kumarappan, A.; Jarrar, M. Discriminatory power of rbcL barcode locus for authentication of some of United Arab Emirates (UAE) native plants. 3 Biotech 2017, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Yao, H.; Song, J.; Liu, C.; Zhu, Y.; Ma, X.; Pang, X.; Xu, H.; Chen, S. Identification of medicinal plants in the family Fabaceae using a potential DNA barcode ITS2. J. Ethnopharmacol. 2010, 130, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, J.; Liu, C.; Luo, K.; Han, J.; Li, Y.; Pang, X.; Xu, H.; Zhu, Y.; Xiao, P.; et al. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, P.M.; Graham, S.W.; Little, D.P. Choosing and using a plant DNA barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.B.; Huang, P.H.; Ree, R.H.; Liu, M.L.; Li, D.Z.; Wang, H. DNA barcoding of Pedicularis L.(Orobanchaceae): Evaluating four universal barcode loci in a large and hemiparasitic genus. J. Syst. Evol. 2011, 49, 425–437. [Google Scholar] [CrossRef]
- Li, H.Q.; Chen, J.Y.; Wang, S.; Xiong, S.Z. Evaluation of six candidate DNA barcoding loci in Ficus (Moraceae) of China. Mol. Ecol. Resour. 2012, 12, 783–790. [Google Scholar] [CrossRef]
- Little, D.P.; Knopf, P.; Schulz, C. DNA barcode identification of Podocarpaceae—The second largest conifer family. PLoS ONE 2013, 8, e81008. [Google Scholar] [CrossRef] [PubMed]
- Hai-Fei, Y.; Yun-Jiao, L.; Xie, X.F.; Cai-Yun, Z.; Hu, C.M.; Hao, G.; Xue-Jun, G. DNA Barcoding Evaluation and Its Taxonomic Implications in the Species-Rich Genus Primula L. in China. PLoS ONE 2015, 10, e0122903. [Google Scholar] [CrossRef]
- Saarela, J.M.; Sokoloff, P.C.; Gillespie, L.J.; Consaul, L.L.; Bull, R.D. DNA Barcoding the Canadian Arctic Flora: Core Plastid Barcodes (rbcL + matK) for 490 Vascular Plant Species. PLoS ONE 2013, 8, e77982. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, X.; Zhu, S.; Zhao, H.; Fu, L. Species-specific identification from incomplete sampling: Applying DNA barcodes to monitoring invasive Solanum plants. PLoS ONE 2013, 8, 367–375. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ng, W.L.; Mahat, M.N.; Nazre, M.; Mohamed, R. DNA barcoding of the endangered Aquilaria (Thymelaeaceae) and its application in species authentication of agarwood products traded in the market. PLoS ONE 2016, 11, e0154631. [Google Scholar] [CrossRef]
- Costion, C.M.; Kress, W.J.; Crayn, D.M. DNA barcodes confirm the taxonomic and conservation status of a species of tree on the brink of extinction in the Pacific. PLoS ONE 2016, 11, e0155118. [Google Scholar] [CrossRef][Green Version]
- Zhao, M.C.; Yu, X.Z.; Li, N.Z.; Lian-Ming, G.; De Zhu, L. Testing four candidate barcoding markers in temperate woody bamboos (Poaceae: Bambusoideae). J. Syst. Evol. 2012, 50, 527–539. [Google Scholar]
- Steven, G.N.; Subramanyam, R. Testing plant barcoding in a sister species complex of pantropical Acacia (Mimosoideae, Fabaceae). Mol. Ecol. Resour. 2019, 9, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Das, M.M.; Mahadani, P.; Singh, R.; Karmakar, K.; Ghosh, S.K. matK sequence based plant DNA barcoding failed to identify Bambusa (Family: Poaceae) species from Northeast India. J. Environ. Sociobiol. 2013, 10, 49–54. [Google Scholar]
