Precipitation Mediates the Distribution but Not the Taxonomic Composition of Phytoplankton Communities in a Tributary of Three Gorges Reservoir
Abstract
:1. Introduction
2. Materials and Methods
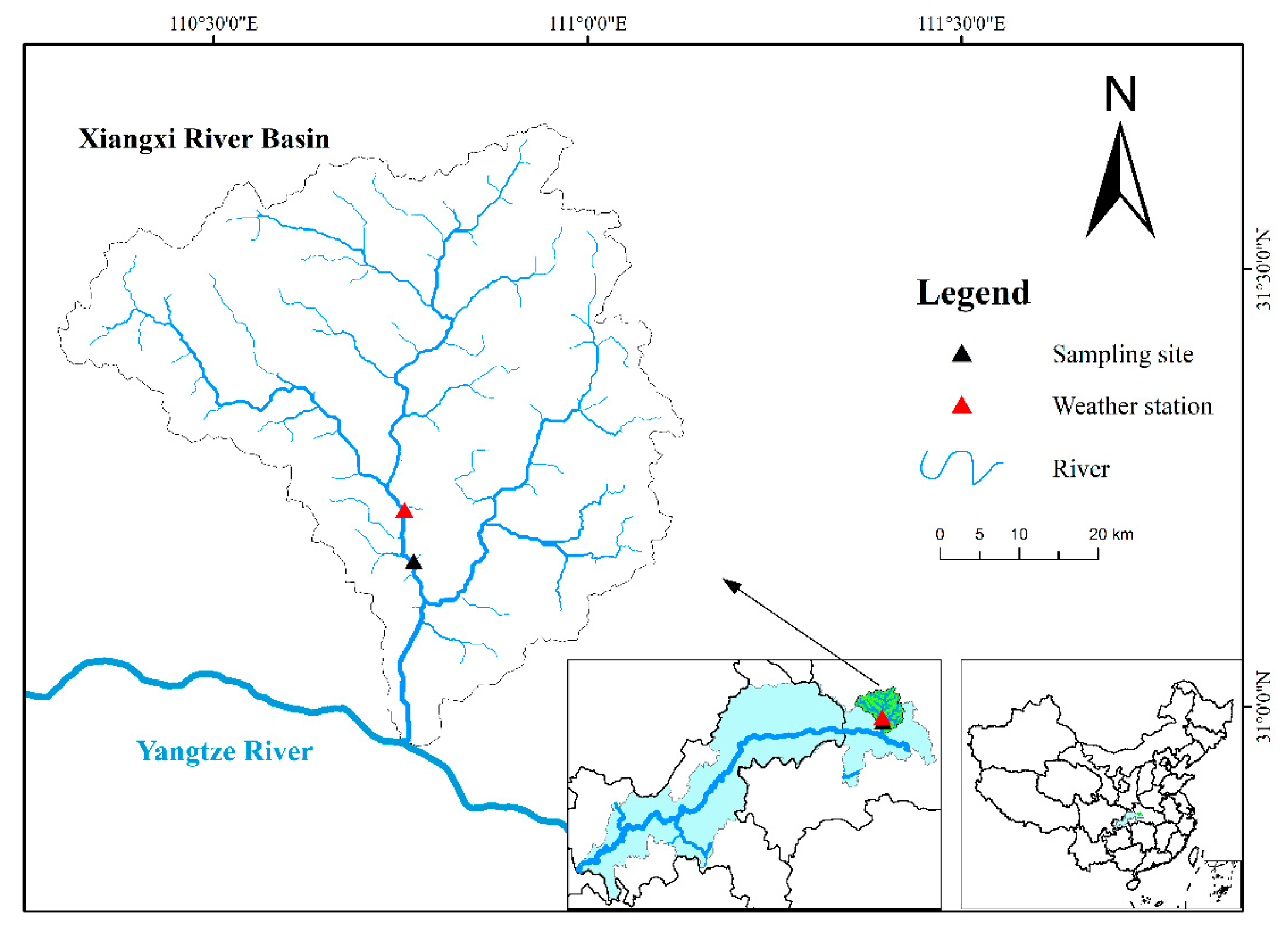
2.1. Sampling Site and Sampling Methods
2.2. Data Analysis
2.3. Statistical Analysis
3. Results
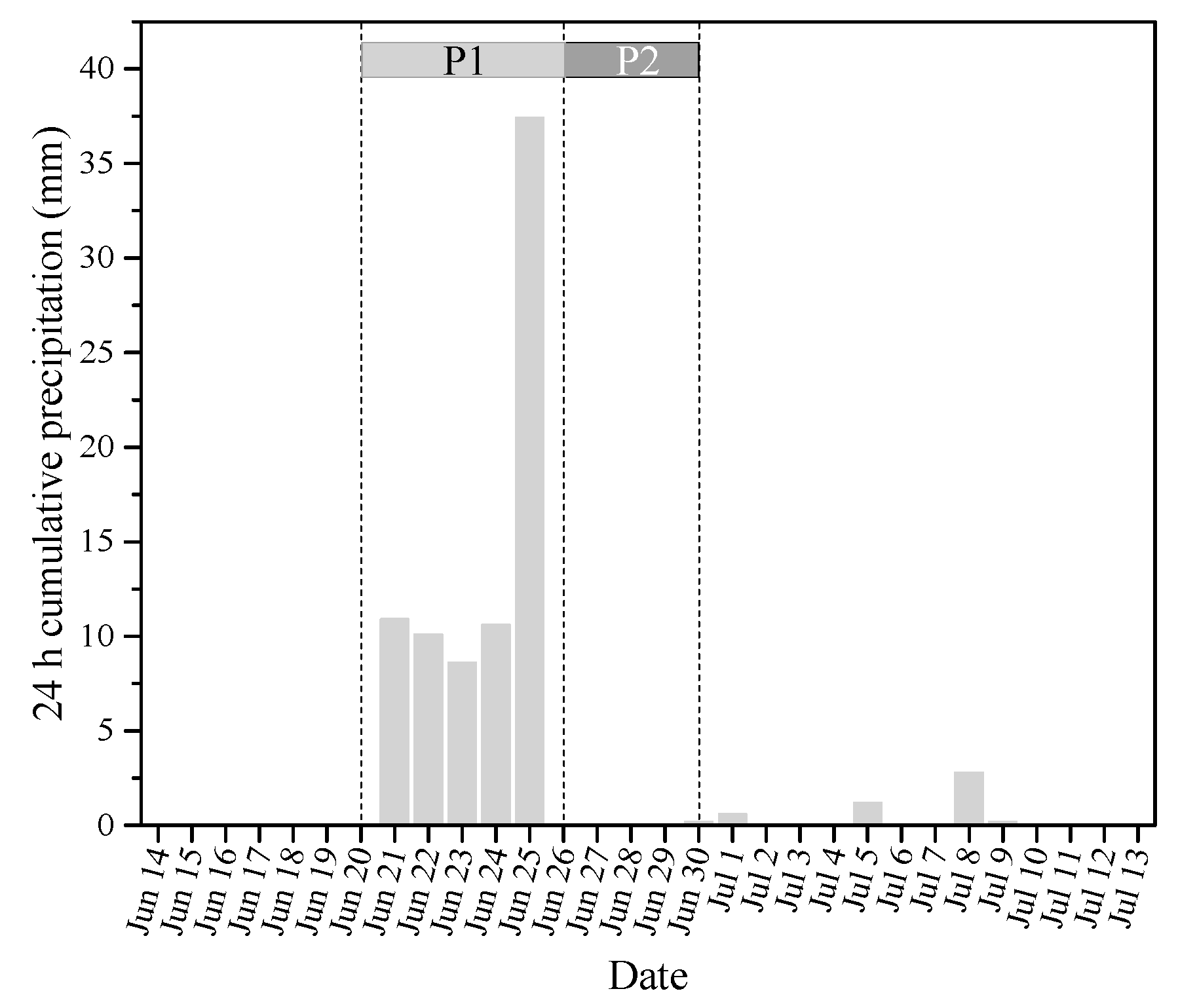
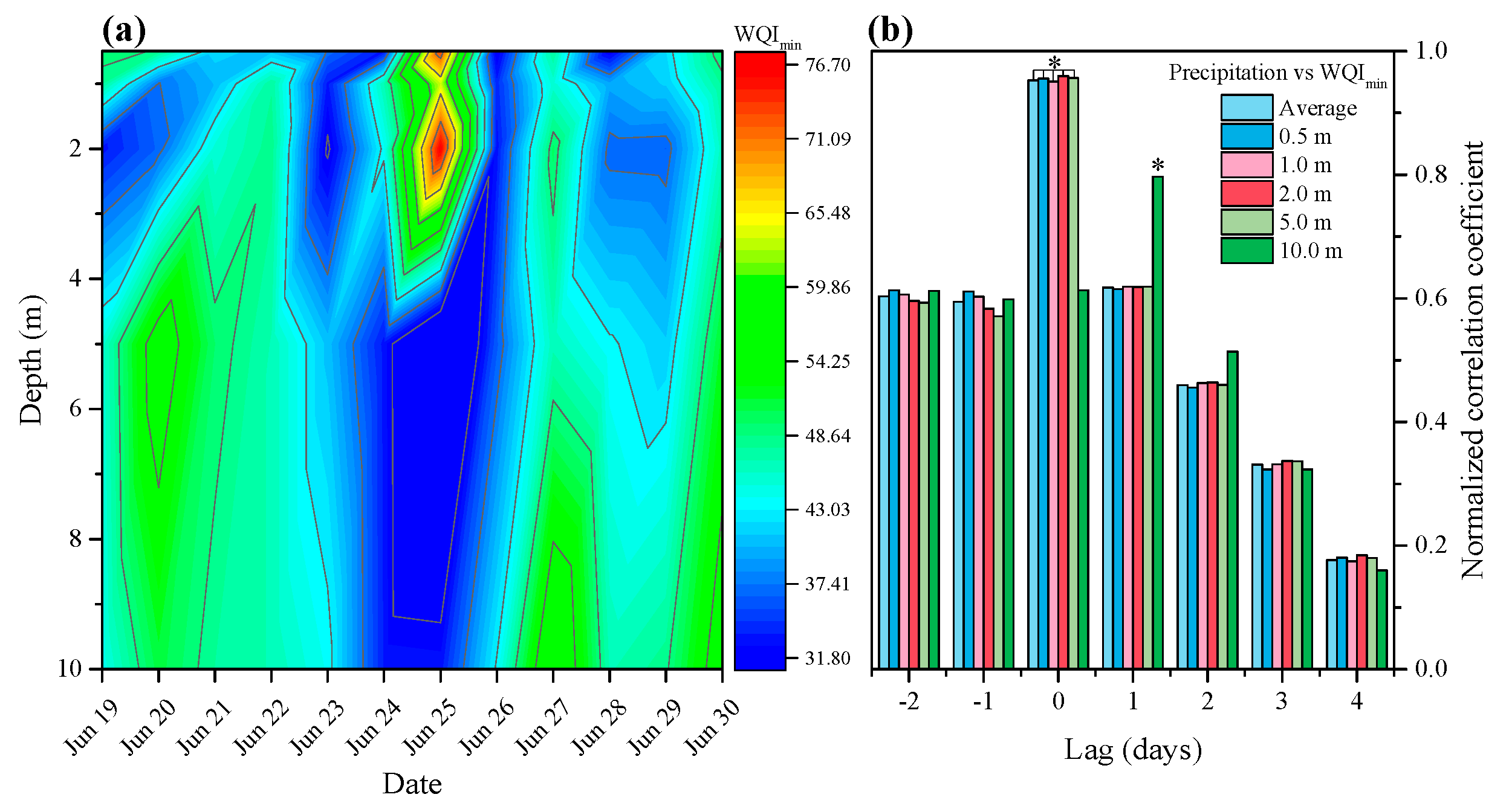
3.1. Effects of Precipitation on Water Quality
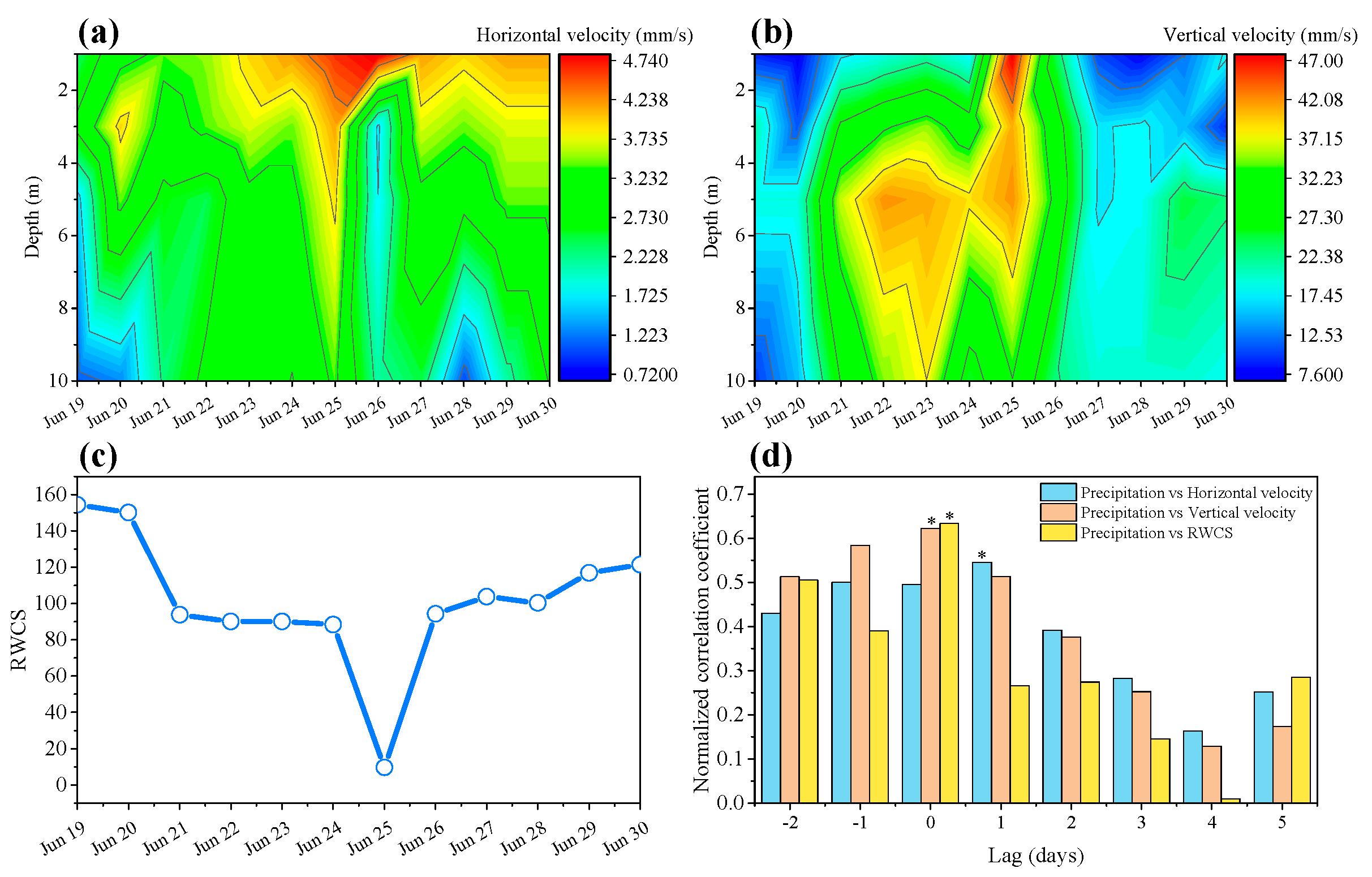
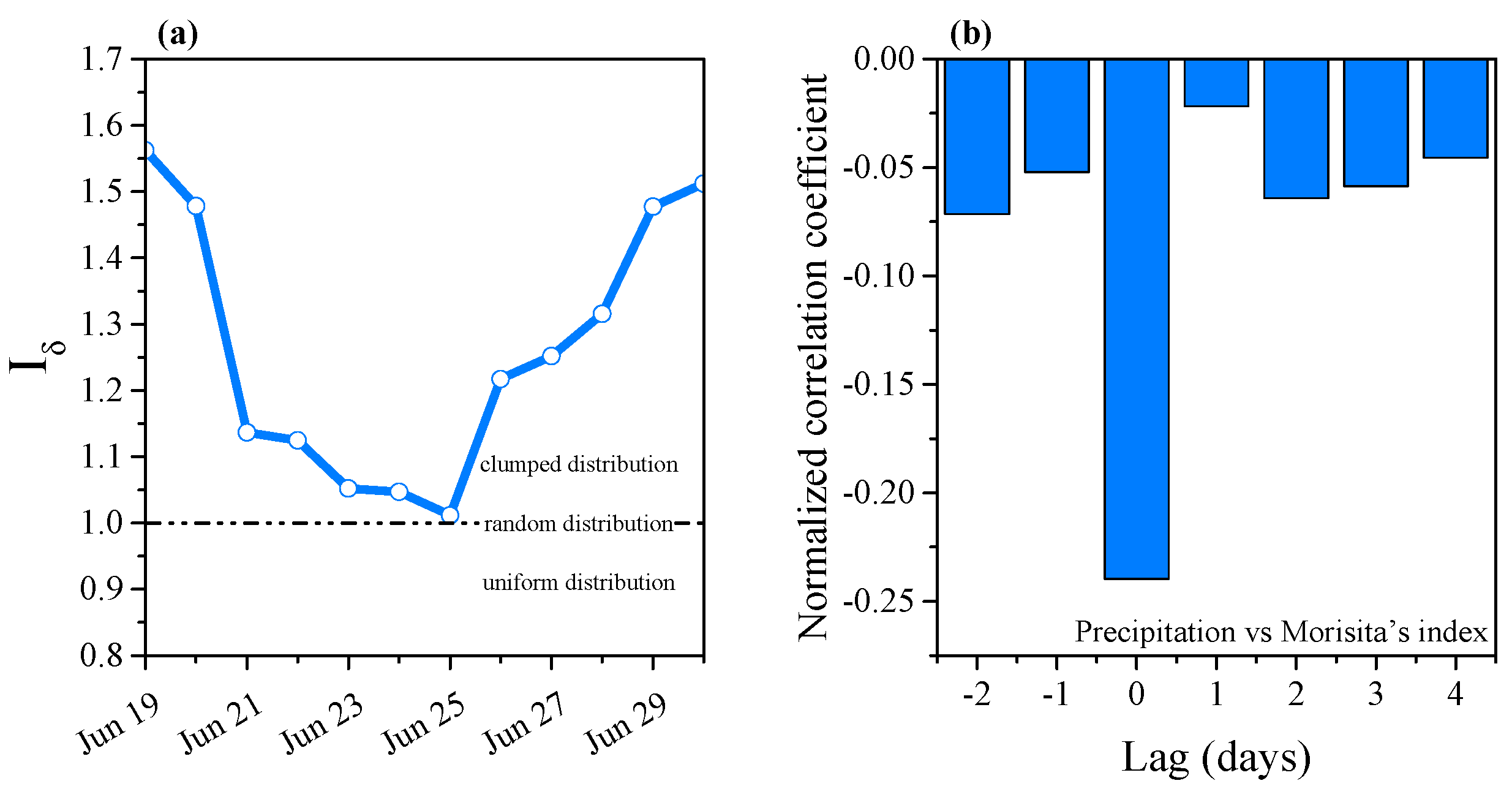
3.2. Effects of Precipitation on Hydrodynamics
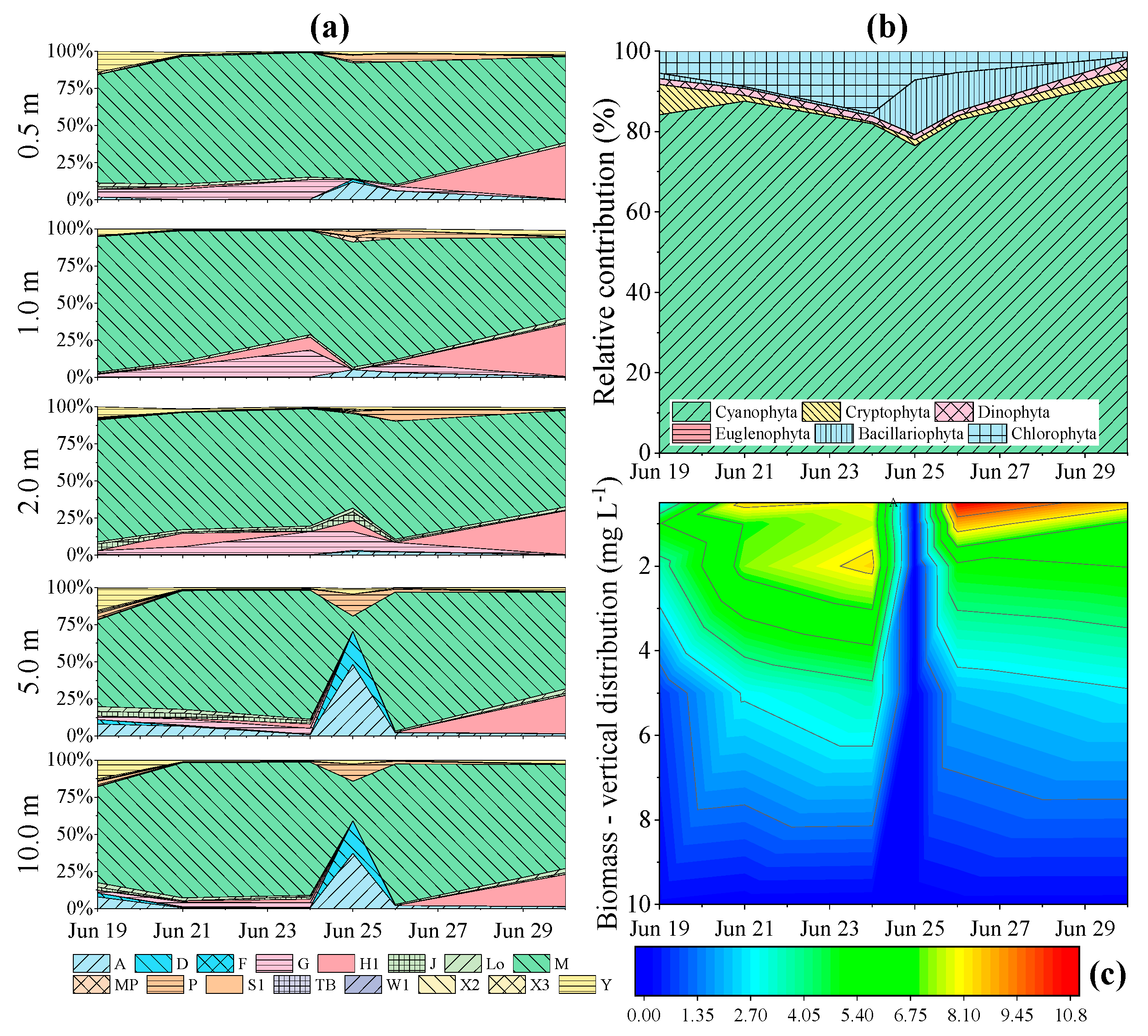
3.3. Phytoplankton Assemblage Dynamics
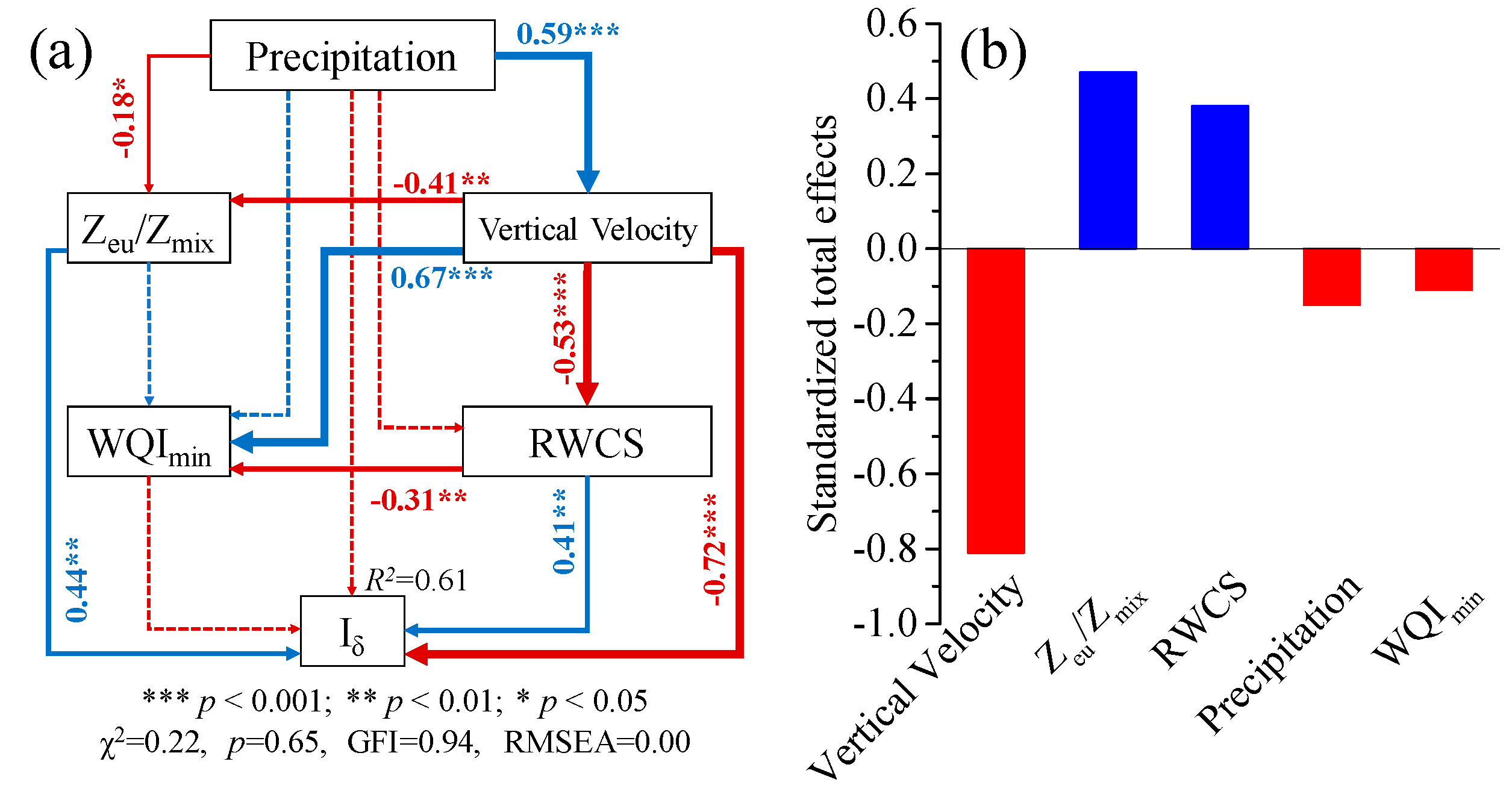
3.4. Structural Equation Model (SEM)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, N.W.; Mo, Q.L.; Kuo, Y.M.; Su, Y.P.; Zhong, Y.P. Hydrochemical controls on reservoir nutrient and phytoplankton dynamics under storms. Sci. Total Environ. 2018, 619, 301–310. [Google Scholar] [CrossRef]
- Havens, K.E.; Ji, G.; Beaver, J.R.; Fulton, R.S.; Teacher, C.E. Dynamics of cyanobacteria blooms are linked to the hydrology of shallow Florida lakes and provide insight into possible impacts of climate change. Hydrobiologia 2017, 829, 1–17. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Wu, J.T. Phytoplankton dynamics of a subtropical reservoir controlled by the complex interplay among hydrological, abiotic, and biotic variables. Environ. Monit. Assess. 2016, 188, 689. [Google Scholar] [CrossRef] [PubMed]
- Cyr, H. Winds and the distribution of nearshore phytoplankton in a stratified lake. Water Res. 2017, 122, 114–127. [Google Scholar] [CrossRef]
- Vidal, J.; Rigosi, A.; Hoyer, A.; Escot, C.; Rueda, F.J. Spatial distribution of phytoplankton cells in small elongated lakes subject to weak diurnal wind forcing. Aquat. Sci. 2014, 76, 83–99. [Google Scholar] [CrossRef]
