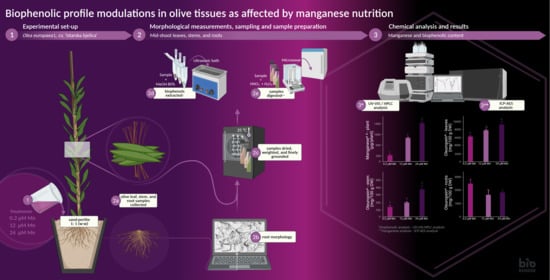
Biophenolic Profile Modulations in Olive Tissues as Affected by Manganese Nutrition
Abstract
1. Introduction
2. Results
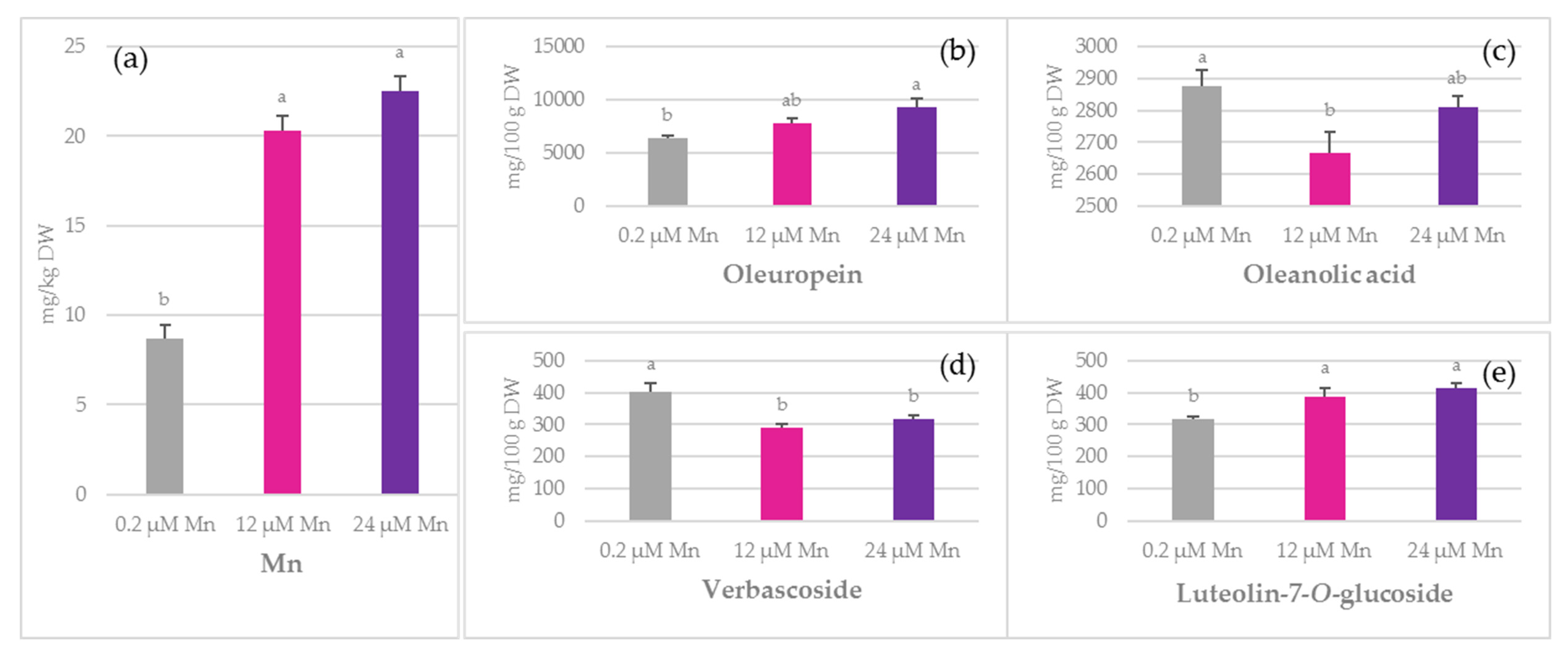
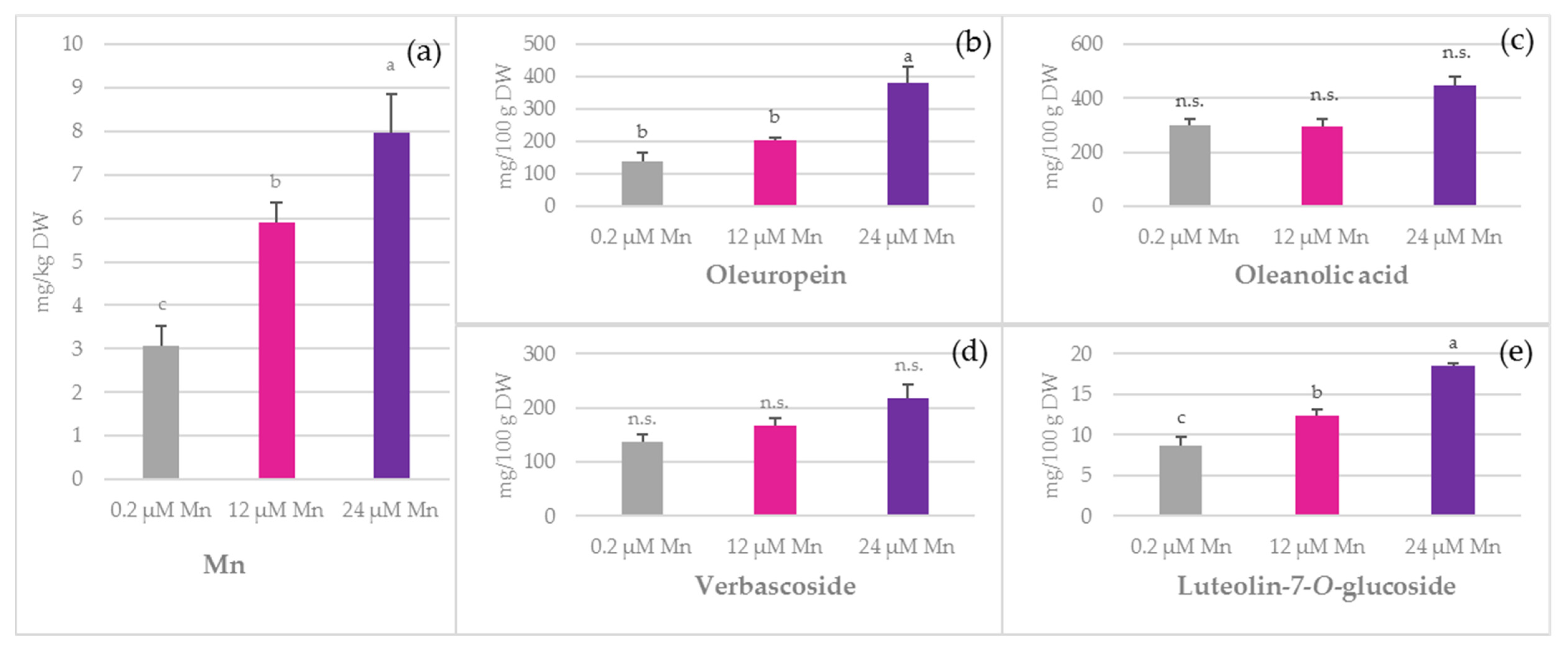
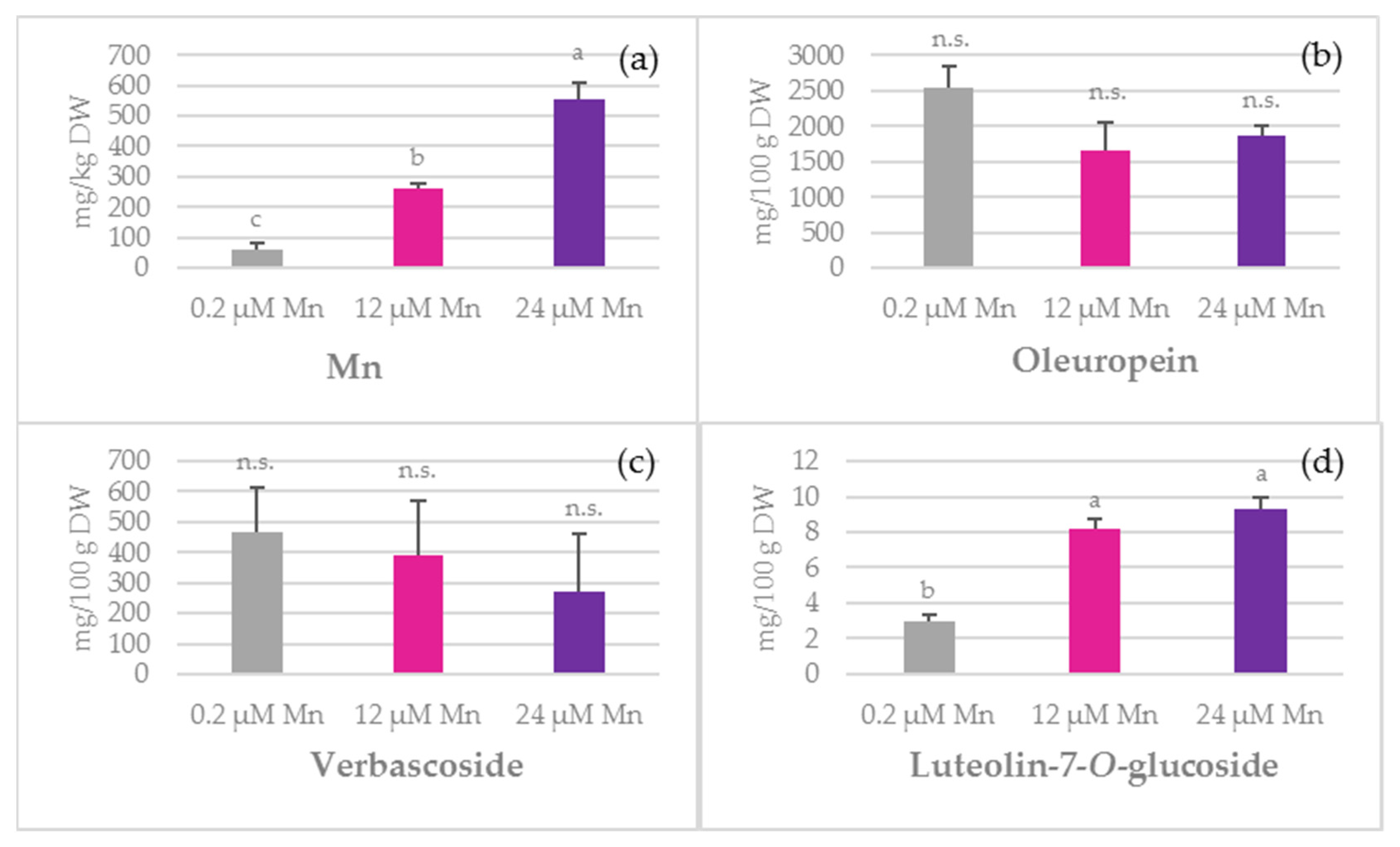
2.1. Concentration of Mn and Biophenols in Olive Leaves, Stems, and Roots
2.2. Morphological Parameters
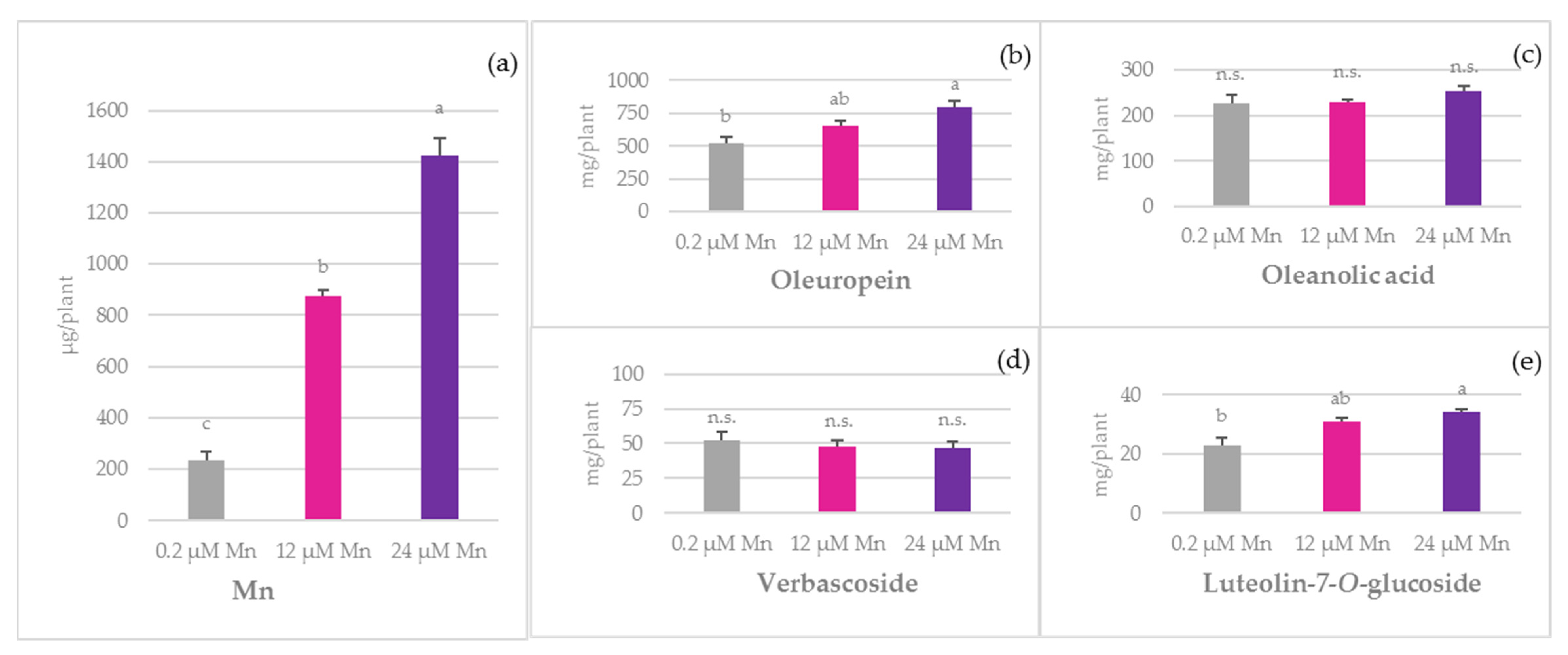
2.3. Total per Plant Quantity of Mn and Biophenols
2.4. Mn Uptake and Use Efficiency
3. Discussion
3.1. Mn Distribution in Olive Tissues and TF
3.2. TPC
3.3. Phenolic Acids
3.4. Oleuropein
3.5. Simple Biophenols
3.6. Oleanolic Acid and Flavonoids
4. Materials and Methods
4.1. Experimental Conditions, Treatments and Sampling
4.2. Chemicals
4.3. Sample Preparation
4.4. Determination of TPC
4.5. High-Performance Liquid Chromatography (HPLC)
4.6. Manganese Concentration Measurement
4.7. Concentration to Quantity Conversion
4.8. TF, MnUE, Mn Distribution
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zarrouk, W.; Baccouri, B.; Taamalli, W.; Trigui, A.; Daoud, D.; Zarrouk, M. Oil fatty acid composition of eighteen Mediterranean olive varieties cultivated under the arid conditions of Boughrara (southern Tunisia). Grasas Y Aceites 2009, 60, 498–506. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Salvador, M.D.; Fregapane, G. Virgin olive oil and olive fruit minor constituents as affected by irrigation management based on SWP and TDF as compared to ETc in medium-density young olive orchards (Olea europaea L. cv. Cornicabra and Morisca). Food Res. Int. 2009, 42, 1067–1076. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; You, F.; Wang, C.Z.; Zhou, H. Reduced-pressure boiling extraction of oleuropein coupled with ultrasonication from olive leaves (Olea europaea L.). Adv. Mater. Sci. Eng. 2015, 2015, 719485. [Google Scholar] [CrossRef][Green Version]
- Žuntar, I.; Putnik, P.; Kovačević, B.D.; Nutrizio, M.; Šupljika, F.; Poljanec, A.; Dubrović, I.; Barba, F.J.; Jambrak, A.R. Phenolic and Antioxidant Analysis of Olive Leaves. Foods 2019, 8, 248. [Google Scholar] [CrossRef]
