Abstract
Cell biology, plant-based extracts, structural chemistry, and laboratory in vitro or in vivo experiments are the principal aspects or interfaces that can contribute to discovering new possibilities in cancer therapy and to developing improved chemotherapeutics. Forestry residues can be used for their wealthy resource in polyphenols and other phytoconstituents known for anticancer properties. This review is designed to bring together information on the in vitro or in vivo anticancer potential of woody vascular plants especially the bark extracts (BE) and biosynthesized metallic nanoparticles (BMN) using bark extracts. Type of extracts, main phytoconstituents found in extracts responsible for the anticancer activity, and targeted cancerous cell lines were followed. The literature data were collected via Clarivate Analytics, Science Direct, PubMed, and Google Academic (2011–2021). The search terms were: bark extracts, metallic nanoparticles, silver nanoparticles, gold nanoparticles, anticancer, cytotoxic activity, antiproliferative effect, and antimetastatic potential in vitro and in vivo. All of the search terms listed above were used in different combinations. The literature data highlight the efficaciousness of the BE and BMN as anticancer agents in in vitro experiments and showed the mechanism of action and their advantage of nontoxicity on normal cells. In vitro testing has shown promising results of the BE and BMN effect on different cancer cell lines. In vivo testing is lacking and more data is necessary for drug development on animal models.
1. Introduction
Today, cancer, or malignant disease is an alarming situation because of the uncontrolled and abnormal growth of cancer cells. The most undesirable complication of cancer is metastasis and unfortunately this causes death. High mortality in cancer patients is still persistent and the statistics are not very encouraging [1]. Drug therapies are more inefficient due to resistance to chemotherapy and because it destroys the cancer cells along with normal cells [2,3,4]. The ideal anticancer agent should be able to selectively target the cancerous cells and do no harm to the normal cells [5,6].
Cancer is one of the pathological conditions in which apoptosis plays an important role, whether it is abnormal apoptosis or a defective one. That leads to unsuppressed proliferation, which results in cancerous cells that will not naturally die. For that, scientists focused their attention on anticancer strategies for targeting the uncontrollable production of malignant cells and the disorder of apoptosis [7,8]. The collaboration between cell biology, in vitro and in vivo experiments, and structural chemistry will improve and build new anticancer agents [9].
There is a real need to discover new botanical products for developing new anticancer agents. The chemical composition of plants is still poorly studied and this should be the most important source because of their potency in the pharmacological field. Because plants have been traditionally used by humans in healing processes for centuries, and many of them have success that should be an imperative calling for assiduous research in this field. It is necessary to develop new bioactive compounds to meet the need for new drug therapies. For example, Paclitaxel (Taxol) obtained from Taxus brevifolia L., and Vincristine, Vinblastine, and Vinorelbine (Vinca alkaloids) extracted from Catharanthus roseus G. Don should remind researchers of the endless source of plants as anticancer agents [10].
The current tendency in biotechnology is to recycle and use environmentally friendly products. In this context, forest waste is now widely appreciated as a rich source of natural polyphenols, with therapeutic potential [11]. Polyphenols extracted from plant bark are widely studied, mostly in vitro, and are recommended as antioxidants, antimicrobial agents, and anticarcinogens [10]. Thus, in vitro and in vivo experiments are necessary to validate their use for the production of novel anticancerous drugs with low cost and energy [5,12].
Besides classical plant bark extracts (BE), biosynthesized metallic nanoparticles (BMN) are synthesized using ecofriendly and green-chemistry ways. These BMNs present a new interest for nanomedicine, as they are being intensively studied. It was found that BMN are potent antioxidants and antimicrobial and anticancer agents [13].
Cancer chemoprevention implicates the use of pharmacological intervention to prevent, inhibit, or reverse carcinogenesis with synthetic or natural chemical products. Less than one percent of an estimated 250.000 plants has been screened pharmacologically. The use of phytochemicals present in medicinal plants as antioxidants possess no toxicity as compared to modern drugs and are efficient against different types of cancers with no side effects [14,15,16]. This study aims to bring together in vitro and in vivo results of the anticancer potential of BE and BMNs using BE.
2. Research Methodology
The literature data were collected via Clarivate Analytics, Science Direct, PubMed, and Google Academic (2011–2021). The search terms were: bark extracts, metallic nanoparticles, silver nanoparticles, gold nanoparticles, anticancer, cytotoxic activity, antiproliferative effect, and antimetastatic potential in vitro and in vivo. All of the search terms listed above were used in different combinations.
BioRender was used for drawing the figures from manuscripts (https://app.biorender.com/, accessed on 31 August 2021) [17].
3. Anticancer Potential of Bioactive Compounds from Bark Extracts (BE) in Different Type of Suppressed Cancer Cells Lines
The plant kingdom includes a multitude of secondary compounds such as polyphenols, alkaloids, flavonoids, lignans, terpenes, and taxanes. The list continues with minerals, vitamins, oils, and other biomolecules with great anticancer potential. The anticancer potential is due to potent antioxidant activity, inhibition of cancer cell-activating proteins, activating the repair DNA mechanism, or stimulating the formation of protective enzymes of biomolecules present in BE [18].
