Himalayan Ficus palmata L. Fruit Extract Showed In Vivo Central and Peripheral Analgesic Activity Involving COX-2 and Mu Opioid Receptors
Abstract
:1. Introduction
2. Methodology
2.1. Collection and Preparation of Plant Extract
2.2. Animals
2.3. Acute Toxicity Study
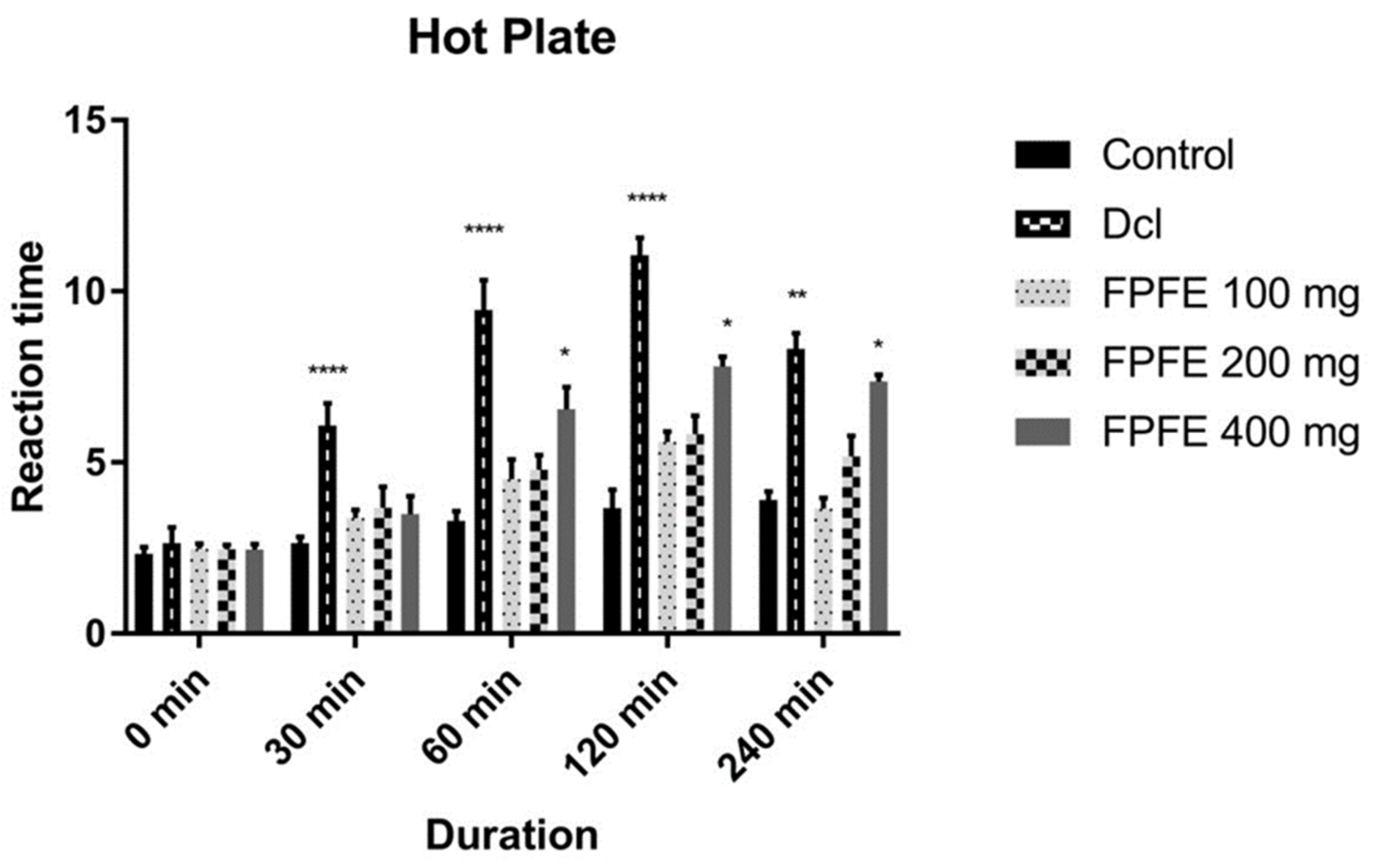
2.4. Hot Plate Test
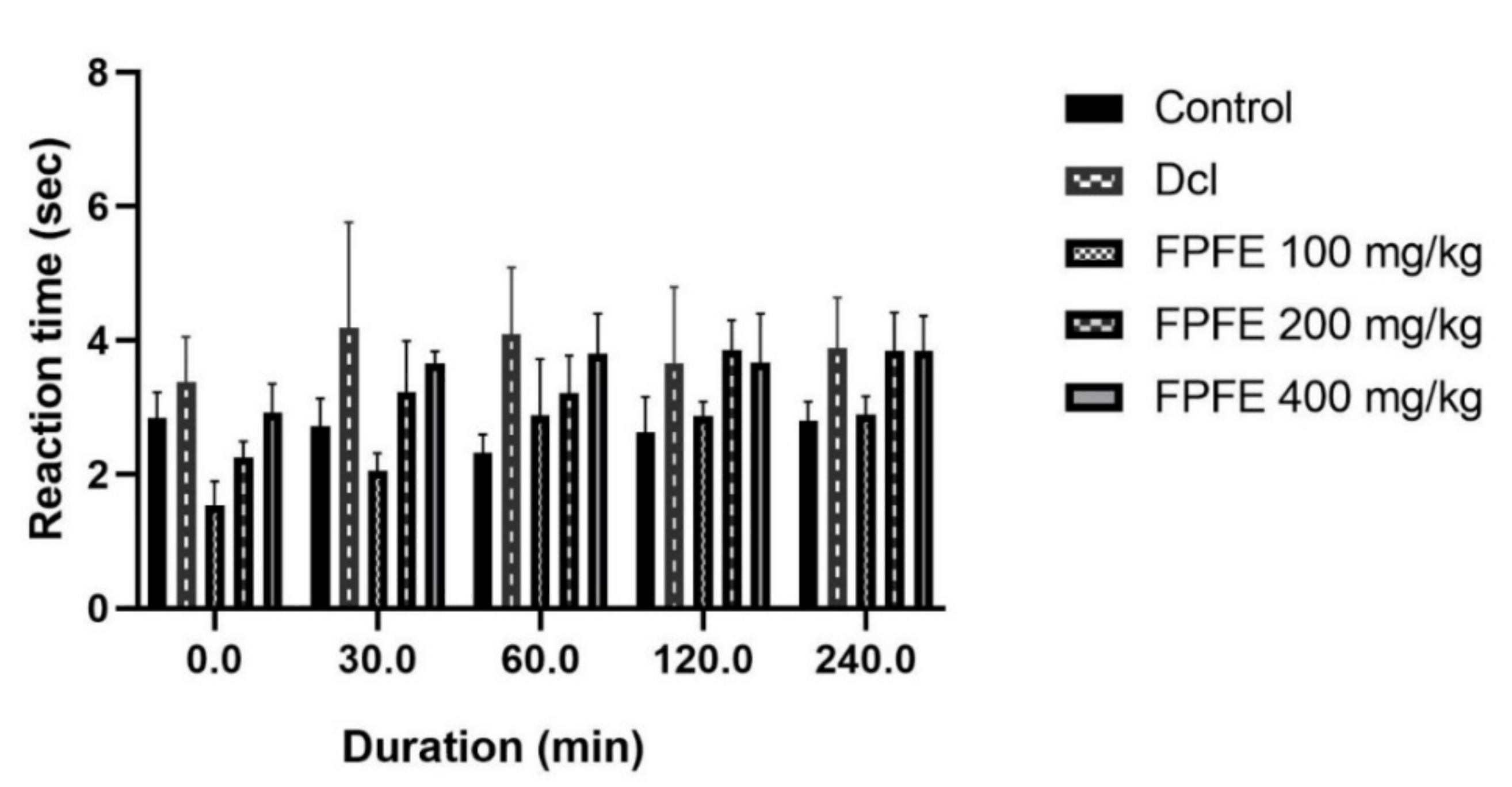
2.5. Tail Flick Test
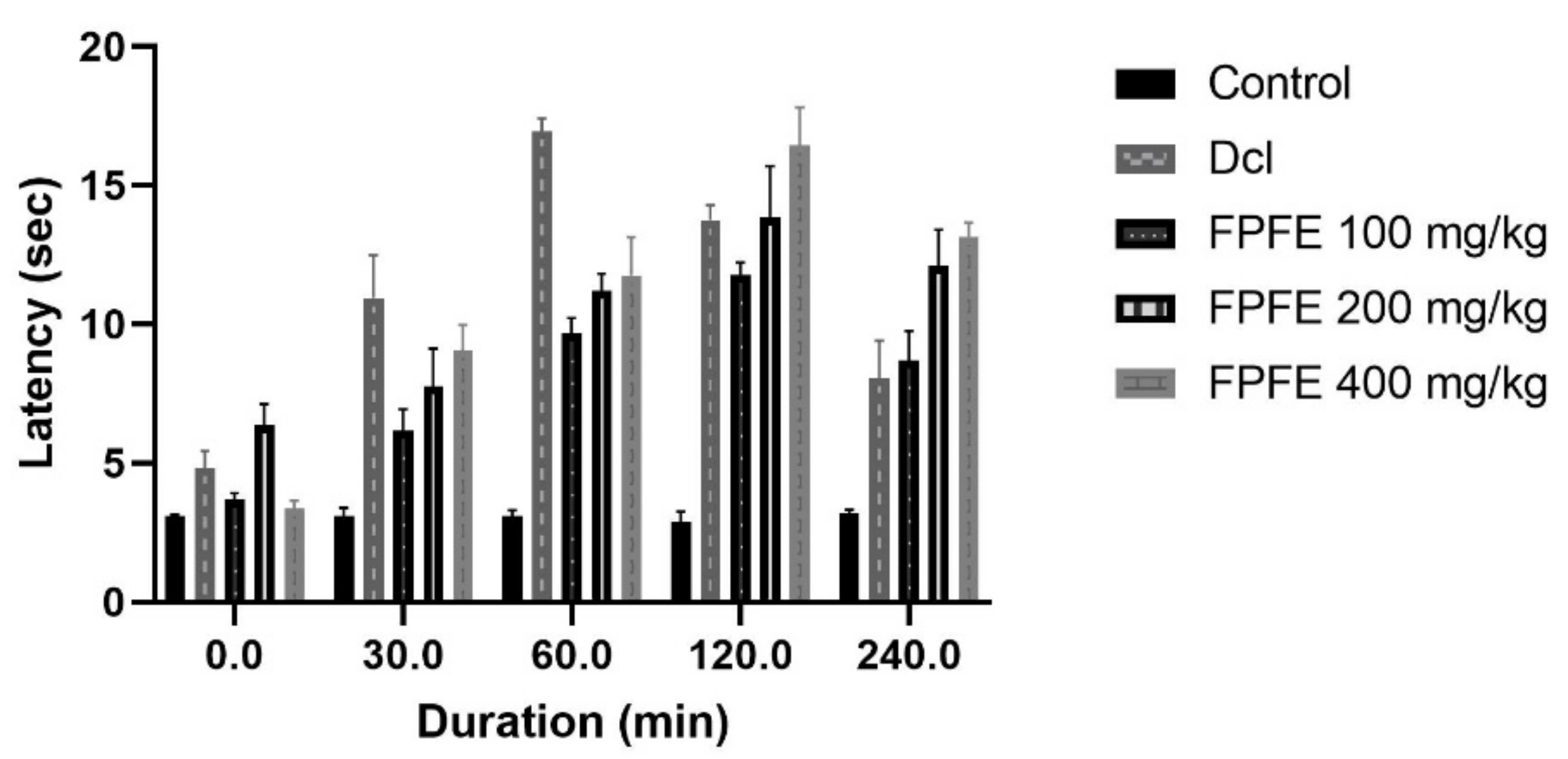
2.6. Tail Immersion Test
2.7. Formaldehyde Induced Pain Model
2.8. Statistical Analysis
2.9. Molecular Docking Studies
3. Results and Discussion
3.1. Chemical Characterization of Plant Extract
3.2. Acute Toxicity Study
3.3. Hot Plate Test
3.4. Tail Flick Test
3.5. Tail Immersion Test
3.6. Formaldehyde Induced Pain Model
3.7. Docking Studies against COX-2 (PDB: 3NT1)
3.8. Docking Studies with the Mu-Opioid (PDB: 4DKL)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FPFE | Ficus palmata L. fruit extract |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| RARI | Regional Ayurveda Research Institute |
| IAEC | Committee of the Department of Pharmaceutical Sciences |
| ATS | Acute toxicity study |
| OECD | Organization for Economic Co-operation and Development |
| NOAEL | non-observable adverse effect dose level |
| SEM | standard error of the mean |
| HPLC | High-performance liquid chromatography |
| ANOVA | Analysis of variance |
| LOD | limit of detection |
| LOQ | limit of quantification |
| UPLC-MS | Ultra performance liquid chromatography-mass spectrometry |
| PDB | The Protein Data Bank |
References