- Yang, J.R.; Lv, H.; Isabwe, A.; Liu, L.M.; Yu, X.Q.; Chen, H.H.; Yang, J. Disturbance-induced phytoplankton regime shifts and recovery of cyanobacteria dominance in two subtropical reservoirs. Water Res. 2017, 120, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Vidal, J.; Casamitjana, X.; Soler, M.; Colomer, J. The role of surface vertical mixing in phytoplankton distribution in a stratified reservoir. Limnol. Oceanogr. 2007, 52, 620–634. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.S.; Kim, D.K.; Joo, G.J. Delayed influence of dam storage and discharge on the determination of seasonal proliferations of Microcystis aeruginosa and Stephanodiscus hantzschii in a regulated river system of the lower Nakdong River (South Korea). Water Res. 2007, 41, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-S.; Bang, S.W.; Kim, Y.O.; Han, M.S. Effects of rainfall on the hydrological conditions and phytoplankton community structure in the riverine zone of the Pal’tang Reservoir, Korea. J. Freshwater Ecol. 2002, 17, 507–520. [Google Scholar] [CrossRef]
- Ahn, C.Y.; Chung, A.S.; Oh, H.M. Rainfall, phycocyanin, and N: P ratios related to cyanobacterial blooms in a Korean large reservoir. Hydrobiologia 2002, 474, 117–124. [Google Scholar] [CrossRef]
- Znachor, P.; Zapomelova, E.; Rehakova, K.; Nedoma, J.; Simek, K. The effect of extreme rainfall on summer succession and vertical distribution of phytoplankton in a lacustrine part of a eutrophic reservoir. Aquat. Sci. 2008, 70, 77–86. [Google Scholar] [CrossRef]
- Iriarte, A.; Purdie, D.A. Factors controlling the timing of major spring bloom events in an UK south coast estuary. Estuar. Coast. Shelf S. 2004, 61, 679–690. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis; IPCC: Geneva, Switzerland, 2013. [Google Scholar]
- Lehmann, J.; Coumou, D.; Frieler, K. Increased record-breaking precipitation events under global warming. Clim. Chang. 2015, 132, 501–515. [Google Scholar] [CrossRef]
