Evaluation of Preemergent Herbicides for Chloris virgata Control in Mungbean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1. Field Study
2.2. Experiment 2. Pot Study (PRE Herbicides)
2.3. Experiment 3. Pot Study (Imazapic Doses)
2.4. Statistical Analyses
3. Results
3.1. Experiment 1. Field Study
3.2. Experiment 2. Pot Study (PRE Herbicides)
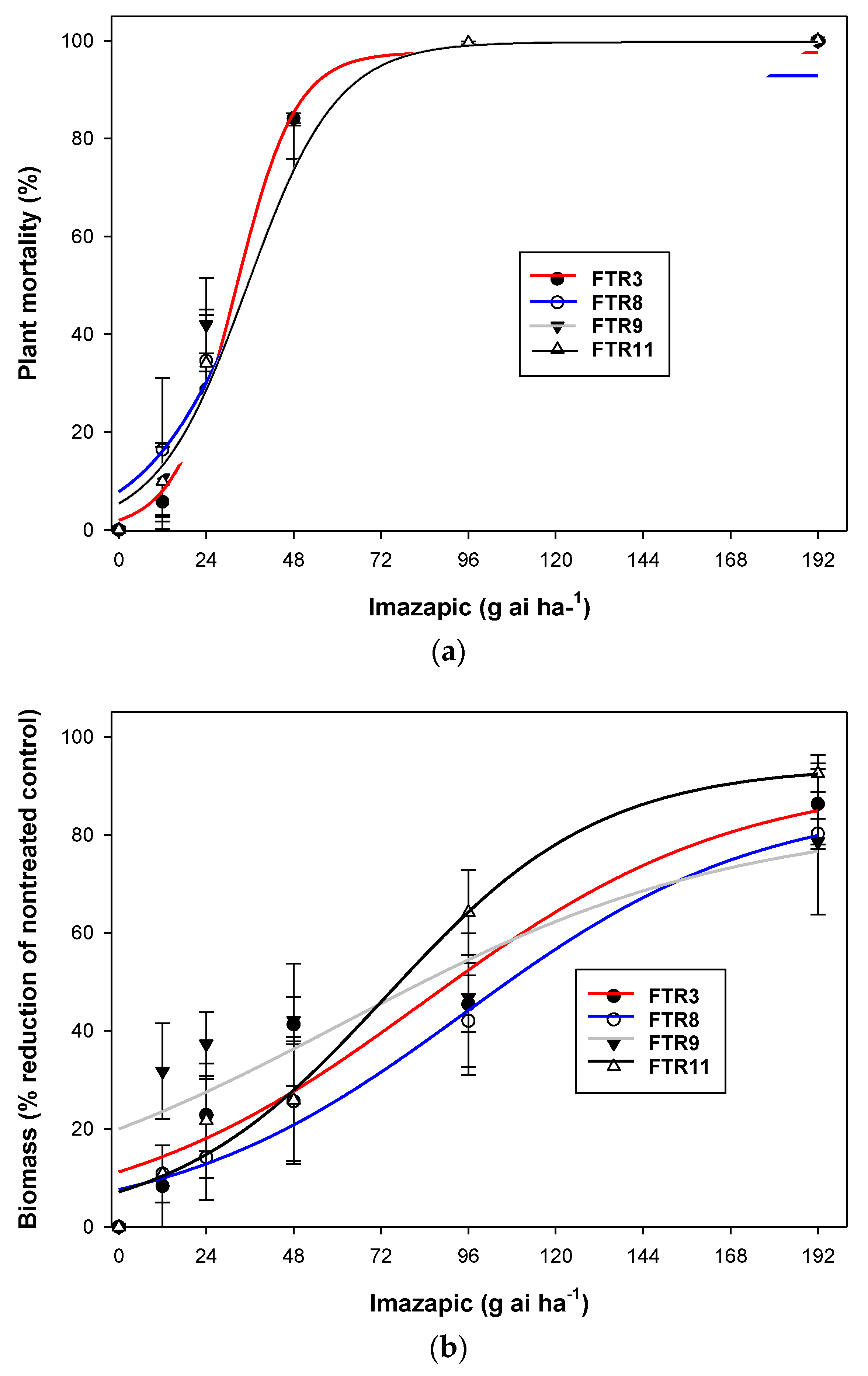
3.3. Experiment 3. Pot Study (Imazapic Doses)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarry, S. The Rise and Rise of Mungbeans. GroundCover Issue 125—Pulse Breeding Advances. Available online: https://grdc.com.au/Media-Centre/Ground-Cover-Supplements/Ground-CoverIssue-125--Pulse-breeding-advances/The-rise-and-rise-of-mungbeans2016 (accessed on 26 May 2021).