One of the important sources of bioactive compounds is forestry waste, especially bark [19,20]. Phenolic compounds as phenolic acids, flavonoids, lignans, and stilbenes, can capture free radicals and neutralize them and can prevent the aging process of cells and protect them from carcinogens [21]. There were more than 8000 phenolic compounds present in plants and their antioxidant effect has been observed in many studies [22]. The results suggested that an increased number of phenolic compounds from BE was correlated with their anticancer activity [23]. It is shown in the literature that a combination of two or three polyphenols has a synergistic and more efficacious effect in cancer treatment than using a single one as therapy [24]. One of the combinations used was EGCG (epigallocatechin-3-gallate) and quercetin [25,26] or ECGC and resveratrol against prostate cancer cells [27]. When using similar or complementary bio compounds the anticancer effect is enhanced; the anticancer effect is managed on multiple mechanisms of action [24]. Elansary et al. [28] studied expressly the polyphenolic characterization of Malus baccata and Malus toringoides. The three main polyphenols found in BE were protocatechuic acid, gallic acid, and catechin. They exerted potent anticancer activity against breast cancer, cervical cancer, and Jurkat T cell leukemia cancer cell lines, also having antioxidant and antimicrobial capability. The same authors found in another study that Quercus species showed an increased profile of polyphenol acids such as ellagic acid, gallic acid, protocatechuic acid, vanillic acid, and caffeic acid. These bio compounds showed necrosis and apoptotic effect in cancerous cells after treatment in a dose-dependent manner [29].
The alkaloids showed a beneficial effect on normal cells and cytotoxic activity against cancerous cells. This chemical family is one of the most important biologically active secondary metabolites found in the plant kingdom with multilateral biological activities [30,31]. Gabunamine, gabunine, and tabernamine from Tabernaemontana johnstonii BE exhibited important cytotoxicity against the P-388 mouse lymphocytic leukemia cell culture system [32]. Paclitaxel obtained from BE a few decades ago is part of this family of active anticancer bio compounds and was used in the treatment of ovarian cancer. Over time this isolate compound was approved to be used in other types of cancer [33].
Terpenes are another class of bio compounds in the alkaloid family with anticancer properties. Monoterpenes can act synergistically with polyphenols or alone with great potential in anticancer activity [34]. Over 20.000 terpenes were isolated and tested as anticarcinogens and the most important are lupeol, betulin, betulinic acid, oleanoic acid, etc. [35]. It was found that monoterpenes and sesquiterpenes found in Khaya senegalensis BE exhibited anticancer activity against colorectal and cervical cancer cell lines. Another study reported that terpenes have been introduced already in clinical testing for the treatment of solid tumors [36]. The BE biocompounds summarized in this review showed their potent activity as anticarcinogens. Some phytochemicals already are used in clinical therapy while others are used in clinical trials and some are barely discovered as potent anticarcinogens [37]. The BEs are found to be a valuable source of biologically active compounds with anticancer potential (Table 1).

Table 1.
Type of cancer cells lines suppressed by bioactive compounds from BE.
Being the most pronounced cancer among women, breast cancer is one of the most studied cancer types. MCF-7 and MDA-MB-231 human breast cancer cell lines are often used in experiments. Table 1 shows the variety of BE with potential against breast cancer cell lines. For example, Ydav et al. [56] showed that Saraca indica L. (Fabaceae) alcoholic BE inhibited the proliferation of breast cancer cell lines. Its activity was more prominent in MCF-7 than in MDA-MB-231 cells.
Human lung carcinoma cell migration was inhibited in a dose-dependent manner by an ultrasound-assisted extract from Fagus sylvatica L. (Fagaceae) and Picea abies L. (Pinaceae) bark. In this study, the cytotoxic effect was not observed in the A549 lung carcinoma cell line but a slight modification was observed by increasing the concentration of the samples. In another study methanolic BE of Costus pictus D.Don (Costaceae) remarkably decreased cell viability in A549 lung carcinoma cell line in a dose-dependent manner [46].
PANC-1 was one of the pancreatic cancer cell lines inhibited by the β-caryophyllene compound. This was obtained by Dahham et al. [41] from essential oil from stem BE of Aquilaria crassna. The effect was similar to one of 5-fluorouracil, betulinic acid, and tamoxifen. Dahham et al. [41] showed that β-caryophyllene from the Aquilaria crassna bark had an antiproliferative effect mostly against HCT 116 and HT29 colon cancer cell lines. Because of the effect against colon cancer cell lines, they tested the efficacy of β-caryophyllene on the HCT 116 cell line. The recorded result was that β-caryophyllene can cease colonization with its cytotoxic effect.
4. Biosynthesized Metallic Nanoparticles (BMN) Mediated by Bark Extracts as Anticancer Agents
BEs are increasingly used in metallic nanoparticle biosynthesis as bioactive compounds that have the ability to reduce metallic ions. Because these biomolecules are ecofriendly and safe for human therapeutic use, the low cost makes it suitable for large-scale production over other bioactive products [13,62,63]. BMNs are used for their potential applications as antimicrobial, antioxidant, and anticancer agents [64].