- Richardson, J.; Feuchtmayr, H.; Miller, C.; Hunter, P.D.; Maberly, S.C.; Carvalho, L. Response of cyanobacteria and phytoplankton abundance to warming, extreme rainfall events and nutrient enrichment. Glob. Chang. Biol. 2019, 25, 3365–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.X.; Zhu, G.W.; Paerl, H.W.; Zhu, M.Y.; Yu, L.; Zhang, Y.B.; Liu, M.L.; Zhang, Y.L.; Qin, B.Q. Extreme weather event may induce Microcystis blooms in the Qiantang River, Southeast China. Environ. Sci. Pollut. R. 2018, 25, 22273–22284. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, X.; Bi, Y.; Hu, Z. Effects of rainfall on spring phytoplankton community structure in Xiangxi Bay of the Three-Gorges Reservoir, China. Fresen. Environ. Bull. 2012, 21, 3533–3541. [Google Scholar]
- Wu, T.F.; Qin, B.Q.; Zhu, G.W.; Luo, L.C.; Ding, Y.Q.; Bian, G.Y. Dynamics of cyanobacterial bloom formation during short-term hydrodynamic fluctuation in a large shallow, eutrophic, and wind-exposed Lake Taihu, China. Environ. Sci. Pollut. R. 2013, 20, 8546–8556. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-S.; Kim, D.-K.; Shin, H.-S.; Yoon, J.-D.; Kim, H.-W.; Joo, G.-J. Impact of summer rainfall on the seasonal water quality variation (chlorophyll a) in the regulated Nakdong River. KSCE J. Civ. Eng. 2011, 15, 983–994. [Google Scholar] [CrossRef]
- Sadro, S.; Melack, J.M. The Effect of an Extreme Rain Event on the Biogeochemistry and Ecosystem Metabolism of an Oligotrophic High-Elevation Lake. Arct. Antarct. Alp. Res. 2012, 44, 222–231. [Google Scholar] [CrossRef]
- Badylak, S.; Phlips, E.; Dix, N.; Hart, J.; Srifa, A.; Haunert, D.; He, Z.L.; Lockwood, J.; Stofella, P.; Sun, D.T.; et al. Phytoplankton dynamics in a subtropical tidal creek: Influences of rainfall and water residence time on composition and biomass. Mar. Freshwater Res. 2016, 67, 466–482. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.Q.