- Rachaputi, R.C.N.; Sands, D.; McKenzie, K.; Agius, P.; Lehane, J.; Seyoum, S. Eco-physiological drivers influencing mungbean Vigna radiata (L.) Wilczek productivity in subtropical Australia. Field Crop. Res. 2019, 238, 74–81. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Florentine, S.K.; Ferguson, J.C.; Chechetto, R.G. Implications of narrow crop row spacing in managing weeds in mungbean (Vigna radiata). Crop. Prot. 2017, 95, 116–119. [Google Scholar] [CrossRef]
- Yadav, S.K.; Bhan, V.M.; Singh, S.P. Crop-weed competition studies in mung beans (Vigna radiata). Exp. Agric. 1983, 19, 337–340. [Google Scholar] [CrossRef]
- Davidson, B.; Cook, T.; Chauhan, B.S. Alternative options to glyphosate for control of large Echinochloa colona and Chloris virgata plants in cropping fallows. Plants 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, H.S.; Thompson, M.; Chauhan, B.S. Target-site resistance to glyphosate in Chloris virgata biotypes and alternative herbicide options for its control. Agronomy 2020, 10, 1266. [Google Scholar] [CrossRef]
- Fernando, N.; Humphries, T.; Florentine, S.K.; Chauhan, B.S. Factors affecting seed germination of feather fingergrass (Chloris virgata). Weed Sci. 2016, 64, 605–612. [Google Scholar] [CrossRef]
- Ngo, T.D.; Krishnan, M.; Boutsalis, P.; Gill, G.; Preston, C. Target-site mutations conferring resistance to glyphosate in feathertop Rhodes grass (Chloris virgata) populations in Australia. Pest. Manag. Sci. 2018, 74, 1094–1100. [Google Scholar] [CrossRef]
- Widderick, M.; McLean, A. Optimal intervals differ for double knock application of paraquat after glyphosate or haloxyfop for improved control of Echinochloa colona, Chloris virgata and Chloris truncata. Crop. Prot. 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Llewellyn, R.S.; Ronning, D.; Ouzman, J.; Walker, S.; Mayfield, A.; Clarke, M. Impact of Weeds on Australian Grain Production: The Cost of Weeds to Australian Grain Growers and the Adoption of Weed Management and Tillage Practices; Report for GRDC; CSIRO: Kingston, ACT, Australia, 2016. [Google Scholar]
- Ngo, T.D.; Boutsalis, P.; Preston, C.; Gill, G. Growth, development, and seed biology of feather fingergrass (Chloris virgata) in Southern Australia. Weed Sci. 2017, 65, 413–425. [Google Scholar] [CrossRef]
- Manalil, S.; Mobli, A.; Chauhan, B.S. Competitiveness of windmill grass (Chloris truncata) and feathertop Rhodes grass (Chloris virgata) in mungbean (Vigna radiata). Crop. Pasture Sci. 2020, 71, 916–923. [Google Scholar] [CrossRef]
- Werth, J.; Boucher, L.; Thornby, D.; Walker, S.; Charles, G. Changes in weed species since the introduction of glyphosate-resistant cotton. Crop. Pasture Sci. 2013, 64, 791–798. [Google Scholar] [CrossRef]
- Manalil, S.; Werth, J.; Jackson, R.; Chauhan, B.S.; Preston, C. An assessment of weed flora 14 years after the introduction of glyphosate-tolerant cotton in Australia. Crop. Pasture Sci. 2017, 68, 773–780. [Google Scholar] [CrossRef]
- Grain Research and Development Corporation. Available online: https://grdc.com.au/resources-and-publications/all-publications/publications/2020/integrated-weed-management-of-feathertop-rhodes-grass?utm_medium=short_url&utm_content=Integrated%20Weed%20Management%20of%20Feathertop%20Rhodes%20Grass&utm_source=website&utm_term=North (accessed on 5 June 2021).