More than 65% of nanoparticles used in general biological applications are silver NPs as earlier studies have shown. That is because they have the smallest dimension and have easy access to permeate the cellular membrane and affect cellular physiology. When the diameter of a nanoparticle decreases the touching surface increases, and that has an explicit influence on their cell entrance and efficacy [63]. Beyond their nanosize, the BMN has another advantage. The biomolecules present in BE are potent stabilizing agents that are attached to the surface of the nanoparticles and also have a cytotoxic effect. That effect is combined with the size advantage and the cytotoxic effect is highlighted [65].
Silver nanoparticles have an important role in therapy for wound healing, not only in skin but also in breast cancer. In this review, the majority of cited studies are on silver NPs and the principally tested cells are breast cancer cell lines. For example, the BE AgNPs from Elaeodendron croceum displayed an IC50 value of 138.8 µg/mL against the MDA-MB-231 breast cancer cell line, comparable with the IC50 value of 80 µg/dL obtained after treatment with Paclitaxel [66].
Another study showed that approximately 82.5% of A549 lung cancer cells were dead after treatment with Toxicodendron vernicifluum (Stokes) F.A. Barkley (Anacardiaceae) AgNPs [67]. Table 2 summarizes the potential anticancer activity of BMN, specifying the type of suppressed cancer cells.

Table 2.
Type of cancer cell lines suppressed by biosynthesized metalic nanoparticles (BMN) mediated by bark extracts.
5. BE and BMNs Mechanism of AntiCancer Action
It has been reported that plant-derived extracts show a selective decrease in the growth activity of cancer cells compared to normal cells [38]. This selective targeting is due to phytochemicals present in plants as flavonoids, phenolic acids, terpenes, tannins, and steroids (Figure 1). These compounds are described as key antioxidant agents. The reasons for selective annihilation of cancerous cells and nontoxicity on normal ones remain yet unknown [46,80]. The inducing antioxidant activity of natural extracts mainly described for redox activity acts as a reducing agent, hydrogen donor, or oxygen scavenger. That might be helpful in preventing or slowing down the evolution of oxidative stress-related disorders as cancer [46,59].
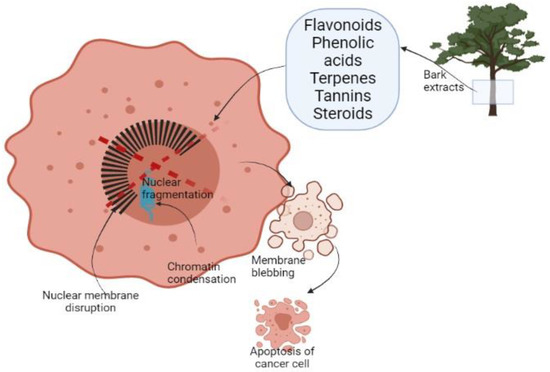
Figure 1.
Schematic representation of cancer cell apoptosis after treatment with BE.
Cell death appears by two alternate modes apoptosis or necrosis. These two ways are different because the first one is programmed managed cell death without a local inflammatory response. Necrosis is characterized as a passive unexpected form of cell death dependent on environmental disturbance with the unrestrained release of inflammatory response. Apoptotic cells go through diverse morphological transformations such as membrane blebbing, chromatin condensation, nuclear membrane disruption, nuclear fragmentation, and dissolution (Figure 1). The first sign of apoptosis is the apoptotic body [81]. Cell apoptosis develops through the mitochondria-mediated intrinsic pathway or death receptor-induced extrinsic pathway. It was observed that BE from Pinus massoniana has a role in both pathways. After the treatment of HeLa cells the expression of Bax, a proapoptotic protein vigorously increased. Cell apoptosis was induced by releasing of cytochrome C [82,83].
The molecular mechanism of BE from Spondias pinnata mediated apoptosis induction was observed in A549 lung carcinoma and MCF-7 breast cancer cell lines. The western blot results showed an increased expression of Bax (proapoptotic) protein and a decreased one for Bcl-2 (antiapoptotic) protein in A549 cells. The same expression was observed after treatment in MCF-7 cells. The only difference was in Bcl-2 expression that was not changed [58]. Alvala et al. [60] found that the BE of Tecomella undulata caused apoptosis on the K562 chronic myeloid leukemia cell line. That was evident after DNA fragmentation assay, increase in FAS (cell surface death receptor), FADD (FAS associated death domain protein) levels, and activation of caspase 8 and 3/7 (a cysteine protease that initiates apoptotic signaling via the extrinsic apoptotic pathway).
Alstoria scholaris and Alstoria venenata BE showed important membrane blebbing, nuclear condensation, and damage, which are manifestations of apoptosis rather than necrosis. The cytotoxic effect of BE on DLA (Dalton`s ascitic lymphoma) cells and their cytoprotective effect on normal ones may be due to their potent antioxidant activity [39].