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Perga, M.E.; Bruel, R.; Rodriguez, L.; Guenand, Y.; Bouffard, D. Storm impacts on alpine lakes: Antecedent weather conditions matter more than the event intensity. Glob. Chang. Biol. 2018, 24, 5004–5016. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Yang, J.; Lv, H.; Yang, J.; Liu, L.M.; Yu, X.Q.; Chen, H.H. Decline in water level boosts cyanobacteria dominance in subtropical reservoirs. Sci. Total Environ. 2016, 557, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, L.; Zheng, Y.; Li, D. Seasonal succession of phytoplankton in response to the variation of environmental factors in the Gaolan River, Three Gorges Reservoir, China. Chin. J. Oceanol. Limnol. 2013, 31, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.X.; Bi, Y.H.; Hu, Z.Y. Responses of phytoplankton functional groups to the hydrologic regime in the Daning River, a tributary of Three Gorges Reservoir, China. Sci. Total Environ. 2013, 450, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, D.F.; Johnson, D.M.; Yi, Z.Q.; Huang, Y.L. Effects of vertical mixing on phytoplankton blooms in Xiangxi Bay of Three Gorges Reservoir: Implications for management. Water Res. 2012, 46, 2121–2130. [Google Scholar] [CrossRef]
- Han, J.C.; Huang, G.H.; Zhang, H.; Li, Z.; Li, Y.P. Heterogeneous Precipitation and Streamflow Trends in the Xiangxi River Watershed, 1961-2010. J. Hydrol. Eng. 2014, 19, 1247–1258. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association(APHA): Washington, DC, USA, 2012. [Google Scholar]
- Hu, H.; Wei, Y. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The freshwater algal flora of the British Isles: An identification guide to freshwater and terrestrial algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Brierley, B.; Carvalho, L.; Davies, S.; Krokowski, J. Report to SNIFFER (Project WFD80), Edinburgh, December 2007; Guidance on the Quantitative Analysis of Phytoplankton in Freshwater Samples; Sniffer: Edinburgh, UK, 2007. [Google Scholar]