- Talhaoui, N.; Vezza, T.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Gálvez, J.; Segura-Carretero, A. Phenolic compounds and in vitro immunomodulatory properties of three Andalusian olive leaf extracts. J. Funct. Foods 2016, 22, 270–277. [Google Scholar] [CrossRef]
- Hong, Y.H.; Song, C.; Shin, K.K.; Choi, E.; Hwang, S.H.; Jang, Y.J.; Taamalli, A.; Yum, J.; Kim, J.H.; Kim, E.; et al. Tunisian Olea europaea L. leaf extract suppresses Freund’s complete adjuvant-induced rheumatoid arthritis and lipopolysaccharide-induced inflammatory responses. J. Ethnopharmacol. 2021, 268, 113602. [Google Scholar] [CrossRef]
- Thielmann, J.; Kohnen, S.; Hauser, C. Antimicrobial activity of Olea europaea Linné extracts and their applicability as natural food preservative agents. Int. J. Food Microbiol. 2017, 251, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Lorzadeh, N.; Kazemirad, Y.; Kazemirad, N. Treatment of genital herpes using olive leaf extract. Clin. Case Rep. 2021, 9, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez Román, D.; Gómez Caravaca, A.M.; Zarrouk, M.; Segura Carretero, A. Geographical Characterization of Tunisian Olive Tree Leaves (cv. Chemlali) Using HPLC-ESI-TOF and IT/MS Fingerprinting with Hierarchical Cluster Analysis. J. Anal. Methods Chem. 2018, 2018, 6789704. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K.; Antonopoulou, S.; Kaliora, A.C. Oleuropein inhibits LDL oxidation induced by cooking oil frying by-products and platelet aggregation induced by platelet-activating factor. LWT Food Sci. Technol. 2002, 35, 479–484. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Sanz, M.T.; Blanco, B.; Beltrán, S.; Niknam, S.M. Freeze dried extract from olive leaves: Valorisation, extraction kinetics and extract characterization. Food Bioprod. Process. 2020, 124, 196–207. [Google Scholar] [CrossRef]
- Kritikou, E.; Kalogiouri, N.P.; Kolyvira, L.; Thomaidis, N.S. Target and Suspect HRMS Metabolomics for the Determination of Functional Ingredients in 13 Varieties of Olive Leaves and Drupes from Greece. Molecules 2020, 25, 4889. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Ammar, S.; del Contreras, M.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS based metabolic profiling of the potential Olea europaea by-product “wood” and its comparison with leaf counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef]