Nayak et al. [65] studied the mechanism of silver nanoparticles obtained from the bark extracts of Azadirachta indica on the MG-63 osteosarcoma cell line after 24 h of treatment. They observed the formation of granulations in the nucleus, which is a measure of chromatin condensation. That reaction is well documented in nanoparticle treated samples before. The cytotoxic activity of metallic nanoparticles is due to their small size and affinity for the acidic pH of the cancer cells [84]. It is known that a characteristic of apoptosis is the loss of mitochondria membrane integrity and potential (Figure 2). The fluorescence signal decreases when the mitochondria lose their membrane potential and the absorption of rhodamine 123 by the affected cells is low. β-caryophyllene from Aquilaria crassna essential oil BE had a potent capacity in apoptosis-inducing. The fluorescence signal decreased in treated cancer cells PANC-1 pancreatic cancer, HCT-116, and HT29 colorectal cancer. The untreated cells showed a substantial fluorescence intensity. These results present the unaffected state of normal cells [40]. In the same way, another study revealed that β-caryophyllene oxide produces apoptosis. They found that apoptosis is produced through suppression of PI3K/AKT/mTOR/S6K1 pathways and also following ROS-mediated MAPKs activation. The first signaling pathway is not only conducting toward apoptosis but is closely related to angiogenesis and the second pathway ROS-mediated mitogen-activated protein kinases activation regulates diverse cellular actions as proliferation, motility, and survival [85].
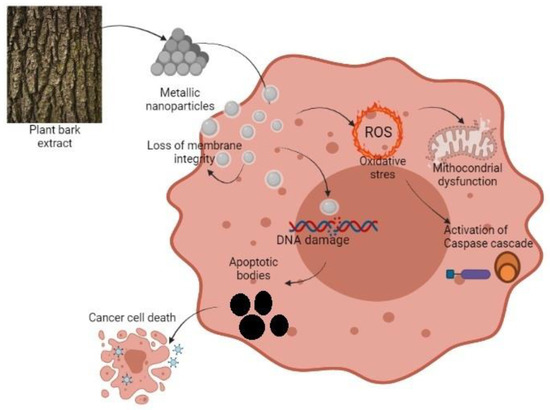
Figure 2.
Schematic representation of BMN action against a cancer cell.
In another study [86], the effect of BE from Acanthopanax sessiliflorus on MDA-MB-231 and MCF-7 breast cancer cell lines was shown. The intracellular ROS (reactive oxygen species) levels were determined by inspecting the fluorescent intensity of a redox reagent. It was observed that the BE elevated the proportion of green fluorescence in both cell lines dose-dependently. That suggests that the studied BE stimulates the production of intracellular ROS after the selective interruption of the mitochondrial respiratory chain. This is evidence of apoptosis with the involvement of mitochondrial membrane depolarization. In general, ROS contributed to cell death inducing the cell membrane lipid oxidation and the expression of genes associated with DNA disruption, which promotes DNA damage in apoptosis or necrosis at levels above the cellular antioxidant defense systems [8,86].
Another way for cancer cell extinction is interrupting the cell cycle. After the analysis of cellular cycles, BE from Euphorbia umbellata, showed that chloroform fraction promotes cell cycle arrest at G0/G1 phase. Moreover, treatment with this potent extract showed a decreased percentage of cells in S-phase [48]. Another study conducted by Gonzalez-Sarrias et al. [38] revealed that proliferation of colon cancer cells can be inhibited by the compounds present in the maple BE, by arresting the advancement of the cell cycle at the S-phase. In addition, cancer cells own an extra survival mechanism such as colonization, migration, tumor angiogenesis, cell adhesion, epithelial-mesenchymal transition, and transendothelial migration known as a metastatic cascade. The suppression of colonization and migration of HCT 116 colon cancer cells was demonstrated by β-caryophyllene. It also had the ability to prevent metastatic propagation of malignant cells and to suspend cell invasion, the main characteristic of metastatic cascade [41,87,88].
Toxicity on different normal cells was realized to observe the behavior of the BE. For example, cytotoxicity of the Toxicodendron vernicifluum BE was studied. The results indicated that NIH3T3 mouse embryonic fibroblasts growth was not significantly reduced and cell death was not observed at different concentrations of the extract [67].
The β-caryophyllene found in essential oil from Aquilaria crassna bark exhibited low toxicity against 3T3-L1 and RGC-5 normal cell lines. The results showed that even the higher concentration of β-caryophyllene did not affect the morphology of a normal cell line (3T3-L1) [41]. In the case of the HaCaT human keratinocytes normal cell line, the BE from Fagus sylvatica and Picea abies did not show cytotoxic activity. Instead, BE stimulated normal cells’ proliferation [49].
The in vivo toxicity of AgNPs bark extract of Elaedendron croceum was assessed on male Wister rats. The authors revealed that there was no mortality or any toxic reaction after the acute oral test. There was no toxicity observed in the heart, liver, and kidney after the morphological investigation [66]. Another efficacy advantage is that during chemical reactions in the synthesis of nanoparticles, some harmful reactants from BE are involved in capping and reducing the AgNPs. Therefore, the cytotoxic effect of AgNPs on normal cells is reduced [66]. In addition, it was observed in the literature that NPs with extremely small diameters appear to have a higher cytotoxic effect in normal cells due to swift internalization than NPs with higher diameter [89].
Accordingly, with data shown in studies, the tests effectuated on normal cells with BE and BEM exhibited no harmful or toxic effects.