- Rainsford, K.D. Anti-inflammatory drugs in the 21st century. Subcell Biochem. 2007, 42, 3–27. [Google Scholar] [PubMed]
- Ahmadiani, A.; Fereidoni, M.; Semnanian, S.; Kamalinejad, M.; Saremi, S. Antinociceptive and anti-inflammatory effects of Sambucus ebulus rhizome extract in rats. J. Ethnopharmacol. 1998, 61, 229–235. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Shirasaka, M.; Konno, S.-I.; Kikuchi, S.-I. Analgesic effect of percutaneously absorbed non-steroidal anti-inflammatory drugs: An experimental study in a rat acute inflammation model. BMC Musculoskelet. Disord. 2008, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malairajan, P.; Gopalakrishnan, G.; Narasimhan, S.; Jessi Kala Veni, K. Analgesic activity of some Indian medicinal plants. J. Ethnopharmacol. 2006, 106, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.N.; Nayar, S.L. Glossary of Indian Medicinal Plants; Council of Scientific and Industrial Research: New Delhi, India, 1956. [Google Scholar]
- Tewari, D.; Mocan, A.; Parvanov, E.D.; Sah, A.N.; Nabavi, S.M.; Huminiecki, L.; Ma, Z.F.; Lee, Y.Y.; Horbanczuk, J.O.; Atanasov, A.G. Ethnopharmacological approaches for therapy of jaundice: Part I. Front. Pharmacol. 2017, 8, 518. [Google Scholar] [CrossRef] [Green Version]
- Tewari, D.; Sah, A.N.; Bawari, S.; Bussmann, R.W. Ethnobotanical investigations on plants used in folk medicine by native people of Kumaun Himalayan Region of India. Ethnobot. Res. Appl. 2020, 20, 1–35. [Google Scholar] [CrossRef]
- Langerman, L.; Zakowski, M.I.; Piskoun, B.; Grant, G.J. Hot plate versus tail flick: Evaluation of acute tolerance to continuous morphine infusion in the rat model. J. Pharmacol. Toxicol. Methods 1995, 34, 23–27. [Google Scholar] [CrossRef]
