P2K1 Receptor, Heterotrimeric Gα Protein and CNGC2/4 Are Involved in Extracellular ATP-Promoted Ion Influx in the Pollen of Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
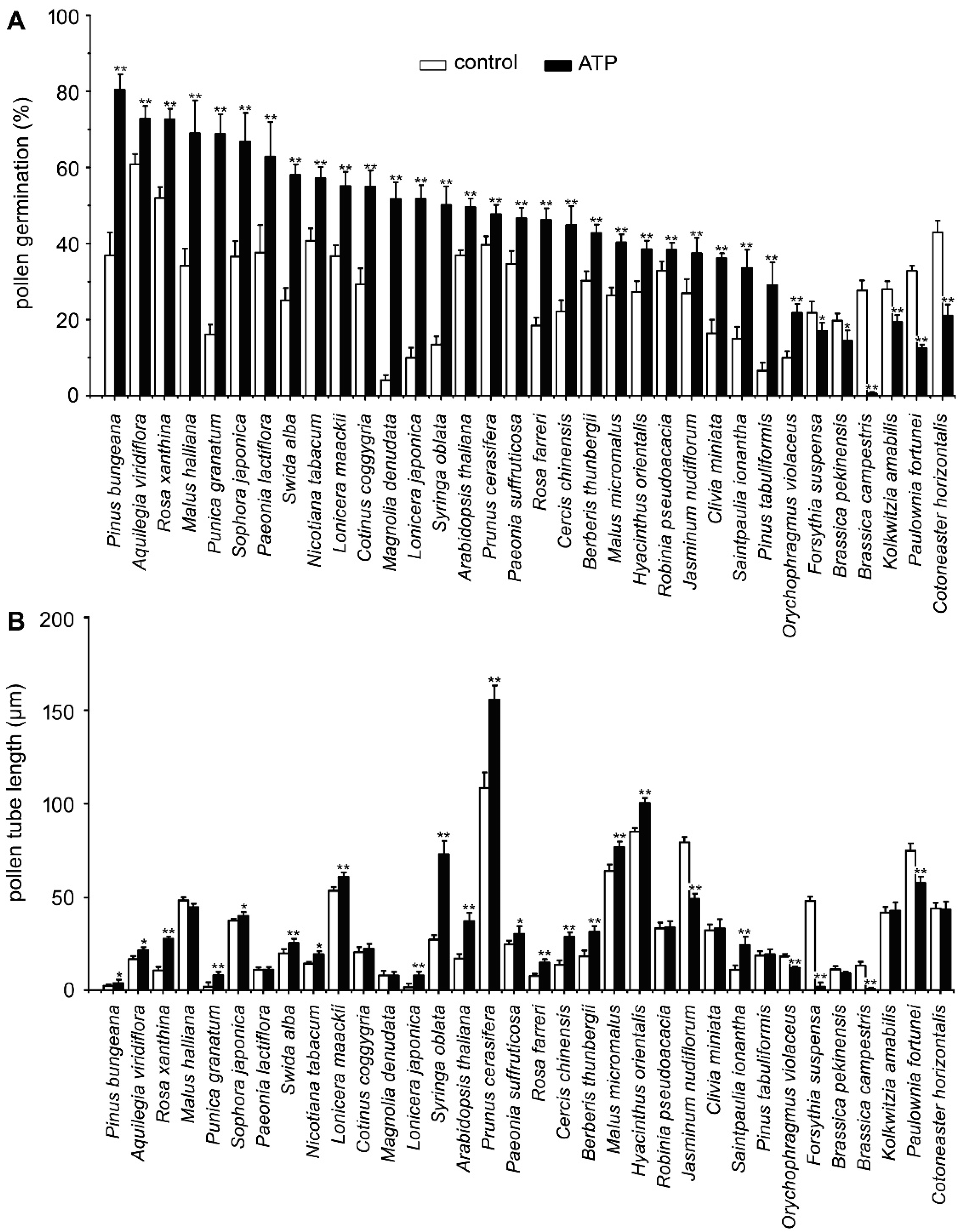
2.1. ATP Addition Impacts PG and PTG in Pollens from 34 Species
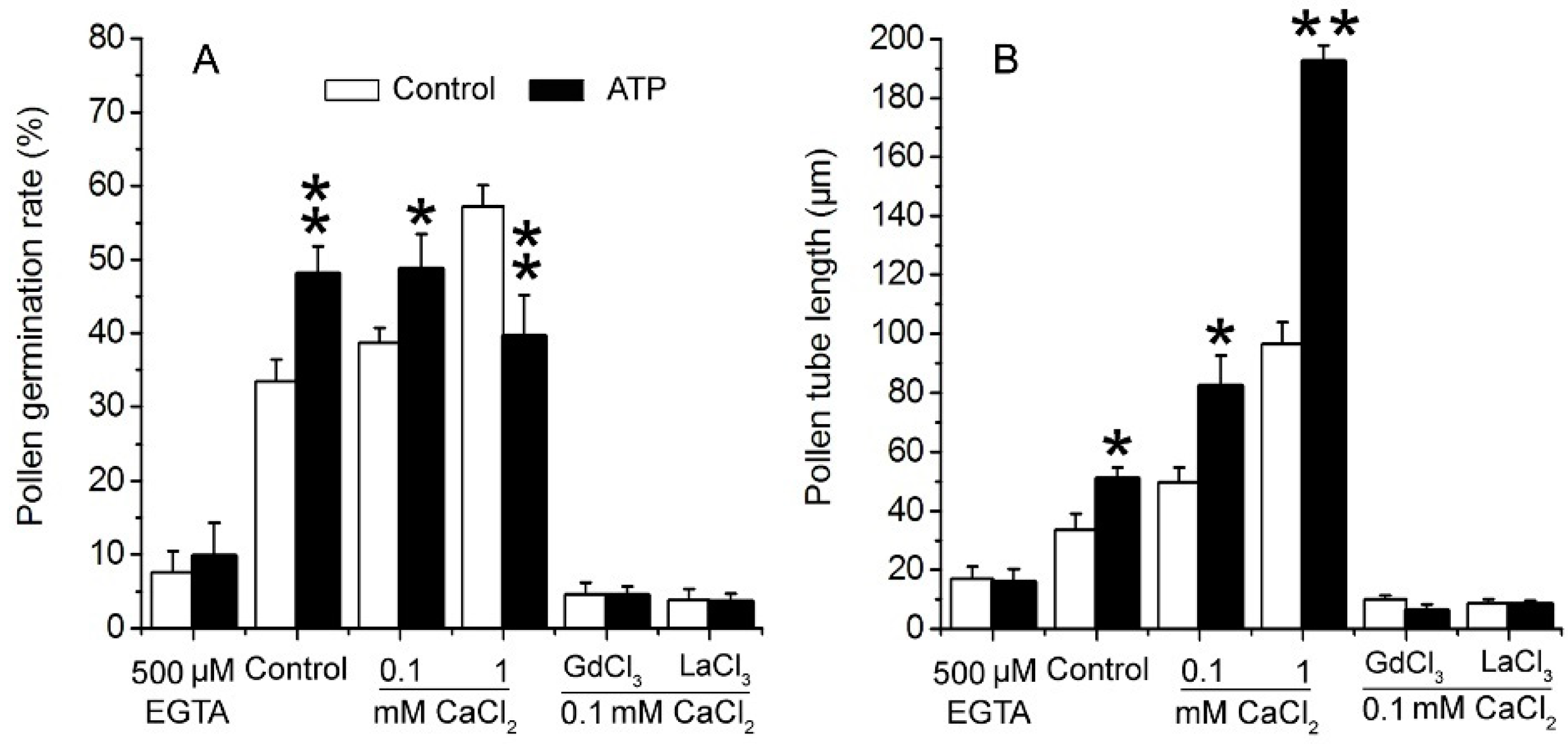
2.2. eATP Regulates PG and PTG of Arabidopsis thaliana via K+ and Ca2+ Intake
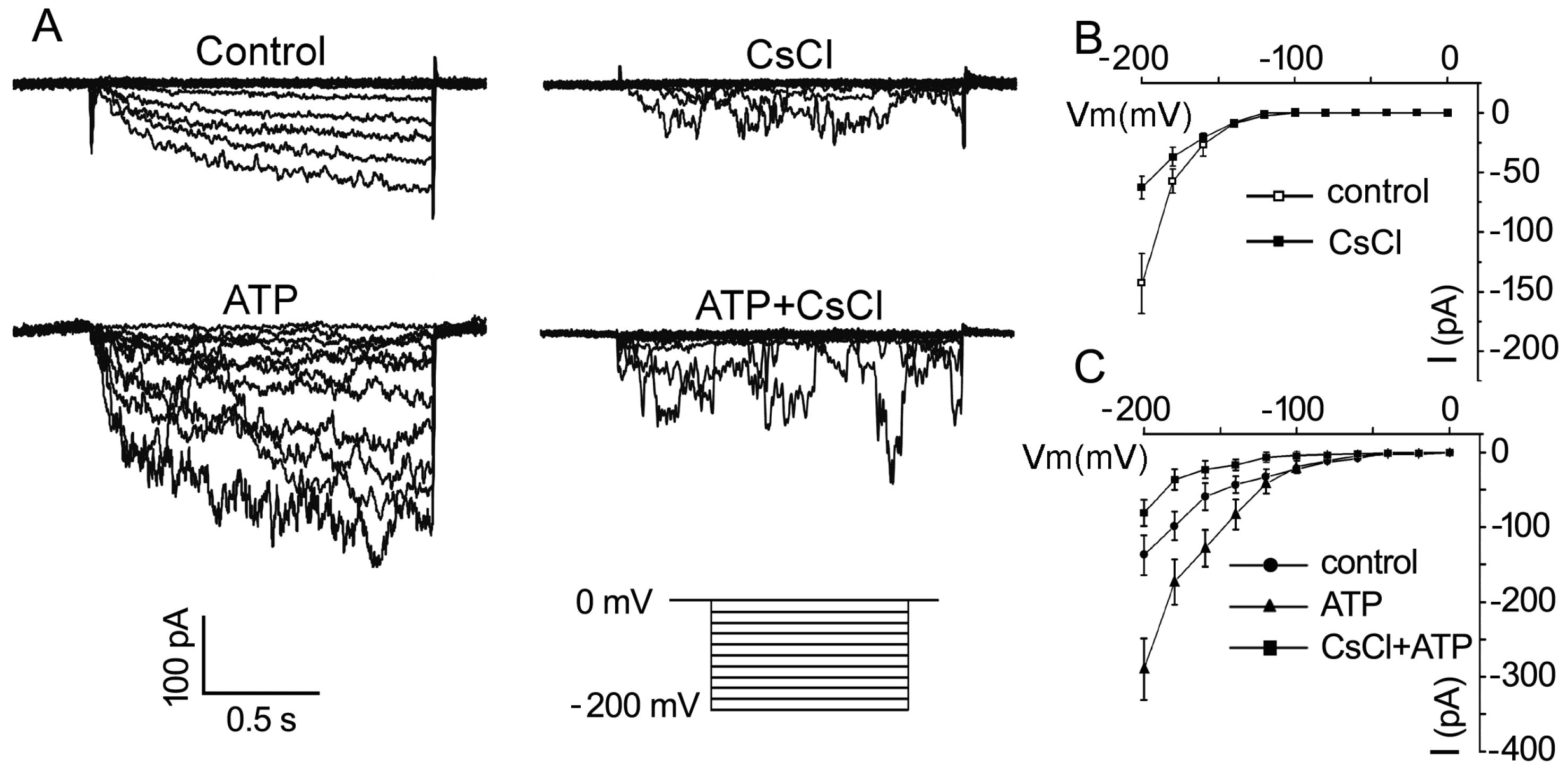
2.3. ATP Stimulates K+ and Ca2+ Influx in Arabidopsis thaliana Pollen Protoplast
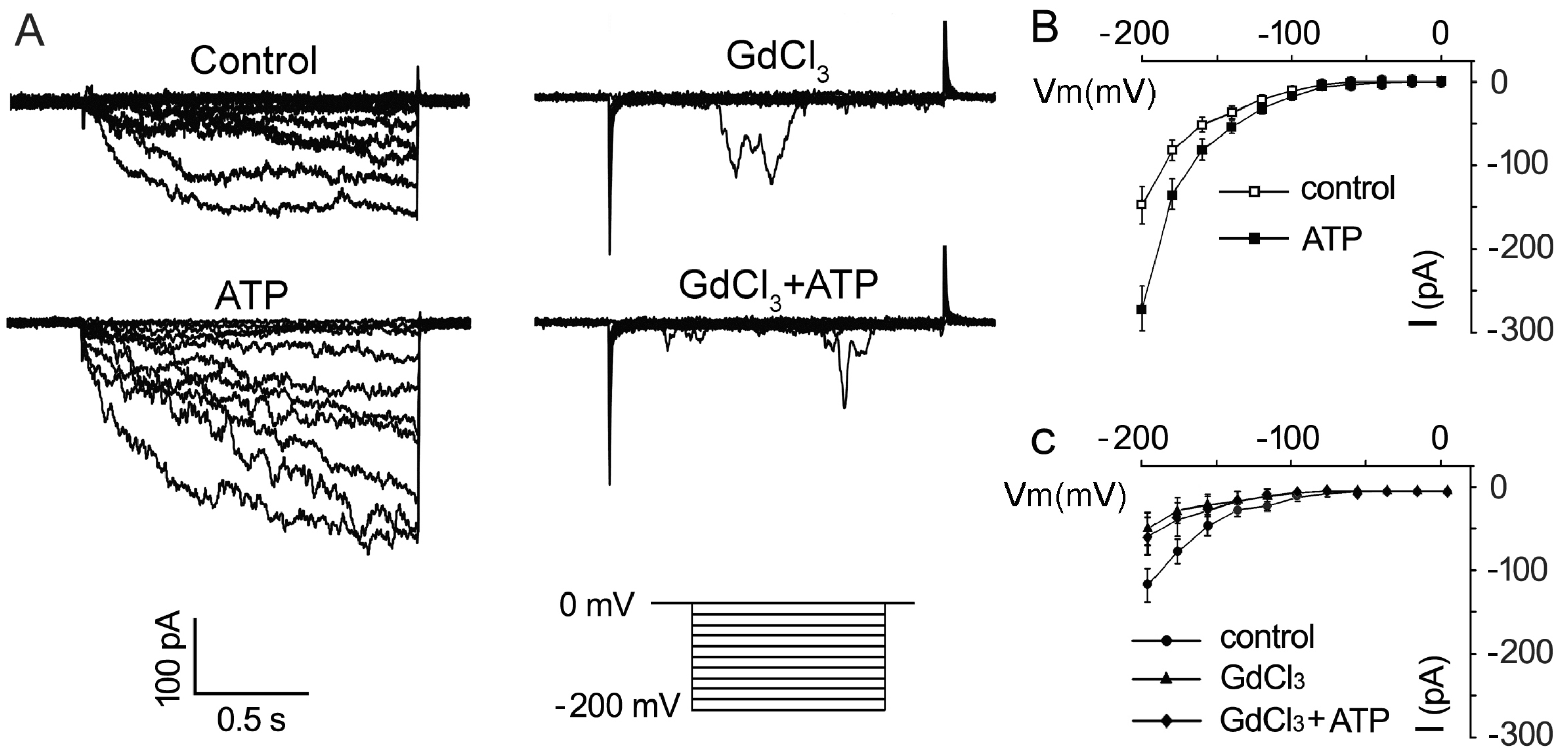
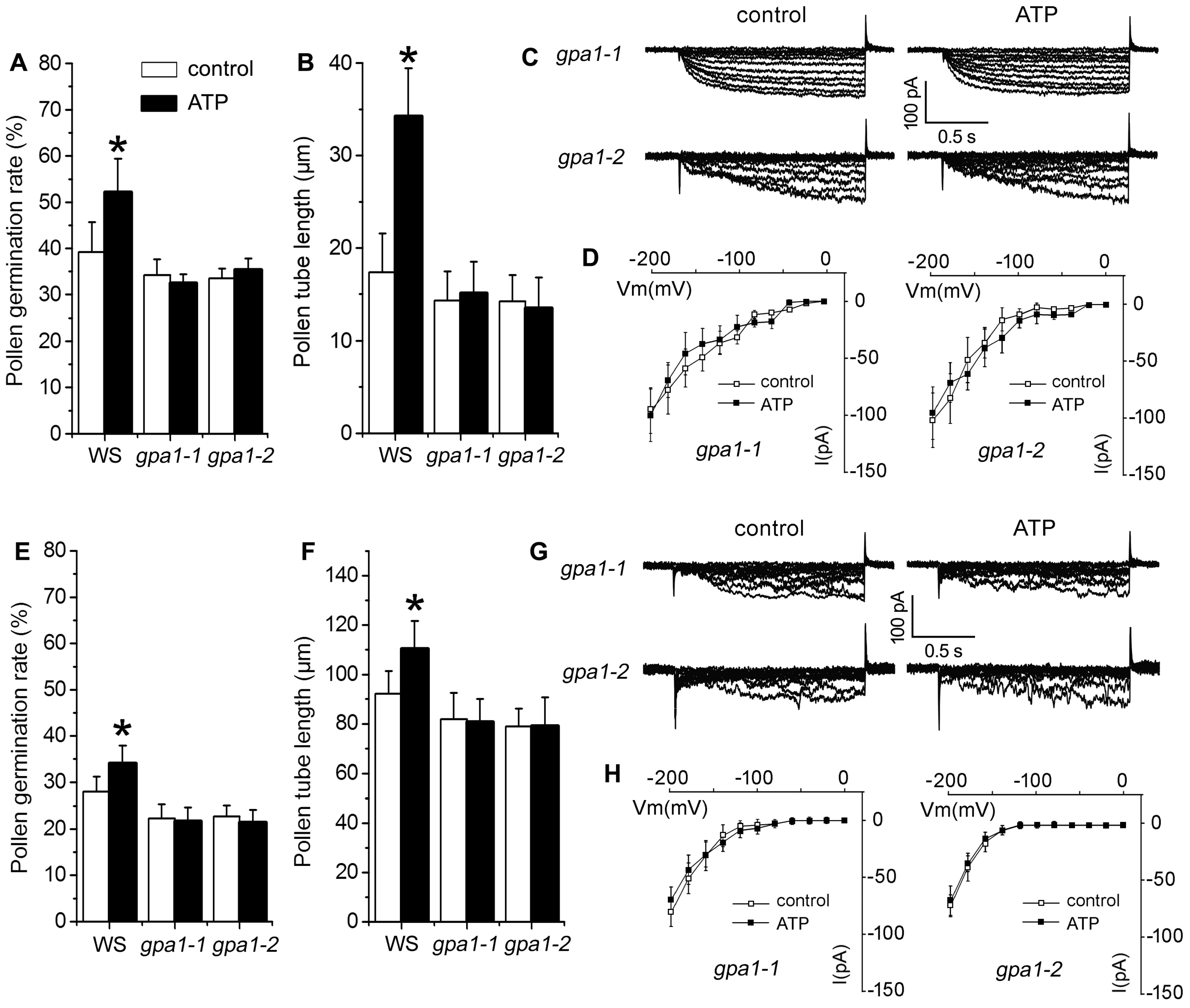
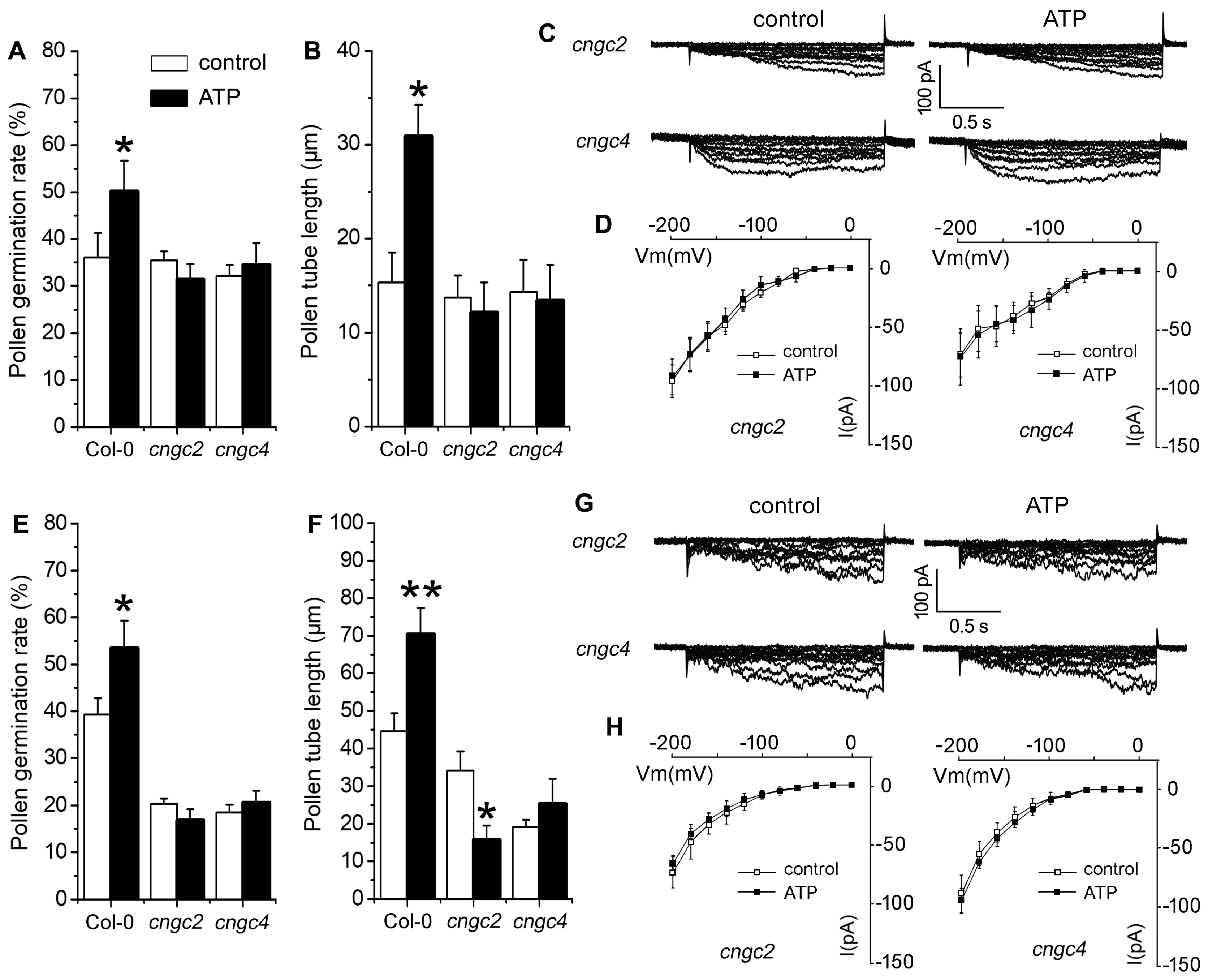
2.4. P2K1 Receptor, Heterotrimeric G Protein α Subunit and Two CNGCs Are Involved in eATP-Regulated PG and PTG by Modulating K+ and Ca2+ Influx
3. Discussion
3.1. eATP Regulates PG and PTG in Dozens of Plant Species
3.2. ATP Regulates PG and PTG of Arabidopsis thaliana
3.3. K+/Ca2+ Influx Mediates ATP Regulation of PG and PTG
3.4. Signaling Underlying ATP-Regulated PG and PTG of Arabidopsis thaliana
4. Materials and Methods
4.1. Plant Materials
4.2. In Vitro Pollen Germination
4.3. Protoplast Isolation
4.4. Patch-Clamp Recording
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Tanaka, K.; Nguyen, C.T.; Stacey, G. Extracellular ATP is a central signaling molecule in plant stress responses. Curr. Opin. Plant Biol. 2014, 20, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Choi, J.; Cao, Y.; Stacey, G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant Sci. 2014, 5, 446. [Google Scholar] [CrossRef] [Green Version]
- Clark, G.; Roux, S.J. Role of Ca2+ in mediating plant responses to extracellular ATP and ADP. Int. J. Mol. Sci. 2018, 19, 3590. [Google Scholar] [CrossRef] [Green Version]
- Pietrowska-Borek, M.; Dobrogojski, J.; Sobieszczuk-Nowicka, E.; Borek, S. New insight into plant signaling: Extracellular ATP and uncommon nucleotides. Cells 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chivasa, S.; Ndimba, B.K.; Simon, W.J.; Lindsey, K.; Slabas, A.R. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell 2005, 17, 3019–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.J.; Liu, Y.S.; Wu, J.Y. The signaling role of extracellular ATP and its dependence on Ca2+ flux in elicitation of Salvia miltiorrhiza hairy root cultures. Plant Cell Physiol. 2008, 49, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Sivaguru, M.; Stacey, G. Extracellular ATP in plants. Visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol. 2006, 142, 984–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonon, C.; Cecilia Terrile, M.; Jose Iglesias, M.; Lamattina, L.; Casalongue, C. Extracellular ATP, nitric oxide and superoxide act coordinately to regulate hypocotyl growth in etiolated Arabidopsis seedlings. J. Plant Physiol. 2010, 167, 540–546. [Google Scholar] [CrossRef]