6. Conclusions and Future Perspectives
Cancer has become a front line between resistance to chemotherapy, aggressive invasion, secondary toxic effects, and scientists who struggle to find a better chemical bioactive extract to maintain homeostasis, to avoid the unwanted secondary toxic effects, and to prevent metastatic cascade. BEs and BMNs are a future promise source of antioxidant compounds, which can fight against cancerous cells. All standardized extracts, shown in the listed studies above, had an impressive antioxidant capacity that was linked with anticancer ability. For maximal certainty, researchers tested in vitro the BEs and BMNs cytotoxic effect on cancer cells and normal cells. Unfortunately, in vivo studies for the anticancer potential of BEs are few. The results are encouraging and in vivo experiments should be supported. BMNs showed also antioxidant potency with great in vitro cytotoxic effect on cancer cells but no in vivo experiment was found. The toxicity on normal cells was shown as harmless. This is a promising result for the application of BEs and BMNs on animal subjects and bypasses the secondary undesirable effect of chemotherapy. There remains a need for in vivo studies to confirm the anticancer action of BEs and BMNs.
Author Contributions
Conceptualization, C.T.; methodology, C.T.; writing—original draft preparation, E.B.; writing—review and editing, C.T.; supervision, C.T.; project administration, C.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Romanian Ministry of Education and Research, CNS-UEFISCDI, project number PN-III-P1-1.1-TE-2019-1549, within PNCDI III.
Acknowledgments
This work was supported by a grant from the Romanian Ministry of Education and Research, CNS-UEFISCDI, project number PN-III-P1-1.1-TE-2019-1549, within PNCDI III.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R.; Thabrew, I.; De Silva, E.D. A study of the potential anticancer activity of Mangifera zeylanica bark: Evaluation of cytotoxic and apoptotic effects of the hexane extract and bioassay-guided fractionation to identify phytochemical constituents. Oncol. Lett. 2016, 11, 1335–1344. [Google Scholar] [CrossRef]
- Chanda, S.; Nagani, K. In Vitro and in Vivo Methods for Anticancer Activity Evaluation and Some Indian Medicinal Plants Possessing Anticancer Properties: An Overview. J. Pharm. Phytochem. 2013, 2, 140–152. [Google Scholar]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Fock, K.M. Review article: The epidemiology and prevention of gastric cancer. Aliment. Pharmacol. Ther. 2014, 40, 250–260. [Google Scholar] [CrossRef]
- Alawode, T. An Overview of the Anticancer Properties of Some Plants Used in Traditional Medicine in Nigeria. Int. Res. J. Biochem. Bioinform. 2013, 3, 7–14. [Google Scholar]
- Mishra, T.; Arya, R.K.; Meena, S.; Joshi, P.; Pal, M.; Meena, B.; Upreti, D.K.; Rana, T.S.; Datta, D. Isolation, Characterization and Anticancer Potential of Cytotoxic Triterpenes from Betula utilis Bark. PLoS ONE 2016, 11, e0159430. [Google Scholar] [CrossRef]
- De Wilt, L.H.; Jansen, G.; Assaraf, Y.G.; van Meerloo, J.; Cloos, J.; Schimmer, A.; Chan, E.T.; Kirk, C.J.; Peters, G.J.; Kruyt, F.A. Proteasome-based mechanisms of intrinsic and acquired bortezomib resistance in non-small cell lung cancer. Biochem. Pharmacol. 2012, 83, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Thamizhiniyan, V.; Young-Woong, C.; Young-Kyoon, K. The cytotoxic nature of Acanthopanax sessiliflorus stem bark extracts in human breast cancer cells. Saudi J. Biol. Sci. 2015, 22, 752–759. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism; IntechOpen: London, UK, 2019. [Google Scholar]
- Greenwell, M.; Rahman, P. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Jerez, M.; Selga, A.; Sineiro, J.; Torres, J.L.; Núñez, M.J. A comparison between bark extracts from Pinus pinaster and Pinus radiata: Antioxidant activity and procyanidin composition. Food Chem. 2007, 100, 439–444. [Google Scholar] [CrossRef]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.; Song, D.; et al. A systematic review on ethnomedicines of anti-cancer plants. Phytother. Res. 2017, 31, 202–264. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Kim, G.-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A.; Aboul-Enein, A.M.; Aboul-Enein, K.M.; Fujita, K. Willow Leaves’ Extracts Contain Anti-Tumor Agents Effective against Three Cell Types. PLoS ONE 2007, 2, e178. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Muhammad, B.; Thapa, P.; Shrestha, B.G. Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. BMC Complement. Altern. Med. 2017, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Prema, R.; Sekar, S.D.; Chandra Sekhar, K.B. Review On: Herbs as Anticancer Agents. Int. J. Pharma Ind. Res. 2011, 1, 105. [Google Scholar]
- BioRender. Available online: https://App.Biorender.Com/ (accessed on 31 August 2021).