- Vasudewa, N.S.; Abeytunga, D.T.U.; Ratnasooriya, W.D. Antinociceptive Activity of Pleurotus ostreatus., an Edible Mushroom, in Rats. Pharm. Biol. 2007, 45, 533–540. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [Google Scholar]
- Chakraborty, A.; Devi, R.K.B.; Rita, S.; Sharatchandra, K.H.; Singh, T.I. Preliminary studies on antiinflammatory and analgesic activities of Spilanthes acmella in experimental animal models. Indian J. Pharmacol. 2004, 36, 148. [Google Scholar]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Gou, K.-J.; Zeng, R.; Dong, Y.; Hu, Q.-Q.; Hu, H.-W.-Y.; Maffucci, K.G.; Dou, Q.-L.; Yang, Q.-B.; Qin, X.-H.; Qu, Y. Anti-inflammatory and Analgesic Effects of Polygonum orientale L. Extracts. Front. Pharmacol. 2017, 8, 562. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yuzurihara, M.; Hibino, T.; Yano, S.; Kase, Y. Aqueous extract of Asiasari radix inhibits formalin-induced hyperalgesia via NMDA receptors. J. Ethnopharmacol. 2009, 123, 128–133. [Google Scholar] [CrossRef]
- Marahel, S.; Umesha, S. Anti-inflammatory and antinociceptive effect of Pachygone ovata leaves. Pharm. Biol. 2016, 54, 3046–3054. [Google Scholar] [CrossRef] [Green Version]
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef]
- Alqasoumi, S.I.; Basudan, O.A.; Al-Rehaily, A.J.; Abdel-Kader, M.S. Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia. Saudi Pharm. J. 2014, 22, 460–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Gupta, P.; Gupta, J. Virtual structural similarity elucidates bioactivity of Fenchone: Enriched phytochemical in fennel essential oil. Curr. Drug Discov. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Zengin, G.; Ak, G.; Sinan, K.I.; Cziáky, Z.; Mishra, S.T.; Jekő, J. Phenolic Profiling, Antioxidants, Multivariate, and Enzyme Inhibitory Properties of Wild Himalayan Fig (Ficus palmata Forssk.): A Potential Candidate for Designing Innovative Nutraceuticals and Related Products. Anal. Lett. 2020, 54, 1439–1456. [Google Scholar] [CrossRef]
- Tewari, D.; Sah, A.N.; Bawari, S.; Sharma, H.; Mangal, A.K. Pharmacognostical Evaluation and HPTLC Fingerprinting Identification of Ficus palmata Forssk. (Bedu) from Western Himalaya. Curr. Bioact. Compd. 2018, 14, 180–190. [Google Scholar] [CrossRef]
- Oniga, S.D.; Pacureanu, L.; Stoica, C.I.; Palage, M.D.; Craciun, A.; Rusu, L.R.; Crisan, E.-L.; Araniciu, C. COX Inhibition Profile and Molecular Docking Studies of Some 2-(Trimethoxyphenyl)-Thiazoles. Molecules 2017, 22, 1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Yaksh, T.L. Comparison of the antinociceptive effects of pre- and posttreatment with intrathecal morphine and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology 1992, 77, 757–763. [Google Scholar] [CrossRef]
- Dirig, D.M.; Yaksh, T.L. Intrathecal baclofen and muscimol, but not midazolam, are antinociceptive using the rat-formalin model. J. Pharmacol. Exp. Ther. 1995, 275, 219–227. [Google Scholar]
- Beecher, H.K. The measurement of pain: Prototype for the quantitative study of subjective responses. Pharmacol. Rev. 1957, 9, 59–209. [Google Scholar]