- Zhu, R.; Dong, X.; Xue, Y.; Xu, J.; Zhang, A.; Feng, M.; Zhao, Q.; Xia, S.; Yin, Y.; He, S.; et al. Redox-Responsive Transcription Factor 1 (RRFT1) is involved in extracellular ATP-regulated Arabidopsis thaliana seedling growth. Plant Cell Physiol. 2020, 61, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.; Torres, J.; Finlayson, S.; Guan, X.; Handley, C.; Lee, J.; Kays, J.E.; Chen, Z.J.; Roux, S.J. Apyrase (nucleoside triphosphate-diphosphohydrolase) and extracellular nucleotides regulate cotton fiber elongation in cultured ovules. Plant Physiol. 2010, 152, 1073–1083. [Google Scholar] [CrossRef] [Green Version]
- Clark, G.; Fraley, D.; Steinebrunner, I.; Cervantes, A.; Onyirimba, J.; Liu, A.; Torres, J.; Tang, W.; Kim, J.; Roux, S.J. Extracellular nucleotides and apyrases regulate stomatal aperture in Arabidopsis. Plant Physiol. 2011, 156, 1740–1753. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.H.; Wang, W.X.; Chen, C.; Wang, Y.F.; Liu, T.; Li, X.; Shang, Z.L. Extracellular ATP promotes stomatal opening of Arabidopsis thaliana through heterotrimeric G protein α subunit and reactive oxygen species. Mol. Plant 2012, 5, 852–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Cao, Y.; Li, H.; Kim, D.; Ahsan, N.; Thelen, J.; Stacey, G. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat. Commun. 2017, 8, 2265. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Tanaka, K.; Liang, Y.; Cao, Y.; Lee, S.Y.; Stacey, G. Extracellular ATP, a danger signal, is recognized by DORN1 in Arabidopsis. Biochem. J. 2014, 463, 429–437. [Google Scholar] [CrossRef]
- Chivasa, S.; Murphy, A.M.; Hamilton, J.M.; Lindsey, K.; Carr, J.P.; Slabas, A.R. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009, 60, 436–448. [Google Scholar] [CrossRef]
- Kim, S.H.; Yang, S.H.; Kim, T.J.; Han, J.S.; Suh, J.W. Hypertonic stress increased extracellular ATP levels and the expression of stress-responsive genes in Arabidopsis thaliana seedlings. Biosci. Biotechnol. Biochem. 2009, 73, 1252–1256. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, X.; Deng, S.; Zhang, C.; Wang, M.; Ding, M.; Zhao, R.; Shen, X.; Zhou, X.; Lu, C.; et al. Extracellular ATP signaling is mediated by H2O2 and cytosolic Ca2+ in the salt response of Populus euphratica cells. PLoS ONE 2012, 7, e53136. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Sun, J.; Zhao, R.; Ding, M.; Zhang, Y.; Sun, Y.; Wang, W.; Tan, Y.; Liu, D.; Ma, X.; et al. Populus euphratica APYRASE2 enhances cold tolerance by modulating vesicular trafficking and extracellular ATP in Arabidopsis plants. Plant Physiol. 2015, 169, 530–548. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Tanaka, K.; Cao, Y.; Qi, Y.; Qiu, J.; Liang, Y.; Lee, S.Y.; Stacey, G. Identification of a plant receptor for extracellular ATP. Science 2014, 343, 290–294. [Google Scholar] [CrossRef]
- Pham, A.Q.; Cho, S.H.; Nguyen, C.T.; Stacey, G. Arabidopsis lectin receptor kinase P2K2 is a second plant receptor for extracellular ATP and contributes to innate immunity. Plant Physiol. 2020, 183, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hao, F.; Mu, H.; Ahsan, N.; Thelen, J.J.; Stacey, G. S-acylation of P2K1 mediates extracellular ATP-induced immune signaling in Arabidopsis. Nat. Commun. 2021, 12, 2750. [Google Scholar] [CrossRef]