- Altarejos, J.; Salido, S.; Pérez-Bonilla, M.; Linares-Palomino, P.J.; Van Beek, T.A.; Nogueras, M.; Sánchez, A. Preliminary assay on the radical scavenging activity of olive wood extracts. Fitoterapia 2005, 76, 348–351. [Google Scholar] [CrossRef]
- Pérez-Bonilla, M.; Salido, S.; Van Beek, T.A.; Altarejos, J. Radical-scavenging compounds from olive tree (Olea europaea L.) wood. J. Agric. Food Chem. 2014, 62, 144–151. [Google Scholar] [CrossRef]
- Entus, R.; Poling, M.; Herrmann, K.M. Redox Regulation of Arabidopsis 3-Deoxy-D-arabino-Heptulosonate 7-Phosphate Synthase. Plant Physiol. 2015. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 1–23. [Google Scholar] [CrossRef]
- Brown, P.H.; Graham, R.D.; Nicholas, D.J.D. The effects of manganese and nitrate supply on the levels of phenolics and lignin in young wheat plants. Plant Soil 1984, 81, 437–440. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Kleiber, T.; Kaczmarek, A. Effect of increasing manganese concentration in nutrient solution on the antioxidant activity, vitamin C, lycopene and polyphenol contents of tomato fruit. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Zarinkamar, F.; Niknam, V. Changes in primary and secondary metabolites of Mentha aquatica L. exposed to different concentrations of manganese. Environ. Sci. Pollut. Res. 2018, 25, 7575–7588. [Google Scholar] [CrossRef] [PubMed]
- Farzadfar, S.; Zarinkamar, F.; Hojati, M. Magnesium and manganese affect photosynthesis, essential oil composition and phenolic compounds of Tanacetum parthenium. Plant Physiol. Biochem. 2017, 112, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Pasković, I.; Herak Ćustić, M.; Pecina, M.; Bronić, J.; Ban, D.; Radić, T.; Pošćić, F.; Jukić Špika, M.; Soldo, B.; Palčić, I.; et al. Manganese soil and foliar fertilization of olive plantlets: The effect on leaf mineral and phenolic content and root mycorrhizal colonization. J. Sci. Food Agric. 2019, 99, 360–367. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Therios, I.; Alifragis, D. Differential uptake, distribution within tissues, and use efficiency of Manganese, Iron, and Zinc by olive cultivars Kothreiki and Koroneiki. HortScience 2009, 44, 1994–1999. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 476–494. [Google Scholar] [CrossRef]