- Leelavathy, S.; Sankar, P.D. Maturase K (matK) as a Barcode in Bamboos. Res. J. Pharm. Technol. 2021, 14, 955–958. [Google Scholar] [CrossRef]
- Yu-xiao, Z.; Yu-xing, X.; Peng-fei, M.; Lina, Z.; De-zhu, L. Selection of potential plastid DNA barcodes for Bambusoideae (Poaceae). Plant Divers. Resour. 2013, 35, 743–750. [Google Scholar] [CrossRef]
- Dev, S.A.; Sijimol, K.; Prathibha, P.S.; Sreekumar, V.B.; Muralidharan, E.M. DNA barcoding as a valuable molecular tool for the certification of planting materials in bamboo. 3 Biotech 2020, 10, 59. [Google Scholar] [CrossRef]
- Gui, Y.; Wang, S.; Quan, L.; Zhou, C.; Long, S.; Zheng, H.; Jin, L.; Zhang, X.; Ma, N.; Fan, L. Genome size and sequence composition of moso bamboo: A comparative study. Science in China Series C. Life Sci. 2007, 50, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Lin, Y.T.; Lin, C.W.; Chen, W.Y.; Yang, C.H.; Ku, H.M. Transferability of rice SSR markers to bamboo. Euphytica 2010, 175, 23–33. [Google Scholar] [CrossRef]
- Peng, Z.; Lu, Y.; Li, L.; Zhao, Q.; Feng, Q.I.; Gao, Z.; Lu, H.; Hu, T.; Yao, N.; Liu, K.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Gen. 2013, 45, 456–461. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, Z.; Fei, B.; Li, L.; Hu, T.; Gao, Z.; Jiang, Z. BambooGDB: A bamboo genome database with functional annotation and an analysis platform. Database J. Biol. Databases Curation 2014, 2014, bau006. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Zhang, C.; Qi, F.; Li, X.; Mu, S.; Peng, Z. Characterization of the floral transcriptome of Moso bamboo (Phyllostachys edulis) at different flowering developmental stages by transcriptome sequencing and RNA-seq analysis. PLoS ONE 2014, 9, e98910. [Google Scholar] [CrossRef]
- Zhou, M.B.; Wu, J.J.; Ramakrishnan, M.; Meng, X.W.; Vinod, K.K. Prospects for the study of genetic variation among Moso bamboo wild-type and variants through genome resequencing. Trees 2019, 33, 371–381. [Google Scholar] [CrossRef]
- Li, S.; Ramakrishnan, M.; Vinod, K.K.; Kalendar, R.; Yrjälä, K.; Zhou, M. Development and Deployment of High-Throughput Retrotransposon-Based Markers Reveal Genetic Diversity and Population Structure of Asian Bamboo. Forests 2020, 11, 31. [Google Scholar] [CrossRef]
- Liu, H.L.; Wu, M.; Li, F.; Gao, Y.M.; Chen, F.; Xiang, Y. TCP transcription factors in moso bamboo (Phyllostachys edulis): Genome-wide identification and expression analysis. Front. Plant Sci. 2018, 9, 1263. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Li, K.; Zhang, Q.J.; Tong, Y.; Zhang, Y.; Wang, J.; Clark, L.; Gao, L.Z. Draft genome of the herbaceous bamboo Raddia distichophylla. G3 2021, 11, jkaa049. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, H.; Yan, H.; Sheng, M.; Cao, Y.; Yang, K.; Xu, H.; Xu, W.; Gao, Z.; Su, Z. Refinement of bamboo genome annotations through integrative analyses of transcriptomic and epigenomic data. Comput. Struct. Biotechnol. J. 2021, 19, 2708–2718. [Google Scholar] [CrossRef]