- Kaur, S.; Kaur, T.; Bhullar, M.S. Imidazolinone herbicides for weed control in greengram. Indian J. Weed Sci. 2016, 48, 37–39. [Google Scholar] [CrossRef]
- Khairnar, C.B.; Goud, V.V.; Sethi, H.N. Pre- and post-emergence herbicides for weed management in mungbean. Indian J. Weed Sci. 2014, 46, 392–395. [Google Scholar]
- Patel, B.D.; Chaudhari, D.D.; Patel, V.J.; Patel, R.B. Pre- and post-emergence herbicides for weed control in greengram and their residual effect on succeeding crops. Indian J. Weed Sci. 2016, 48, 40–43. [Google Scholar] [CrossRef]
- Mahajan, G.; Walsh, M.; Chauhan, B.S. Junglerice (Echinochloa colona) and feather fingergrass (Chloris virgata) seed production and retention at sorghum maturity. Weed Technol. 2020, 34, 272–276. [Google Scholar] [CrossRef]
- Heap, I. (Ed.) International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org (accessed on 4 February 2021).
- Grain Research and Development Corporation. Available online: https://grdc.com.au/__data/assets/pdf_file/0014/315311/GRDC-GrowNotes-Mungbeans-Northern.pdf (accessed on 26 May 2021).
- LaRossa, R.A.; Schloss, J.V. The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonella typhimurium. J. Biol. Chem. 1984, 259, 8753–8757. [Google Scholar] [CrossRef]
- BASF. Pursuit Herbicide; BASF: Ludwigshafen, Germany, 2011; Available online: https://agro.basf.ca/basf/agprocan/agsolutions/solutions.nsf/Images/PDC-OAIO-AFWLFC/$File/Pursuit_TechSheet.pdf (accessed on 26 May 2021).
- Mallory-Smith, C.A.; Retzinger, E.J., Jr. Revised classification of herbicides by site of action for weed resistance management strategies. Weed Technol. 2003, 17, 605–619. [Google Scholar] [CrossRef]
- Ali, S.; Patel, J.C.; Desai, L.J.; Singh, J. Effect of herbicides on weeds and yield of rainy season greengram (Vigna radiata L. Wilczek). Legume Res. 2011, 34, 300–303. [Google Scholar]
- Naylor, E.L. Weed Management Handbook; Blackwell Publishing Company: Oxford, UK, 2008. [Google Scholar]
- Senseman, S.A.; Armbrust, K. Herbicide Handbook; Weed Science Society of America: Champaign, IL, USA, 2007. [Google Scholar]
- Buttar, G.S.; Aulakh, C.S.; Mehra, S.P. Chemical weed control in mungbean (Vigna radiata L.)—Farmer’s participatory approach. Indian J. Weed Sci. 2006, 38, 276–277. [Google Scholar]
- Mirjha, P.R.; Prasad, S.K.; Patel, S.; Baghel, P.; Monesh, S. Effect of chemical weed control on weed indices in kharif mungbean (Vigna radiata L. Wilczek). Environ. Ecol. 2013, 31, 1526–1529. [Google Scholar]
- Duke, S.O.; Lydon, J.; José, M.B.; Sherman, T.D.; Lehnen, L.P.; Matsumoto, H. Protoporphyrinogen oxidase-inhibiting herbicides. Weed Sci. 1991, 39, 465–473. [Google Scholar] [CrossRef]
- Soltani, N.; Nurse, R.E.; Shropshire, C.; Sikkema, P.H. Tolerance of adzuki bean to pre-emergence herbicides. Can. J. Plant Sci. 2015, 95, 959–963. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S. Phytotoxicity of selected herbicides to mung bean (Phaseolus aureus Roxb.). Environ. Exp. Bot. 2006, 55, 41–48. [Google Scholar] [CrossRef]
- Bhowmik, P.C.; Swarcewicz, M.K.; Mitra, S. Plant bioassay for isoxaflutole in soil. In Proceedings of the 18th Asian Pacific Weed Science Society Conference, Beijing, China, 28 May–2 June 2001; pp. 663–670. [Google Scholar]