- Ma, G.; Li, J.; Li, J.; Li, Y.; Gu, D.; Chen, C.; Cui, J.; Chen, X. Plant Science OsMTP11, a trans-Golgi network localized transporter, is involved in manganese tolerance in rice. Plant Sci. 2018, 274, 59–69. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, P. The lipid droplet: A conserved cellular organelle. Protein Cell 2017, 8, 796–800. [Google Scholar] [CrossRef]
- Yamada, K.; Nagano, A.J.; Nishina, M.; Hara-nishimura, I.; Nishimura, M. Identification of two novel endoplasmic reticulum body-specific integral membrane proteins. Plant Physiol. 2013, 161, 108–120. [Google Scholar] [CrossRef]
- Schneider, A.C.; Chun, H.; Tefanovic, S.; Baldwin, B.G. Punctuated plastome reduction and host-parasite horizontal gene transfer in the holoparasitic plant genus Aphyllon. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181535. [Google Scholar] [CrossRef]
- Frank, J.; Happeck, R.; Meier, B.; Hoang, M.T.T.; Stribny, J.; Hause, G.; Ding, H.; Morsomme, P.; Baginsky, S.; Peiter, E. Chloroplast-localized BICAT proteins shape stromal calcium signals and are required for efficient photosynthesis. New Phytol. 2019, 221, 866–880. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Palma, J.M.; Rodriguez-Ruiz, M. Plant peroxisomes: A nitro-oxidative cocktail. Redox Biol. 2017, 11, 535–542. [Google Scholar] [CrossRef]
- Eroglu, S.; Giehl, R.F.H.; Meier, B.; Takahashi, M.; Terada, Y.; Ignatyev, K.; Andresen, E.; Küpper, H.; Peiter, E.; von Wirén, N. Metal tolerance protein 8 mediates manganese homeostasis and iron reallocation during seed development and germination. Plant Physiol. 2017, 174, 1633–1647. [Google Scholar] [CrossRef]
- Lanquar, V.; Ramos, M.S.; Lelièvre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Krämer, U.; Thomine, S. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef]
- Connor, D.J.; Fereres, E. The Physiology of Adaptation and Yield Expression in Olive. Hortic. Rev. (Am. Soc. Hortic. Sci). 2010, 31, 155–229. [Google Scholar] [CrossRef]