| Explant | Basal Medium | PGRs (as Indicated in μM Except Otherwise Mentioned) | Results | Reference(s) |
|---|---|---|---|---|
| Node (young first three segment) | MS | * BAP (22.0) ** BAP (22.0) + AdS (216.0) *** IBA (4.90) | Shoot multiplication and rooting | [16] |
| Node | MS | * BAP (15.0) ** BAP (10.0) + AdS (75.0) *** ½ MS + IBA (5.0) + NAA (5.0) | Shoot multiplication and rooting | [19] |
| Seeds, Nodes | MS | * BAP (13.32) ** BAP(13.32) ***NAA (16.11); IBA (49.0) | Shoot multiplication and rooting | [91] |
| Node | MS | ** BAP (31.08) ***NAA (16.11) + IAA (5.71) | Organogenesis, multiple shoots and rooting | [92] |
| Nodes | MS | ** BAP (13.32) + Ads (270.0) *** IBA (4.90) | Shoot multiplication and rooting | [93] |
| Node | MS | * BAP (8.86) ** BAP (8.86) + Ads (13.5) + 3% Suc ***IBA (14.76) + NAA (3.67) + 3% Suc # 2,4-D (14.61) ##, ** 2,4-D (14.61) *** IBA (14.76) + NAA (3.67) | Shoot multiplication and rooting | [94,100] |
| Node | MS | * ¼ MS BAP ** ¾ MS + 3 ppm Kn | Shoot multiplication | [96] |
| Stem cuttings | MS | * BAP (0–8.88) + CW (0–20.0) ** BAP (22.2) *** NAA (2.68) + AA (283.5) + CA (130) + Cyst (206.25) | Shoot multiplication and rooting | [97] |
| Small branch cuttings | MS | ** BA (3 × 10−5) | Shoot multiplication | [98] |
| Inter node segments | MS | * BAP (2.22) ** BAP (8.88) # Kin (23.25) + NAA (16.11) | Shoot multiplication, rooting and callusing | [104] |
| Clump | MS | * BAP (15) ** mT (20) | Shoot multiplication | [101] |
| Immature and mature inflorescence | MS | * BAP (31.08) ** BAP (13.32) *** IBA (49.0) | Shoot multiplication and rooting | [102] |
| Seeds | MS | * BAP (22.2) ** BAP(1.332) * IBA (49.0) + NAA (16.11) | Shoot multiplication and rooting | [105] |
| Seeds | MS | * BAP (22.2) ** BAP (13.32) *** IBA (49.0); NAA (16.11) | Shoot multiplication and rooting | [106] |
| Seeds | MS | * BA (20.0) ** BA (10.0) *** IBA (40.0) | Shoot multiplication and rooting | [107] |
| Node | MS | * TDZ (1.135) + NAA (1.34) + AA (283.5) + CA (130.0) + Cyst (206.25) ** TDZ (1.135) + NAA (1.34) + AA (283.5) + CA (130.0) + Cyst (206.25) *** ¼ MS + IBA (9.80) | Shoot multiplication and rooting | [108] |
| In vitro grown shoots | MS | ** BAP (31.08) • NAA (16.11) + IBA (14.70) + 5% Suc | Shoot multiplication and rhizogenesis | [109] |
| Seeds | MS | * BAP (13.32) ** BAP (13.32) *** IBA (34.30) | Shoot multiplication and rooting | [110] |
| Explant | Basal Medium | PGRs (as Indicated in μM Except Otherwise Mentioned) | Results | Reference(s) |
|---|---|---|---|---|
| Node | MS | # MMS + 2,4-D (30.0) * BAP (20.0) *** NAA (5.0–25.0) | Somatic embryogenesis and germination | [95] |
| Nodal and leaf bases | MS | # 2,4-D (30.0) ## 2,4-D (9) + IAA (2.85) + BAP (0.88) X BAP (4.4) + GA3 (2.8) ** BAP (13.2) *** NAA (16) | Somatic embryogenesis | [111] |
| Seeds | MS | #,## 2,4-D (13.59) or 2,4-D (2.265) *,** BAP (8.88) + NAA (2.68) + Kin (4.65) *** ½ MS + IBA (13.32) | Somatic embryogenesis | [112] |
| Explant | Sterilant Used | Concentration (%) | Duration (Minute) | Reference(s) |
|---|---|---|---|---|
| Nodal segment | Teepol | A few drops | 5 | [16] |
| Bavistin | 1 | 30 | ||