- Napiórkowska-Krzebietke, A.; Kobos, J. Assessment of the cell biovolume of phytoplankton widespread in coastal and inland water bodies. Water Res. 2016, 104, 532–546. [Google Scholar] [CrossRef]
- Padisak, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Pesce, S.F.; Wunderlin, D.A. Use of water quality indices to verify the impact of Cordoba City (Argentina) on Suquia River. Water Res. 2000, 34, 2915–2926. [Google Scholar] [CrossRef]
- Wang, J.L.; Fu, Z.S.; Qiao, H.X.; Liu, F.X. Assessment of eutrophication and water quality in the estuarine area of Lake Wuli, Lake Taihu, China. Sci. Total Environ. 2019, 650, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Amaral, J.H.F.; Borges, A.V.; Melack, J.M.; Sarmento, H.; Barbosa, P.M.; Kasper, D.; de Melo, M.L.; De Fex-Wolf, D.; da Silva, J.S.; Forsberg, B.R. Influence of plankton metabolism and mixing depth on CO2 dynamics in an Amazon floodplain lake. Sci. Total Environ. 2018, 630, 1381–1393. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.P.; Jeppesen, E.; Olrik, K.; Kristensen, P. Impact of Nutrients and Physical Factors on the Shift from Cyanobacterial To Chlorophyte Dominance in Shallow Danish Lakes. Can. J. Fish Aquat. Sci. 1994, 51, 1692–1699. [Google Scholar] [CrossRef]
- Padisák, J.; Barbosa, F.; Koschel, R.; Krienitz, L. Deep layer cyanoprokaryota maxima in temperate and tropical lakes. Arch. Hydrobiol. Spec. Issues Adv. Limnol. 2003, 58, 175–199. [Google Scholar]
- Thackeray, S.J.; George, D.G.; Jones, R.I.; Winfield, I.J. Statistical quantification of the effect of thermal stratification on patterns of dispersion in a freshwater zooplankton community. Aquat. Ecol. 2006, 40, 23–32. [Google Scholar] [CrossRef]