- Zhang, T.; Li, R.; Xing, J.; Yan, L.; Wang, R.; Zhao, Y. The YUCCA-auxin-wox11 module controls crown root development in rice. Front. Plant Sci. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Tracy, J.E. Manganese Toxicity in Avocado (Persea americana Mill.). Calif. Avocado Soc. 1991, 75, 147–158. [Google Scholar]
- Alam, S.; Kamei, S.; Kawai, S. Amelioration of manganese toxicity in barley with iron. J. Plant Nutr. 2001, 24, 1421–1433. [Google Scholar] [CrossRef]
- Quartin, V.M.L.; Antunes, M.L.; Muralha, M.C.; Sousa, M.M.; Nunes, M.A. Mineral imbalance due to manganese excess in triticales. J. Plant Nutr. 2001, 24, 175–189. [Google Scholar] [CrossRef]
- Sarkar, D.; Pandey, S.K.; Sud, K.C.; Chanemougasoundharam, A. In vitro characterization of manganese toxicity in relation to phosphorus nutrition in potato (Solanum tuberosum L.). Plant Sci. 2004, 167, 977–986. [Google Scholar] [CrossRef]
- Chatzistathis, T.A.; Papadakis, I.E.; Therios, I.N.; Giannakoula, A.; Dimassi, K. Is chlorophyll fluorescence technique a useful tool to assess manganese deficiency and toxicity stress in olive plants? J. Plant Nutr. 2011, 34, 98–114. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Verlag, F.; Lidon, F.C. Tolerance of rice to excess manganese in the early stages of vegetative growth. Characterisation Manganese Accumul. 2001, 158, 1341–1348. [Google Scholar]
- Andrew, B.C.S.; Hegarty, M.P. Comparative responses to manganese excess of eight tropical and four temperate pasture legume species. Aust. J. Agric. Res. 1969, 20, 687–696. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Papadakis, I.; Therios, I.; Patakas, A.; Giannakoula, A.; Menexes, G. Differential Response of Two Olive Cultivars To Excess Manganese. J. Plant Nutr. 2012, 35, 784–804. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D.; Pedler, J.F. Manganese nutrition and accumulation of phenolics and lignin as related to differential resistance of wheat genotypes to the take-all fungus. Plant Soil 1993, 151, 255–263. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; de Paiva Foletto-Felipe, M.; Abrahão, J.; et al. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Leopoldini, M.; Chiodo, S.G.; Russo, N.; Toscano, M. Detailed investigation of the OH radical quenching by natural antioxidant caffeic acid studied by quantum mechanical models. J. Chem. Theory Comput. 2011, 7, 4218–4233. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Root verbascoside and oleuropein are potential indicators of drought resistance in olive trees (Olea europaea L.). Plant Physiol. Biochem. 2019, 141, 407–414. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and biotransformations of phenol-conjugated oleosidic secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K. Phenolic compounds in olives. Analyst 1998, 123, 31–44. [Google Scholar] [CrossRef]
- Alagna, F.; Geu-Flores, F.; Kries, H.; Panara, F.; Baldoni, L.; O’Connor, S.E.; Osbourn, A. Identification and characterization of the iridoid synthase involved in oleuropein biosynthesis in olive (Olea europaea) fruits. J. Biol. Chem. 2016, 291, 5542–5554. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal variation of phenolic and mineral composition in olive leaves is cultivar dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Lukić, I.; Pasković, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z.; et al. Determination of the variability of biophenols and mineral nutrients in olive leaves with respect to cultivar, collection period and geographical location for their targeted and well-timed exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef]
- Garcı, P. Effect of Metal Cations on the Chemical Oxidation of Olive o-Diphenols in Model Systems. J. Agric. Food Chem. 1996, 44, 2101–2105. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Ghannadnia, M.; Haddad, R.; Zarinkamar, F.; Sharifi, M. Manganese treatment effects on terpene compounds of Cuminum cyminum flowers. Ind. Crop. Prod. 2014, 53, 65–70. [Google Scholar] [CrossRef]
- de Alvarenga, E.S.; Silva, S.A.; Barosa, L.C.A.; Demuner, A.J.; Parreira, A.G.; Ribeiro, R.I.M.A.; Marcussi, S.; Ferreira, J.M.S.; Resende, R.R.; Granjeiro, P.A.; et al. synthesis and evaluation of sesquiterpene lactone inhibitors of phospholipase A2 from Bothrops jararacussu. Toxicon 2011, 57, 100–108. [Google Scholar] [CrossRef]