- Zhu, R.; Dong, X.; Hao, W.; Gao, W.; Zhang, W.; Xia, S.; Liu, T.; Shang, Z. Heterotrimeric G protein-regulated Ca2+ influx and PIN2 asymmetric distribution are involved in Arabidopsis thaliana roots’ avoidance response to extracellular ATP. Front. Plant Sci. 2017, 8, 1522. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Shang, Z.; Shin, R.; Thompson, E.; Rubio, L.; Laohavisit, A.; Mortimer, J.C.; Chivasa, S.; Slabas, A.R.; Glover, B.J.; et al. Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. 2009, 58, 903–913. [Google Scholar] [CrossRef]
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232. [Google Scholar] [CrossRef] [Green Version]
- Shang, Z.; Laohavisit, A.; Davies, J.M. Extracellular ATP activates an Arabidopsis plasma membrane Ca2+-permeable conductance. Plant Signal. Behav. 2009, 4, 989–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.M.; Stacey, G.; Leblanc-Fournier, N.; Legue, V.; Moulia, B.; Davies, J.M. Early extracellular ATP signaling in Arabidopsis root epidermis: A multi-conductance process. Front. Plant Sci. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Nichols, C.; Oliynyk, M.; Dark, A.; Glover, B.J.; Davies, J.M. Is ATP a signaling agent in plants? Plant Physiol. 2003, 133, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Foresi, N.P.; Laxalt, A.M.; Tonon, C.V.; Casalongue, C.A.; Lamattina, L. Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol. 2007, 145, 589–592. [Google Scholar] [CrossRef] [Green Version]
- Matthus, E.; Sun, J.; Wang, L.M.; Bhat, M.G.; Mohammad-Sidik, A.B.; Wilkins, K.A.; Leblanc-Fournier, N.; Legue, V.; Moulia, B.; Stacey, G.; et al. DORN1/P2K1 and purino-calcium signalling in plants: Making waves with extracellular ATP. Ann. Bot. 2019, 124, 1227–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdaway-Clarke, T.L.; Hepler, P.K. Control of pollen tube growth: Role of ion gradients and fluxes. New Phytol. 2003, 159, 539–563. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. Calcium—A central regulator of pollen germination and tube growth. Biochim. Biophys. Acta 2013, 1833, 1573–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, R.H.; Su, S.; Xiao, H.; Tian, H.Q. Calcium: A critical factor in pollen germination and tube elongation. Int. J. Mol. Sci. 2019, 20, 420. [Google Scholar] [CrossRef] [Green Version]
- Li, D.D.; Guan, H.; Li, F.; Liu, C.Z.; Dong, Y.X.; Zhang, X.S.; Gao, X.Q. Arabidopsis shaker pollen inward K+ channel SPIK functions in SnRK1 complex-regulated pollen hydration on the stigma. J. Integr. Plant. Biol. 2017, 59, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Amien, S.; Kliwer, I.; Marton, M.L.; Debener, T.; Geiger, D.; Becker, D.; Dresselhaus, T. Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biol. 2010, 8, e1000388. [Google Scholar] [CrossRef] [Green Version]