| Mercuric chloride | 0.1 | 6–8 | ||
| Nodal segment | Teepol | 3 | 10 | [19] |
| Streptomycin sulphate | 0.2 | 10 | ||
| Tetracycline hydrochloride | 0.2 | 10 | ||
| Bavistin | 0.2 | 15 | ||
| Mercuric chloride | 0.1 | 3–5 | ||
| Ethyl ethanol | 70 | 1 | ||
| Nodal segment | Mercuric chloride | 0.1 | 7–10 | [91] |
| Cetavelon | 5 | 15 | ||
| Nodal segment | Bavistin | 0.2 | 20 | [93] |
| Tween 20 | A few drops | 20 | ||
| Mercuric chloride | 0.1 | 20 | ||
| Immature inflorescences | Ethyl ethanol | 70 | 1 | [102] |
| Mercuric chloride | 0.1 | 8–15 | ||
| Seeds | Sodium hypochlorite | 4 | 20 | [106] |
| Nodal segment | Bavistin | 0.1 | 20 | [104] |
| Streptomycin sulphate | 0.04 | 20 | ||
| Ethyl ethanol | 70 | 1 | ||
| Mercuric chloride | 0.04 | 6 |
| Tools Used | Achievements | Reference(s) |
|---|---|---|
| Genetic diversity studies | ||
| RAPD, SCAR and multiplex PCR | The analysis and screening for the polymorphism Dendrocalamus bamboos using the SCAR primer was rapid and efficient. | [219] |
| RAPD and AFLP | The first report to provide essential basic genetic information of D. asper. RAPD and AFLP were found to be efficient enough to reveal usable levels of DNA polymorphisms and to investigate genetic information. | [232] |
| RAPD | Using 31 RAPD primers producing 64 polymorphic bands. | [233] |
| Genetic fidelity testing of in vitro-raised D. asper. | ||
| Morphological descriptors | Compared in vitro-raised plants with mother plants and found no variation. | [19] |
| RAPD, ISSR, SSR and AFLP | Tested clonal fidelity of propagated in vitro for over 2 years (at least 30 passages) via enhanced axillary branching with mother plant and found no somaclonal variation. | [234] |
| RAPD and ISSR | DNA samples from in vitro-grown shoots under various stages of subculture, hardened plants growing in the greenhouse, plants growing in the field and the mother plant were subjected for clonal fidelity. No polymorphism was found, confirming true to type nature in vitro raised plants. | [243] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, A.A.; Derise, M.R.; Yong, W.T.L.; Rodrigues, K.F. A Concise Review of Dendrocalamus asper and Related Bamboos: Germplasm Conservation, Propagation and Molecular Biology. Plants 2021, 10, 1897. https://doi.org/10.3390/plants10091897
Mustafa AA, Derise MR, Yong WTL, Rodrigues KF. A Concise Review of Dendrocalamus asper and Related Bamboos: Germplasm Conservation, Propagation and Molecular Biology. Plants. 2021; 10(9):1897. https://doi.org/10.3390/plants10091897
Chicago/Turabian StyleMustafa, Anis Adilah, Mohammad Rahmat Derise, Wilson Thau Lym Yong, and Kenneth Francis Rodrigues. 2021. "A Concise Review of Dendrocalamus asper and Related Bamboos: Germplasm Conservation, Propagation and Molecular Biology" Plants 10, no. 9: 1897. https://doi.org/10.3390/plants10091897
APA StyleMustafa, A. A., Derise, M. R., Yong, W. T. L., & Rodrigues, K. F. (2021). A Concise Review of Dendrocalamus asper and Related Bamboos: Germplasm Conservation, Propagation and Molecular Biology. Plants, 10(9), 1897. https://doi.org/10.3390/plants10091897