- Jiménez-Herrera, R.; Pacheco-López, B.; Peragón, J. Water stress, irrigation and concentrations of pentacyclic triterpenes and phenols in Olea europaea L. Cv. picual olive trees. Antioxidants 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Perica, S.; Goreta, S.; Selak, G.V. Growth, biomass allocation and leaf ion concentration of seven olive (Olea europaea L.) cultivars under increased salinity. Sci. Hortic. 2008, 117, 123–129. [Google Scholar] [CrossRef]
- Pasković, I.; Pecina, M.; Bronić, J.; Perica, S.; Ban, D.; Ban, S.G.; Pošćić, F.; Palčić, I.; Herak Ćustić, M. Synthetic Zeolite A as Zinc and Manganese Fertilizer in Calcareous Soil. Commun. Soil Sci. Plant Anal. 2018, 49, 1072–1082. [Google Scholar] [CrossRef]
- Popović, M.; Jukić Špika, M.; Veršić Bratinčević, M.; Ninčević, T.; Matešković, A.; Mandušić, M.; Rošin, J.; Nazalić, M.; Dunkić, V.; Vitanović, E. Essential Oil Volatile Fingerprint Differentiates Croatian cv. Oblica from Other Olea europaea L. Cultivars. Molecules 2021, 26, 3533. [Google Scholar] [CrossRef]
- Pasković, I.; Soldo, B.; Talhaoui, N.; Palčić, I.; Brkljača, M.; Koprivnjak, O.; Majetić Germek, V.; Ban, D.; Klanjac, J.; Franić, M.; et al. Boron foliar application enhances oleuropein level and modulates volatile compound composition in olive leaves. Sci. Hortic. 2019, 257, 108688. [Google Scholar] [CrossRef]
- Zvobgo, G.; Lwalaba, J.L.W.; Sehar, S.; Mapodzeke, J.M.; Shamsi, I.H.; Zhang, G. The Tolerance Index and Translocation Factor Were Used to Identify the Barley Genotypes with High Arsenic Stress Tolerance. Commun. Soil Sci. Plant Anal. 2018, 49, 50–62. [Google Scholar] [CrossRef]
| Source of Variation | Total Length (cm/plant) | Surface Area (cm2/plant) | Diameter (mm/plant) | Volume (cm3/plant) |
|---|---|---|---|---|
| Treatment | ||||
| 0.2 µM Mn | 2020.03 ± 142.60 b | 410.91 ± 24.18 b | 0.65 ± 0.01 | 6.67 ± 0.32 b |
| 12 µM Mn | 2444.86 ± 62.72 a | 505.71 ± 15.93 a | 0.66 ± 0.02 | 8.35 ± 0.44 a |
| 24 µM Mn | 1954.38 ± 25.86 b | 392.32 ± 7.61 b | 0.63 ± 0.01 | 6.28 ± 0.17 b |
| p-value | * | ** | n.s. | * |
| Simple Biophenols | Phenolic Acids | Triterpene | Secoiridoid | Flavonoids | TPQ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTYR | TYR | Vanillin | VERB | Vanillic acid | Caffeic acid | Oleanolic acid | Oleuropein | Catechin | Luteolin | Apigenin | Apigenin-O | Rutin | Luteolin-O | ||