- Mouline, K.; Very, A.A.; Gaymard, F.; Boucherez, J.; Pilot, G.; Devic, M.; Bouchez, D.; Thibaud, J.B.; Sentenac, H. Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev. 2002, 16, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.M.; Wang, Y.F.; Wang, H.; Wu, W.H. In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J. Exp. Bot. 2001, 52, 1603–1614. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zheng, C.; Kuang, B.; Wei, L.; Yan, L.; Wang, T. Receptor-like kinase RUPO interacts with potassium transporters to regulate pollen tube growth and integrity in rice. PLoS Genet. 2016, 12, e1006085. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.M.; Wu, W.H.; Yang, H.Y. Identification and characterization of the inward K+ channel in the plasma membrane of Brassica pollen protoplasts. Plant Cell Physiol. 1999, 40, 859–865. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.N.; Shen, L.K.; Zhang, W.Z.; Zhang, W.; Wang, Y.; Wu, W.H. Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 2013, 25, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wang, T.; Liu, L. Pollen germination is impaired by disruption of a Shaker K+ channel OsAKT1.2 in rice. J. Plant Physiol. 2020, 248, 153140. [Google Scholar] [CrossRef]
- Wang, S.S.; Diao, W.Z.; Yang, X.; Qiao, Z.; Wang, M.; Acharya, B.R.; Zhang, W. Arabidopsis thaliana CML25 mediates the Ca2+ regulation of K+ transmembrane trafficking during pollen germination and tube elongation. Plant Cell Environ. 2015, 38, 2372–2386. [Google Scholar] [CrossRef]
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; Shipley, A.M.; Rivers, B.A.; Cresti, M.; Hepler, P.K. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 1994, 6, 1815–1828. [Google Scholar]
- Malho, R.; Trewavas, A.J. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 1996, 8, 1935–1949. [Google Scholar] [CrossRef]
- Cole, R.A.; Fowler, J.E. Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant Biol. 2006, 9, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Iwano, M.; Entani, T.; Shiba, H.; Kakita, M.; Nagai, T.; Mizuno, H.; Miyawaki, A.; Shoji, T.; Kubo, K.; Isogai, A.; et al. Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol. 2009, 150, 1322–1334. [Google Scholar] [CrossRef] [Green Version]
- Iwano, M.; Shiba, H.; Miwa, T.; Che, F.S.; Takayama, S.; Nagai, T.; Miyawaki, A.; Isogai, A. Ca2+ dynamics in a pollen grain and papilla cell during pollination of Arabidopsis. Plant Physiol. 2004, 136, 3562–3571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, K.S.; Cork, R.J.; Robinson, K.R. A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Dev. Biol. 1991, 148, 612–619. [Google Scholar] [CrossRef]
- Konrad, K.R.; Wudick, M.M.; Feijo, J.A. Calcium regulation of tip growth: New genes for old mechanisms. Curr. Opin. Plant Biol. 2011, 14, 721–730. [Google Scholar] [CrossRef]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Parrotta, L.; Faleri, C.; Guerriero, G.; Cai, G. Cold stress affects cell wall deposition and growth pattern in tobacco pollen tubes. Plant Sci. 2019, 283, 329–342. [Google Scholar] [CrossRef]