- Iqbal, J.; Abbasi, B.H.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crop. Prod. 2015, 75, 803–809. [Google Scholar] [CrossRef]
- Kasote, D.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-C.; Wu, J.M. Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals EGCG, genistein and quercetin. Anticancer. Res. 2009, 29, 4025–4032. [Google Scholar] [PubMed]
- Tang, S.-N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5, 14. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Harris, N.H.; Johnson, A.D.; Lindvall, H.C.; Wang, G.; Ahmed, K. Protein kinase CK2 modulates apoptosis induced by resveratrol and epigallocatechin-3-gallate in prostate cancer cells. Mol. Cancer Ther. 2007, 6, 1006–1012. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; El-Ansary, D.O.; Ekiert, H.; Al-Mana, F.A. Malus baccata var. gracilis and Malus toringoides Bark Polyphenol Studies and Antioxidant, Antimicrobial and Anticancer Activities. Processes 2020, 8, 283. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; El-Abedin, T.K.Z.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Lu, J.-J.; Bao, J.-L.; Chen, X.-P.; Huang, M.; Wang, Y.-T. Alkaloids Isolated from Natural Herbs as the Anticancer Agents. Evid. Based Complement. Altern. Med. 2012, 2012, 485042. [Google Scholar] [CrossRef]
- Boyer, N.C.; Morrison, K.C.; Kim, J.; Hergenrother, P.J.; Movassaghi, M. Synthesis and anticancer activity of epipolythiodiketopiperazine alkaloids. Chem. Sci. 2013, 4, 1646–1657. [Google Scholar] [CrossRef]
- Kingston, D.G.I.; Gerhart, B.B.; Ionescu, F.; Mangino, M.M.; Sami, S.M. Plant Anticancer Agents V: New Bisindole Alkaloids from Tabernaemontana johnstonii Stem Bark. J. Pharm. Sci. 1978, 67, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Anticancer alkaloids from trees: Development into drugs. Pharmacogn. Rev. 2016, 10, 90–99. [Google Scholar] [CrossRef]
- Jaafari, A.; Tilaoui, M.; Mouse, H.A.; M’Bark, L.A.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Rev. Bras. Farm. 2012, 22, 534–540. [Google Scholar] [CrossRef]
- Lombrea, A.; Scurtu, A.; Avram, S.; Pavel, I.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.; Soica, C.; Danciu, C. Anticancer Potential of Betulonic Acid Derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zhou, S.-Y.; Qian, Z.-Z.; Zhang, H.-L.; Qiu, L.-H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.-S.; Wang, H.-Q. Evaluation of toxicity and single-dose pharmacokinetics of intravenous ursolic acid liposomes in healthy adult volunteers and patients with advanced solid tumors. Expert Opin. Drug Metab. Toxicol. 2012, 9, 117–125. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Şoica, C.; Ledeţi, I.; Aluaş, M.; Zupko, I.; Gǎluşcan, A.; Cinta-Pinzaru, S.; Munteanu, M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem. Cent. J. 2012, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Li, L.; Seeram, N.P. Effects of Maple (Acer) Plant Part Extracts on Proliferation, Apoptosis and Cell Cycle Arrest of Human Tumorigenic and Non-tumorigenic Colon Cells. Phytother. Res. 2011, 26, 995–1002. [Google Scholar] [CrossRef]
- Bagheri, G.; Mirzaei, M.; Mehrabi, R.; Sharifi-Rad, J. Cytotoxic and Antioxidant Activities of Alstonia scholaris, Alstonia venenata and Moringa oleifera Plants from India. Jundishapur J. Nat. Pharm. Prod. 2016, 11, 31129. [Google Scholar] [CrossRef]
- Dolai, N.; Karmakar, I.; Kumar, R.S.; Kar, B.; Bala, A.; Haldar, P.K. Evaluation of antitumor activity and in vivo antioxidant status of Anthocephalus cadamba on Ehrlich ascites carcinoma treated mice. J. Ethnopharmacol. 2012, 142, 865–870. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Hassan, L.E.A.; Dahham, S.S.; Saghir, S.; Mohammed, A.M.A.; Eltayeb, N.M.; Majid, A.M.S.A.; Majid, A.S.A. Chemotherapeutic potentials of the stem bark of Balanite aegyptiaca (L.) Delile: An antiangiogenic, antitumor and antioxidant agent. BMC Complement. Altern. Med. 2016, 16, 396. [Google Scholar] [CrossRef] [PubMed]
- Basri, D.F.; Alamin, Z.A.Z.; Chan, K.M. Assessment of cytotoxicity and genotoxicity of stem bark extracts from Canarium odontophyllum Miq. (dabai) against HCT 116 human colorectal cancer cell line. BMC Complement. Altern. Med. 2015, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; El-Abedin, T.K.A.Z.; Mattar, M.A.; Ekiert, H. Phenolic Compounds of Catalpa speciosa, Taxus cuspidate, and Magnolia acuminata have Antioxidant and Anticancer Activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef]
- Badrinarayanan, V.; Anand, V.; Karpagam, T.; Bai, J.S.; Manikandan, R. In Vitro Antimicrobial and Anticancer Activity of Cinnamomum Zeylanicum Linn Bark Extracts. Int. J. Pharm. Pharmaceut. Sci. 2014, 6, 12–18. [Google Scholar]