- D’Amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
- Tjolsen, A.; Lund, A.; Berge, O.G.; Hole, K. An improved method for tail-flick testing with adjustment for tail-skin temperature. J. Neurosci. Methods 1989, 26, 259–265. [Google Scholar] [CrossRef]
- Taghi Mansouri, M.; Naghizadeh, B.; Ghorbanzadeh, B.; Farbood, Y. Central and peripheral antinociceptive effects of ellagic acid in different animal models of pain. Eur. J. Pharmacol. 2013, 707, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.P.; Böhmer, A.E.; Antunes, C.; Schallenberger, C.; Porciúncula, L.O.; Elisabetsky, E.; Lara, D.R.; Souza, D.O. Anti-nociceptive properties of the xanthine oxidase inhibitor allopurinol in mice: Role of A(1) adenosine receptors. Br. J. Pharmacol. 2009, 156, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Kunanusorn, P.; Teekachunhatean, S.; Sangdee, C.; Panthong, A. Antinociceptive and anti-inflammatory activities of a chinese herbal recipe (DJW) in animal models. Int. J. Appl. Res. Nat. Prod. 2009, 2, 1–8. [Google Scholar]
- Rezayat, M.; Tabarrai, E.; Parvini, S.; Zarrindast, M.R.; Pirali, M. Effects of CCK antagonists on GABA mechanism-induced antinociception in the formalin test. Eur. Neuropsychopharmacol. 1999, 9, 9–14. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.C.; Shim, S.H.; Lee, E.J.; Jin, W.; Bae, K.; Son, K.H.; Kim, H.P.; Kang, S.S.; Chang, H.W. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch. Pharm. Res. 2006, 29, 617–623. [Google Scholar] [CrossRef]