- Ruan, H.Q.; Li, J.; Wang, T.; Ren, H.Y. Secretory vesicles targeted to plasma membrane during pollen germination and tube growth. Front. Cell Dev. Biol. 2021, 8, 615447. [Google Scholar] [CrossRef] [PubMed]
- Battey, N.H.; James, N.C.; Greenland, A.J.; Brownlee, C. Exocytosis and endocytosis. Plant Cell 1999, 11, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.; Malho, R. Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J. Exp. Bot. 2003, 54, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zonia, L. Spatial and temporal integration of signalling networks regulating pollen tube growth. J. Exp. Bot. 2010, 61, 1939–1957. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.F.; Fan, L.M.; Zhang, W.Z.; Zhang, W.; Wu, W.H. Ca2+-permeable channels in the plasma membrane of arabidopsis pollen are regulated by actin microfilaments. Plant Physiol. 2004, 136, 3892–3904. [Google Scholar] [CrossRef] [Green Version]
- Malho, R.; Liu, Q.; Monteiro, D.; Rato, C.; Camacho, L.; Dinis, A. Signalling pathways in pollen germination and tube growth. Protoplasma 2006, 228, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Jin, C.; Qu, H.Y.; Tao, S.T.; Xu, G.H.; Wu, J.; Wu, H.Q.; Zhang, S.L. Low temperature inhibits pollen viability by alteration of actin cytoskeleton and regulation of pollen plasma membrane ion channels in Pyrus pyrifolia. Environ. Exp. Bot. 2012, 78, 70–75. [Google Scholar] [CrossRef]
- Dutta, R.; Robinson, K.R. Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 2004, 135, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Frietsch, S.; Wang, Y.F.; Sladek, C.; Poulsen, L.R.; Romanowsky, S.M.; Schroeder, J.I.; Harper, J.F. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. USA 2007, 104, 14531–14536. [Google Scholar] [CrossRef] [Green Version]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.H.; Obermeyer, G.; Feijo, J.A. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Xu, X.; Li, S.; Liu, T.; Ma, L.; Shang, Z. Heterotrimeric G-protein participation in Arabidopsis pollen germination through modulation of a plasmamembrane hyperpolarization-activated Ca2+-permeable channel. New Phytol. 2007, 176, 550–559. [Google Scholar] [CrossRef]
- Wu, J.; Shang, Z.; Wu, J.; Jiang, X.; Moschou, P.N.; Sun, W.; Roubelakis-Angelakis, K.A.; Zhang, S. Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant J. 2010, 63, 1042–1053. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, B.; Feng, K.; Yan, R.; Kang, E.; Liu, T.; Shang, Z. Extracellular ATP promoted pollen germination and tube growth of Nicotiana tabacum through promoting K+ and Ca2+ absorption. Plant Reprod. 2018, 31, 399–410. [Google Scholar] [CrossRef]
- Reichler, S.A.; Torres, J.; Rivera, A.L.; Cintolesi, V.A.; Clark, G.; Roux, S.J. Intersection of two signalling pathways: Extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide. J. Exp. Bot. 2009, 60, 2129–2138. [Google Scholar] [CrossRef] [Green Version]