- Hills, J.M.; Thomason, J.C. A multi-scale analysis of settlement density and pattern dynamics of the barnacle Semibalanus balanoides. Mar. Ecol. Prog. Ser. 1996, 138, 103–115. [Google Scholar] [CrossRef]
- Baek, S.H.; Shimode, S.; Kim, H.C.; Han, M.S.; Kikuchi, T. Strong bottom-up effects on phytoplankton community caused by a rainfall during spring and summer in Sagami Bay, Japan. J. Marine Syst. 2009, 75, 253–264. [Google Scholar] [CrossRef]
- Clarke, K.R. Nonparametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Zhang, M.; Niu, Z.P.; Cai, Q.H.; Xu, Y.Y.; Qu, X.D. Effect of Water Column Stability on Surface Chlorophyll and Time Lags under Different Nutrient Backgrounds in a Deep Reservoir. Water 2019, 11, 1504. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Zhu, Z.K.; Shahbaz, M.; Chen, L.; Liu, S.L.; Inubushi, K.; Wu, J.S.; Ge, T.D. Split N and P addition decreases straw mineralization and the priming effect of a paddy soil: A 100-day incubation experiment. Biol. Fert. Soils 2019, 55, 701–712. [Google Scholar] [CrossRef]
- Wolf, K.A.; Gupta, S.C.; Rosen, C.J. Precipitation Drives Nitrogen Load Variability in Three Iowa Rivers. J. Hydrol. Reg. Stud. 2020, 30, 100705. [Google Scholar] [CrossRef]
- Sherson, L.R.; Van Horn, D.J.; Gomez-Velez, J.D.; Crossey, L.J.; Dahm, C.N. Nutrient dynamics in an alpine headwater stream: Use of continuous water quality sensors to examine responses to wildfire and precipitation events. Hydrol. Process 2015, 29, 3193–3207. [Google Scholar] [CrossRef]
- Walker, J.C.G. Biogeochemistry - an Analysis of Global Change. Science 1991, 253, 686–687. [Google Scholar]
- Monismith, S.G.; MacIntyre, S. The Surface Mixed Layer in Lakes and Reservoirs. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Oxford, UK, 2009; pp. 636–650. [Google Scholar]
- Boegman, L. Currents in Stratified Water Bodies 2: Internal Waves. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Oxford, UK, 2009; pp. 539–558. [Google Scholar]