- Hua, J.M.; Moon, T.C.; Hong, T.G.; Park, K.M.; Son, J.K.; Chang, H.W. 5-Methoxy-8-(2-hydroxy-3-buthoxy-3-methylbutyloxy)-psoralen isolated from Angelica dahurica inhibits cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res. 2008, 31, 617–621. [Google Scholar] [CrossRef]
- Timonen, J.M.; Vuolteenaho, K.; Leppänen, T.; Nieminen, R.M.; Aulaskari, P.; Jänis, J.; Vainiotalo, P.; Moilanen, E. Synthesis of Novel Anti-inflammatory Psoralen Derivatives—Structures with Distinct Anti-Inflammatory Activities. J. Heterocycl. Chem. 2018, 55, 2590–2597. [Google Scholar] [CrossRef]
- Moutinho, M.S.S.; Aragão, S.; Carmo, D.; Casaca, F.; Silva, S.; Ribeiro, J.; Sousa, H.; Pires, I.; Queiroga, F.; Colaço, B.; et al. Curcumin and Rutin Down-regulate Cyclooxygenase-2 and Reduce Tumor-associated Inflammation in HPV16-Transgenic Mice. Anticancer Res. 2018, 38, 1461–1466. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.; Ekpo, O. Inhibition of γH2AX, COX-2 and regulation of antioxidant enzymes in MPP(+)-exposed SH-SY5Y cells pre-treated with rutin. Metab. Brain Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Arowoogun, J.; Akanni, O.O.; Adefisan, A.O.; Owumi, S.E.; Tijani, A.S.; Adaramoye, O.A. Rutin ameliorates copper sulfate-induced brain damage via antioxidative and anti-inflammatory activities in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22623. [Google Scholar] [CrossRef]
- Selvaraj, G.; Kaliamurthi, S.; Thirungnasambandam, R.; Vivekanandan, L.; Balasubramanian, T. Anti-nociceptive effect in mice of thillai flavonoid rutin. Biomed. Environ. Sci. 2014, 27, 295–299. [Google Scholar] [PubMed]