- Steinebrunner, I.; Wu, J.; Sun, Y.; Corbett, A.; Roux, S.J. Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol 2003, 131, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.H.; Fan, C.Y.; Liu, K.; Jing, Y.P. Extracellular ATP is involved in the initiation of pollen germination and tube growth in Picea meyeri. Trees Struct. Funct. 2015, 29, 563–574. [Google Scholar] [CrossRef]
- Clark, G.; Roux, S.J. Apyrases, extracellular ATP and the regulation of growth. Curr. Opin. Plant. Biol. 2011, 14, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shang, Z.; Shin, R.; Colaco, R.; Laohavisit, A.; Shabala, S.; Davies, J.M. Receptor-like activity evoked by extracellular ADP in Arabidopsis root epidermal plasma membrane. Plant Physiol. 2011, 156, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Jeter, C.R.; Tang, W.; Henaff, E.; Butterfield, T.; Roux, S.J. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 2004, 16, 2652–2664. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.M.; Wilkins, K.A.; Davies, J.M. Arabidopsis DORN1 extracellular ATP receptor; activation of plasma membrane K+-and Ca2+-permeable conductances. New Phytol. 2018, 218, 1301–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellande, K.; Bono, J.J.; Savelli, B.; Jamet, E.; Canut, H. Plant lectins and Lectin Receptor-Like Kinases: How do they sense the outside? Int. J. Mol. Sci. 2017, 18, 1164. [Google Scholar] [CrossRef]
- Wang, F.; Jia, J.; Wang, Y.; Wang, W.; Chen, Y.; Liu, T.; Shang, Z. Hyperpolization-activated Ca2+ channels in guard cell plasma membrane are involved in extracellular ATP-promoted stomatal opening in Vicia faba. J. Plant Physiol. 2014, 171, 1241–1247. [Google Scholar] [CrossRef]
- Weerasinghe, R.R.; Swanson, S.J.; Okada, S.F.; Garrett, M.B.; Kim, S.Y.; Stacey, G.; Boucher, R.C.; Gilroy, S.; Jones, A.M. Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett. 2009, 583, 2521–2526. [Google Scholar] [CrossRef] [Green Version]
- Brost, C.; Studtrucker, T.; Reimann, R.; Denninger, P.; Czekalla, J.; Krebs, M.; Fabry, B.; Schumacher, K.; Grossmann, G.; Dietrich, P. Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J. 2019, 99, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Urquhart, W.; Ung, H.; Yoshioka, K. The role of cyclic nucleotide-gated ion channels in plant immunity. Mol. Plant 2011, 4, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Duszyn, M.; Swiezawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Cyclic nucleotide gated channels (CNGCs) in plant signalling-Current knowledge and perspectives. J. Plant. Physiol. 2019, 241, 153035. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozdemir, M.; Tang, C.; Ishka, M.R.; Brown, E.; Groves, N.R.; Myers, C.T.; Rato, C.; Poulsen, L.R.; McDowell, S.; Miller, G.; et al. A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 2013, 161, 1010–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, K.; DeFalco, T.A.; Moeder, W.; Yoshioka, K. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol. 2013, 163, 611–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.W.; Schorrak, L.M.; Smith, R.K., Jr.; Bent, A.F.; Sussman, M.R. A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol. 2003, 132, 728–731. [Google Scholar] [CrossRef] [Green Version]