| r | 0.61 | 0.14 | −0.26 | −0.20 | 0.62 | 0.89 | 0.36 | 0.83 | 0.57 | −0.70 | 0.18 | −0.30 | 0.75 | 0.81 | 0.65 |
| p | 0.036 | 0.663 | 0.420 | 0.540 | 0.031 | 0.000 | 0.251 | 0.001 | 0.053 | 0.012 | 0.345 | 0.570 | 0.005 | 0.001 | 0.022 |
| Source of Variation | Distribution (%) | TF | MnUE | |||
|---|---|---|---|---|---|---|
| Leaves | Stems | Roots | Leaves | Stems | (mg of the Total Plant DW/µg of the Total per Plant Quantity of Mn) | |
| Treatment | ||||||
| 0.2 µM Mn | 27.7 ± 0.05 a | 11.0 ± 0.01 a | 61.3 ± 0.05 b | 0.48 ± 0.12 a | 0.18 ± 0.03 a | 82.77 ± 0.01 a |
| 12 µM Mn | 17.8 ± 0.01 ab | 6.2 ± 0.01 b | 76.0 ± 0.02 ab | 0.24 ± 0.02 ab | 0.08 ± 0.01 b | 22.15 ± 0.00 b |
| 24 µM Mn | 12.6 ± 0.01 b | 4.2 ± 0.00 b | 83.1 ± 0.01 a | 0.15 ± 0.02 b | 0.05 ± 0.00 b | 12.71 ± 0.00 b |
| p-value | * | ** | * | * | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidović, N.; Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Grozić, K.; Cukrov, M.; Marcelić, Š.; Ban, D.; Talhaoui, N.; et al. Biophenolic Profile Modulations in Olive Tissues as Affected by Manganese Nutrition. Plants 2021, 10, 1724. https://doi.org/10.3390/plants10081724
Vidović N, Pasković I, Lukić I, Žurga P, Majetić Germek V, Grozić K, Cukrov M, Marcelić Š, Ban D, Talhaoui N, et al. Biophenolic Profile Modulations in Olive Tissues as Affected by Manganese Nutrition. Plants. 2021; 10(8):1724. https://doi.org/10.3390/plants10081724
Chicago/Turabian StyleVidović, Nikolina, Igor Pasković, Igor Lukić, Paula Žurga, Valerija Majetić Germek, Kristina Grozić, Marin Cukrov, Šime Marcelić, Dean Ban, Nassima Talhaoui, and et al. 2021. "Biophenolic Profile Modulations in Olive Tissues as Affected by Manganese Nutrition" Plants 10, no. 8: 1724. https://doi.org/10.3390/plants10081724
APA StyleVidović, N., Pasković, I., Lukić, I., Žurga, P., Majetić Germek, V., Grozić, K., Cukrov, M., Marcelić, Š., Ban, D., Talhaoui, N., Palčić, I., Rubinić, V., & Goreta Ban, S. (2021). Biophenolic Profile Modulations in Olive Tissues as Affected by Manganese Nutrition. Plants, 10(8), 1724. https://doi.org/10.3390/plants10081724