- Hernandez-Leon, A.; Fernández-Guasti, A.; González-Trujano, M.E. Rutin antinociception involves opioidergic mechanism and descending modulation of ventrolateral periaqueductal grey matter in rats. Eur. J. Pain 2016, 20, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Da R Lapa, F.; Gadotti, V.M.; Missau, F.C.; Pizzolatti, M.G.; Marques, M.C.A.; Dafré, A.L.; Farina, M.; Rodrigues, A.L.S.; Santos, A.R.S. Antinociceptive properties of the hydroalcoholic extract and the flavonoid rutin obtained from Polygala paniculata L. in mice. Basic Clin. Pharmacol. Toxicol. 2009, 104, 306–315. [Google Scholar] [CrossRef]
- Tominaga, M.; Ogawa, H.; Takamori, K. Possible roles of epidermal opioid systems in pruritus of atopic dermatitis. J. Investig. Dermatol. 2007, 127, 2228–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]

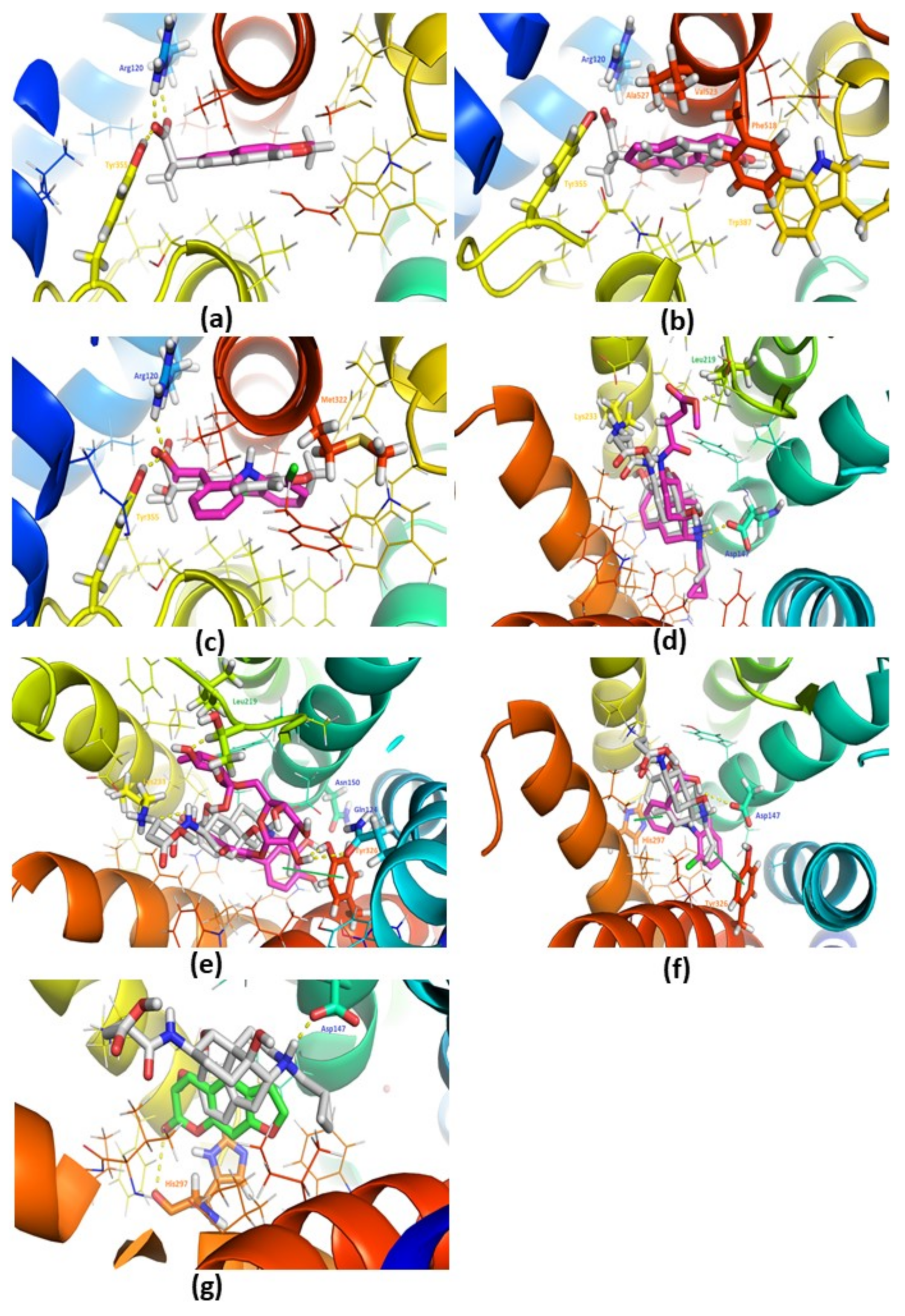
| Mole | PDB ID | Score (Kcal/mol) | Interactions | ||
|---|---|---|---|---|---|
| H-Bonding | Van der Waals | Other | |||
| Naproxen (X-Ray) | 3NT1 | −9.20 | Arg120, Tyr355 | Val394, Leu352, Tyr355, Leu359, Val523, Ala527, Leu531 | Salt Bridge: Arg120 |
| Diclofenac | 3NT1 | −7.20 | Arg120, Tyr355, Val523 | Val349, Leu352, Tyr385, Trp387, Val523, Ala527 | Halogen bond: Met522 Salt Bridge: Arg120 |
| Psoralen | 3NT1 | −8.40 | Arg120 | Leu352, Trp387, Val523 | |
| Rutin | 3NT1 | −6.80 | Arg120, Tyr355, Met522, Val523, Glu524, Ser530 | Val89, Leu93, Trp100, Ile112, Val116, Val349, Leu531 | π-cation: Arg120 Salt Bridge: Arg120 |
| Morphinan Antagonist (X-ray) | 4DKL | −9.00 | Asp147, Leu219, Lys233 | Leu219, Trp293, Ile296, Val300, Ile322, Tyr326, | Salt Bridge: Asp147 |