- Katano, K.; Kataoka, R.; Fujii, M.; Suzuki, N. Differences between seedlings and flowers in anti-ROS based heat responses of Arabidopsis plants deficient in cyclic nucleotide gated channel 2. Plant Physiol. Biochem. 2018, 123, 288–296. [Google Scholar] [CrossRef]
- Gao, Q.F.; Gu, L.L.; Wang, H.Q.; Fei, C.F.; Fang, X.; Hussain, J.; Sun, S.J.; Dong, J.Y.; Liu, H.; Wang, Y.F. Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 3096–3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmati Ishka, M.; Brown, E.; Weigand, C.; Tillett, R.L.; Schlauch, K.A.; Miller, G.; Harper, J.F. A comparison of heat-stress transcriptome changes between wild-type Arabidopsis pollen and a heat-sensitive mutant harboring a knockout of cyclic nucleotide-gated cation channel 16 (cngc16). BMC Genom. 2018, 19, 549. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozdemir, M.; Jones, A.M. Ligand-induced dynamics of heterotrimeric G protein-coupled receptor-like kinase complexes. PLoS ONE 2017, 12, e0171854. [Google Scholar]
- Tunc-Ozdemir, M.; Urano, D.; Jaiswal, D.K.; Clouse, S.D.; Jones, A.M. Direct modulation of heterotrimeric G protein-coupled signaling by a receptor kinase complex. J. Biol. Chem. 2016, 291, 13918–13925. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Song, G.; Werth, E.G.; Walley, J.W.; Hicks, L.M.; Jones, A.M. Receptor-Like kinase phosphorylation of Arabidopsis heterotrimeric G-protein Gα -subunit AtGPA1. Proteomics 2019, 19, e1900265. [Google Scholar] [CrossRef]
- Urano, D.; Chen, J.G.; Botella, J.R.; Jones, A.M. Heterotrimeric G protein signalling in the plant kingdom. Open Biol. 2013, 3, 120186. [Google Scholar] [CrossRef] [Green Version]
- Very, A.A.; Davies, J.M. Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 2000, 97, 9801–9806. [Google Scholar] [CrossRef] [Green Version]


| Latin Name | Family | Class | Phylum | Germination Duration (min) | |
|---|---|---|---|---|---|
| 1 | Aquilegia viridiflora | Ranunculaceae | Dicotyledoneae | Angiospermae | 30 |
| 2 | Arabidopsis thaliana | Brassicaceae | Dicotyledoneae | Angiospermae | 300 |
| 3 | Berberis thunbergii | Berberidaceae | Dicotyledoneae | Angiospermae | 40 |
| 4 | Brassica campestris | Brassicaceae | Dicotyledoneae | Angiospermae | 240 |
| 5 | Brassica pekinensis | Brassicaceae | Dicotyledoneae | Angiospermae | 150 |
| 6 | Cercis chinensis | Leguminosae | Dicotyledoneae | Angiospermae | 90 |
| 7 | Clivia miniata | Amaryllidaceae | Monocotyledoneae | Angiospermae | 420 |
| 8 | Cotinus coggygria | Anacardiaceae | Dicotyledoneae | Angiospermae | 40 |
| 9 | Cotoneaster horizontalis | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 10 | Forsythia suspensa | Oleaceae | Dicotyledoneae | Angiospermae | 120 |
| 11 | Hyacinthus orientalis | Hyacinthaceae | Monocotyledoneae | Angiospermae | 80 |