- Sathuvan, M.; Vignesh, A.; Thangam, R.; Palani, P.; Rengasamy, R.; Murugesan, K. In Vitro Antioxidant and Anticancer potential of Bark of Costus pictus D.DON. Asian Pac. J. Trop. Biomed. 2012, 2, S741–S749. [Google Scholar] [CrossRef]
- Baloglu, M.C.; Llorent-Martínez, E.J.; Aumeeruddy, M.Z.; Mahomoodally, M.F.; Altunoglu, Y.C.; Ustaoglu, B.; Ocal, M.; Gürel, S.; Bene, K.; Sinan, K.I.; et al. Multidirectional insights on Chrysophyllum perpulchrum leaves and stem bark extracts: HPLC-ESI-MSn profiles, antioxidant, enzyme inhibitory, antimicrobial and cytotoxic properties. Ind. Crop. Prod. 2019, 134, 33–42. [Google Scholar] [CrossRef]
- Kanunfre, C.C.; Leffers, T.; Cruz, L.S.; Luz, L.E.; Crisma, A.R.; Wang, M.; Avula, B.; Khan, I.A.; Beltrame, F.L. Euphorbia umbellata bark extracts—an in vitro cytotoxic study. Rev. Bras. Farm. 2016, 27, 206–213. [Google Scholar] [CrossRef]
- Coșarcă, S.-L.; Moacă, E.-A.; Tanase, C.; Muntean, D.L.; Pavel, I.Z.; Dehelean, C.A. Spruce and beech bark aqueous extracts: Source of polyphenols, tannins and antioxidants correlated to in vitro antitumor potential on two different cell lines. Wood Sci. Technol. 2019, 53, 313–333. [Google Scholar] [CrossRef]
- Yessoufou, K.; Elansary, H.O.; Mahmoud, E.A.; Skalicka-Woźniak, K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind. Crop. Prod. 2015, 74, 752–758. [Google Scholar] [CrossRef]
- Prasanthi, D.; Adikay, S. Pharmacognostic Studies and Nephroprotective Potential of Hydroalcoholic Extract of Trichosanthes cucumerina in Acute Renal Failure. Pharmacogn. J. 2017, 9, 176–184. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; AlBalawi, S.M.; Athar, T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.-W. Cytotoxic effects of stem bark extracts and pure compounds from Margaritaria discoidea on human ovarian cancer cell lines. Phytomedicine 2015, 22, 1–4. [Google Scholar] [CrossRef]
- Moirangthem, D.S.; Talukdar, N.C.; Bora, U.; Kasoju, N.; Das, R.K. Differential effects of Oroxylum indicum bark extracts: Antioxidant, antimicrobial, cytotoxic and apoptotic study. Cytotechnology 2012, 65, 83–95. [Google Scholar] [CrossRef]
- Wu, D.-C.; Li, S.; Yang, D.-Q.; Cui, Y.-Y. Effects of Pinus massoniana bark extract on the adhesion and migration capabilities of HeLa cells. Fitoterapia 2011, 82, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.K.; Saini, K.S.; Hossain, Z.; Omer, A.; Sharma, C.; Gayen, J.R.; Singh, P.; Arya, K.R.; Singh, R.K. Saraca indicaBark Extract ShowsIn VitroAntioxidant, Antibreast Cancer Activity and Does Not Exhibit Toxicological Effects. Oxidative Med. Cell. Longev. 2015, 2015, 205360. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Jain, S.K. Screening of in vitro cytotoxic activity of some medicinal plants used traditionally to treat cancer in Chhattisgarh state, India. Asian Pac. J. Trop. Biomed. 2011, 1, S147–S150. [Google Scholar] [CrossRef]
- Ghate, N.B.; Hazra, B.; Sarkar, R.; Mandal, N. In vitro anticancer activity of Spondias pinnata bark on human lung and breast carcinoma. Cytotechnology 2013, 66, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Baldivia, D.D.S.; Leite, D.F.; De Castro, D.T.H.; Campos, J.F.; Dos Santos, U.P.; Paredes-Gamero, E.J.; Carollo, C.A.; Silva, D.B.; Souza, K.D.P.; Dos Santos, E.L. Evaluation of In Vitro Antioxidant and Anticancer Properties of the Aqueous Extract from the Stem Bark of Stryphnodendron adstringens. Int. J. Mol. Sci. 2018, 19, 2432. [Google Scholar] [CrossRef]
- Ravi, A.; Mallika, A.; Sama, V.; Begum, A.S.; Khan, R.S.; Reddy, B.M. Antiproliferative activity and standardization of Tecomella undulata bark extract on K562 cells. J. Ethnopharmacol. 2011, 137, 1353–1359. [Google Scholar] [CrossRef]
- Baharum, Z.; Akim, A.M.; Taufiq-Yap, Y.H.; Hamid, R.A.; Kasran, R. In Vitro Antioxidant and Antiproliferative Activities of Methanolic Plant Part Extracts of Theobroma cacao. Molecules 2014, 19, 18317–18331. [Google Scholar] [CrossRef]
- Nagati, V.; Koyyati, R.; Donda, M.; Alwala, J.; Kudle, K. Green Synthesis and Characterization of Silver Nanoparticles from Cajanus Cajanleaf Extract and Its Antibacterial Activity. Int. J. Nanomater. Biostruct. 2012, 2, 39–43. [Google Scholar]
- Mousavi, B.; Tafvizi, F.; Bostanabad, S.Z. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). Artif. Cells Nanomed. Biotechnol. 2018, 46, 499–510. [Google Scholar] [CrossRef]
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S.-I.; Kalaichelvan, P.T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crop. Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Kumari, M.; Nayak, B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 2016, 58, 44–52. [Google Scholar] [CrossRef]