- Kim, T.W.; Najjar, R.G.; Lee, K. Influence of precipitation events on phytoplankton biomass in coastal waters of the eastern United States. Glob. Biogeochem. Cycles 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Hunter, P.D.; Tyler, A.N.; Willby, N.J.; Gilvear, D.J. The spatial dynamics of vertical migration by Microcystis aeruginosa in a eutrophic shallow lake: A case study using high spatial resolution time-series airborne remote sensing. Limnol. Oceanogr. 2008, 53, 2391–2406. [Google Scholar] [CrossRef]
- Becker, V.; Caputo, L.; Ordonez, J.; Marce, R.; Armengol, J.; Crossetti, L.O.; Huszar, V.L.M. Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res. 2010, 44, 3345–3354. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- An, K.-G.; Jones, J.R. Factors regulating bluegreen dominance in a reservoir directly influenced by the Asian monsoon. Hydrobiologia 2000, 432, 37–48. [Google Scholar] [CrossRef]
- Ye, L.; Han, X.; Xu, Y.; Cai, Q. Spatial analysis for spring bloom and nutrient limitation in Xiangxi bay of three Gorges Reservoir. Environ. Monit. Assess. 2007, 127, 135–145. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, X.; Bi, Y.; Liang, Y.; Hu, J.; Yang, M.; Mei, Y.; Zhu, K.; Zhang, L.; Hu, Z. Phytoplankton variation and its relationship with the environment in Xiangxi Bay in spring after damming of the Three-Gorges, China. Environ. Monit. Assess. 2011, 176, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jheong, W.; Kwon, O.; Ryu, J. Seasonal succession of toxic cyanobacteria and microcystins concentration in Paldang reservoir. Algae 2000, 15, 277–282. [Google Scholar]
- Jacobsen, B.A.; Simonsen, P. Disturbance events affecting phytoplankton biomass, composition and species diversity in a shallow, eutrophic, temperate lake. In Intermediate Disturbance Hypothesis in Phytoplankton Ecology; Springer: Cham, Switzerland, 1993; pp. 9–14. [Google Scholar]