| 12 | Jasminum nudiflorum | Oleaceae | Dicotyledoneae | Angiospermae | 90 |
| 13 | Kolkwitzia amabilis | Caprifoliaceae | Dicotyledoneae | Angiospermae | 60 |
| 14 | Lonicera maackii | Caprifoliaceae | Dicotyledoneae | Angiospermae | 60 |
| 15 | Lonicera japonica | Caprifoliaceae | Dicotyledoneae | Angiospermae | 120 |
| 16 | Magnolia denudata | Magnoliaceae | Dicotyledoneae | Angiospermae | 2880 |
| 17 | Malus halliana | Rosaceae | Dicotyledoneae | Angiospermae | 30 |
| 18 | Malus micromalus | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 19 | Nicotiana tabacum | Solanaceae | Dicotyledoneae | Angiospermae | 30 |
| 20 | Orychophragmus violaceus | Brassicaceae | Dicotyledoneae | Angiospermae | 180 |
| 21 | Paeonia suffruticosa | Paeoniaceae | Dicotyledoneae | Angiospermae | 60 |
| 22 | Paeonia lactiflora | Paeoniaceae | Dicotyledoneae | Angiospermae | 60 |
| 23 | Paulownia fortunei | Scrophulariaceae | Dicotyledoneae | Angiospermae | 60 |
| 24 | Pinus bungeana | Pinaceae | Coniferopsida | Gymnosperm | 4320 |
| 25 | Pinus tabulaeformis | Pinaceae | Coniferopsida | Gymnosperm | 4320 |
| 26 | Punica granatum | Punicaceae | Dicotyledoneae | Angiospermae | 120 |
| 27 | Prunus cerasifera | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 28 | Rosa xanthina | Rosaceae | Dicotyledoneae | Angiospermae | 20 |
| 29 | Robinia pseudoacacia | Leguminosae | Dicotyledoneae | Angiospermae | 30 |
| 30 | Rosa farreri | Rosaceae | Dicotyledoneae | Angiospermae | 30 |
| 31 | Saintpaulia ionantha | Gesneriaceae | Dicotyledoneae | Angiospermae | 180 |
| 32 | Sophora japonica | Leguminosae | Dicotyledoneae | Angiospermae | 30 |
| 33 | Swida alba | Corneceae | Dicotyledoneae | Angiospermae | 60 |
| 34 | Syringa oblata | Oleaceae | Dicotyledoneae | Angiospermae | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yin, H.; Liu, X.; Xu, J.; Qin, B.; Feng, K.; Kang, E.; Shang, Z. P2K1 Receptor, Heterotrimeric Gα Protein and CNGC2/4 Are Involved in Extracellular ATP-Promoted Ion Influx in the Pollen of Arabidopsis thaliana. Plants 2021, 10, 1743. https://doi.org/10.3390/plants10081743
Wu Y, Yin H, Liu X, Xu J, Qin B, Feng K, Kang E, Shang Z. P2K1 Receptor, Heterotrimeric Gα Protein and CNGC2/4 Are Involved in Extracellular ATP-Promoted Ion Influx in the Pollen of Arabidopsis thaliana. Plants. 2021; 10(8):1743. https://doi.org/10.3390/plants10081743
Chicago/Turabian StyleWu, Yansheng, Hongmin Yin, Xinyue Liu, Jiawei Xu, Baozhi Qin, Kaili Feng, Erfang Kang, and Zhonglin Shang. 2021. "P2K1 Receptor, Heterotrimeric Gα Protein and CNGC2/4 Are Involved in Extracellular ATP-Promoted Ion Influx in the Pollen of Arabidopsis thaliana" Plants 10, no. 8: 1743. https://doi.org/10.3390/plants10081743
APA StyleWu, Y., Yin, H., Liu, X., Xu, J., Qin, B., Feng, K., Kang, E., & Shang, Z. (2021). P2K1 Receptor, Heterotrimeric Gα Protein and CNGC2/4 Are Involved in Extracellular ATP-Promoted Ion Influx in the Pollen of Arabidopsis thaliana. Plants, 10(8), 1743. https://doi.org/10.3390/plants10081743