- Odeyemi, S.W.; De La Mare, J.; Edkins, A.L.; Afolayan, A.J. In vitro and in vivo toxicity assessment of biologically synthesized silver nanoparticles from Elaeodendron croceum. J. Complement. Integr. Med. 2019, 16, 20180184. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; MubarakAli, D.; Oh, D.-H.; Kathiresan, K.; Wang, M.-H. Unveiling the potentials of biocompatible silver nanoparticles on human lung carcinoma A549 cells and Helicobacter pylori. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Khan, S.A.; Bello, B.A.; Khan, J.A.; Anwar, Y.; Mirza, M.B.; Qadri, F.; Farooq, A.; Adam, I.K.; Asiri, A.; Khan, S.B. Albizia chevalier based Ag nanoparticles: Anti-proliferation, bactericidal and pollutants degradation performance. J. Photochem. Photobiol. B Biol. 2018, 182, 62–70. [Google Scholar] [CrossRef]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 2018, 14, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Kanjikar, A.P.; Hugar, A.L.; Londonkar, R.L. Characterization of phyto-nanoparticles from Ficus krishnae for their antibacterial and anticancer activities. Drug Dev. Ind. Pharm. 2018, 44, 377–384. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, C. Biofabrication of silver nanoparticles and their combined effect with low intensity ultrasound for treatment of lung cancer. J. Photochem. Photobiol. B Biol. 2018, 181, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surfaces B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Barai, A.C.; Paul, K.; Dey, A.; Manna, S.; Roy, S.; Bag, B.G.; Mukhopadhyay, C. Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.; Kumari, R.M.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Green synthesis of silver nanoparticles using Prosopis juliflora bark extract: Reaction optimization, antimicrobial and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Koshy, E.P.; Mathew, B. Green synthesis of Stereospermum suaveolens capped silver and gold nanoparticles and assessment of their innate antioxidant, antimicrobial and antiproliferative activities. Bioprocess Biosyst. Eng. 2018, 41, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Yugandhar, P.; Vasavi, T.; Devi, P.U.M.; Savithramma, N. Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: Characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl. Nanosci. 2017, 7, 417–427. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L.; Vishwanatha, U.; Ravishankar, B.; Gururaj, H.; Niranjana, P.; Hungund, B.S. Phytochemically Functionalized Cu and Ag Nanoparticles Embedded in MWCNTs for Enhanced Antimicrobial and Anticancer Properties. Nano-Micro Lett. 2015, 8, 120–130. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sharma, J.R.; Sibuyi, N.R.S.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of Biogenic Gold Nanoparticles from Terminalia mantaly Extracts and the Evaluation of Their In Vitro Cytotoxic Effects in Cancer Cells. Molecules 2020, 25, 4469. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Deore, S.; Dhamecha, D.; Jagwani, S.; Jalalpure, S.; Bohara, R. Phytosynthesis of Silver Nanoparticles: Characterization, Biocompatibility Studies, and Anticancer Activity. ACS Biomater. Sci. Eng. 2018, 4, 892–899. [Google Scholar] [CrossRef]
- Bhattacharya, S. Anticarcinogenic Property of Medicinal Plants: Involvement of Antioxidant Role; Research Signpost: Kerala, India, 2012; pp. 83–96. [Google Scholar]
- Bold, R.J.; Termuhlen, P.M.; McConkey, D.J. Apoptosis, cancer and cancer therapy. Surg. Oncol. 1997, 6, 133–142. [Google Scholar] [CrossRef]
- Bellucci, M.; Agostini, F.; Masin, M.; Tartaglia, G.G. Predicting protein associations with long noncoding RNAs. Nat. Methods 2011, 8, 444–445. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, X.-L.; Li, Y.-Y.; Cui, Y.-Y.; Chen, Y.-H. Pinus massoniana Bark Extract: Structure–Activity Relationship and Biomedical Potentials. Am. J. Chin. Med. 2016, 44, 1559–1577. [Google Scholar] [CrossRef]
- Burlacu, E.; Tanase, C.; Coman, N.-A.; Berta, L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules 2019, 24, 4354. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, J.D.; Subramanian, T.; Liu, K.; King, M.R. Rafting Down the Metastatic Cascade: The Role of Lipid Rafts in Cancer Metastasis, Cell Death, and Clinical Outcomes. Cancer Res. 2021, 81, 5–17. [Google Scholar] [CrossRef]
- Hocman, G. Chemoprevention of cancer: Phenolic antioxidants (BHT, BHA). Int. J. Biochem. 1988, 20, 639–651. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).