- Moustaka-Gouni, M. Phytoplankton succession and diversity in a warm monomictic, relatively shallow lake: Lake Volvi, Macedonia, Greece. In Intermediate Disturbance Hypothesis in Phytoplankton Ecology; Springer: Dordrecht, The Netherlands.
- Ministry of Environmental Protection of China. Bulletin on the Ecological and Environmental Monitoring Results of the Three Gorges Project(2003-2018); Ministry of Environmental Protection of China: Beijing, China, 2019. [Google Scholar]
- Paillisson, J.M.; Marion, L. Water level fluctuations for managing excessive plant biomass in shallow lakes. Ecol. Eng. 2011, 37, 241–247. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, D.F.; Ji, D.B.; Xiao, S.B. Influence of the impounding process of the Three Gorges Reservoir up to water level 172.5 m on water eutrophication in the Xiangxi Bay. Sci. China Technol. Sci. 2010, 53, 1114–1125. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Qin, H.; Wang, K.; Bi, Y. Precipitation Mediates the Distribution but Not the Taxonomic Composition of Phytoplankton Communities in a Tributary of Three Gorges Reservoir. Plants 2021, 10, 1800. https://doi.org/10.3390/plants10091800
Peng C, Qin H, Wang K, Bi Y. Precipitation Mediates the Distribution but Not the Taxonomic Composition of Phytoplankton Communities in a Tributary of Three Gorges Reservoir. Plants. 2021; 10(9):1800. https://doi.org/10.3390/plants10091800
Chicago/Turabian StylePeng, Chengrong, Hongjie Qin, Kan Wang, and Yonghong Bi. 2021. "Precipitation Mediates the Distribution but Not the Taxonomic Composition of Phytoplankton Communities in a Tributary of Three Gorges Reservoir" Plants 10, no. 9: 1800. https://doi.org/10.3390/plants10091800
APA StylePeng, C., Qin, H., Wang, K., & Bi, Y. (2021). Precipitation Mediates the Distribution but Not the Taxonomic Composition of Phytoplankton Communities in a Tributary of Three Gorges Reservoir. Plants, 10(9), 1800. https://doi.org/10.3390/plants10091800