| Diclofenac | 4DKL | −7.40 | Asp147 | Trp293, Ile296, Val300 | π-Stacking: His297, Tyr326 |
| Psoralen | 4DKL | −7.20 | His297 | Val236, Ile296, Val300 | Salt Bridge: His297 |
| Rutin | 4DKL | −9.60 | Gln124, Asn150, Leu219, Lys233, Tyr326 | Lys233, Ile296, Val300, Ile322 | π-Stacking: Tyr326 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tewari, D.; Gupta, P.; Bawari, S.; Sah, A.N.; Barreca, D.; Khayatkashani, M.; Khayat Kashani, H.R. Himalayan Ficus palmata L. Fruit Extract Showed In Vivo Central and Peripheral Analgesic Activity Involving COX-2 and Mu Opioid Receptors. Plants 2021, 10, 1685. https://doi.org/10.3390/plants10081685
Tewari D, Gupta P, Bawari S, Sah AN, Barreca D, Khayatkashani M, Khayat Kashani HR. Himalayan Ficus palmata L. Fruit Extract Showed In Vivo Central and Peripheral Analgesic Activity Involving COX-2 and Mu Opioid Receptors. Plants. 2021; 10(8):1685. https://doi.org/10.3390/plants10081685
Chicago/Turabian StyleTewari, Devesh, Pawan Gupta, Sweta Bawari, Archana N. Sah, Davide Barreca, Maryam Khayatkashani, and Hamid Reza Khayat Kashani. 2021. "Himalayan Ficus palmata L. Fruit Extract Showed In Vivo Central and Peripheral Analgesic Activity Involving COX-2 and Mu Opioid Receptors" Plants 10, no. 8: 1685. https://doi.org/10.3390/plants10081685
APA StyleTewari, D., Gupta, P., Bawari, S., Sah, A. N., Barreca, D., Khayatkashani, M., & Khayat Kashani, H. R. (2021). Himalayan Ficus palmata L. Fruit Extract Showed In Vivo Central and Peripheral Analgesic Activity Involving COX-2 and Mu Opioid Receptors. Plants, 10(8), 1685. https://doi.org/10.3390/plants10081685