- Soltani, N.; Shropshire, C.; Sikkema, P.H. Sensitivity of dry bean to dimethenamid-p, saflufenacil and dimethenamid-p/saflufenacil. Am. J. Plant Sci. 2014, 5, 50931. [Google Scholar] [CrossRef] [Green Version]
- Macleod, I.J.; Frost, P.R. Dimethenamid-P—A new selective herbicide for Australian horticulture. In Proceedings of the 13th Australian Weeds Conference, Perth, WA, Australia, 8–13 September 2002. [Google Scholar]
- Poling, K.W.; Renner, K.A.; Penner, D. Dry edible bean class and cultivar response to dimethenamid and metolachlor. Weed Technol. 2009, 23, 73–80. [Google Scholar] [CrossRef]
- Walsh, M.J.; Fowler, T.M.; Crowe, B.; Ambe, T.; Powles, S.B. The potential for pyroxasulfone to selectively control resistant and susceptible rigid ryegrass (Lolium rigidum) biotypes in Australian grain crop production systems. Weed Technol. 2011, 25, 30–37. [Google Scholar] [CrossRef]
- Devine, M.; Duke, S.O.; Fedtke, C. Physiology of Herbicide Action; Prentice Hall: Englewood Cliffs, NJ, USA, 1993. [Google Scholar]
| Herbicides | Dose (g ai ha−1) | Emergence (Plants per Meter Row Length) | Crop Phytotoxicity Score | Grain Yield (kg ha−1) |
|---|---|---|---|---|
| Nontreated control | - | 14b | 0 | 247a |
| Dimethenamid-P | 720 | 9b | 20 | 805cd |
| Diuron | 747 | 11b | 10 | 462ab |
| Flumioxazin | 90 | 7ab | 40 | 590b |
| Imazapic | 48 | 14b | 10 | 483b |
| Imazethapyr | 70 | 13b | 10 | 534b |
| Isoxaflutole | 75 | 3a | 95 | 349ab |
| Linuron | 1125 | 12b | 10 | 448ab |
| Metribuzin | 360 | 9b | 15 | 201a |
| Pendimethalin | 1000 | 13b | 10 | 807cd |
| Prosulfocarb + S-metolachlor | 2300 | 10b | 10 | 751cd |
| Pyroxasulfone | 100 | 11b | 15 | 900d |
| S-metolachlor | 1440 | 13b | 10 | 677cd |
| Terbuthylazine | 752 | 10b | 10 | 398ab |
| Trifluralin | 600 | 10b | 15 | 650bc |
| LSD (0.05) | - | 5 | - | 226 |
| Herbicides | Dose (g ai ha−1) | C. virgata Density (Plants m−2) | C. virgata Biomass (g m−2) | C. virgata Seed (no. m−2) |
|---|---|---|---|---|
| Nontreated | - | 8.0bc | 350c | 64,480c |
| Dimethenamid-P | 720 | 2.7a | 30a | 2080a |
| Diuron | 747 | 10.7c | 152b | 11,440ab |
| Flumioxazin | 90 | 10.7c | 74a | 12,480ab |
| Imazapic | 48 | 12.0c | 241bc | 19,760ab |
| Imazethapyr | 70 | 8.0bc | 181b | 13,520ab |
| Isoxaflutole | 75 | 2.7a | 147b | 6240ab |
| Linuron | 1125 | 10.7c | 179b | 17,680ab |
| Metribuzin | 360 | 10.7c | 298bc | 49,920bc |
| Pendimethalin | 1000 | 4.0ab | 29a | 4160a |
| Prosulfocarb + S-metolachlor | 2300 | 4.0ab | 78a | 8320ab |
| Pyroxasulfone | 100 | 4.0ab | 22a | 4160a |
| S-metolachlor | 1440 | 4.0ab | 43a | 3120a |
| Terbuthylazine | 752 | 10.7c | 321c | 39,520bc |
| Trifluralin | 600 | 4.0ab | 37a | 2080a |
| LSD (0.05) | - | 5.2 | 120 | 34,848 |
| Herbicides | Dose (g ai ha−1) | Survival % (Run 1) | Survival % (Run 2) | Biomass (g per Pot) (Run 1) | C. virgata Biomass (g per Pot) (Run 2) |
|---|---|---|---|---|---|
| Nontreated control | - | 35b | 67d | 6.8c | 3.8c |
| Atrazine | 2700 | 35b | 52cd | 4.2b | 1.3b |
| Dimethenamid-P | 720 | 0a | 0a | 0.0a | 0.0a |
| Diuron | 747 | 35b | 58cd | 5.6c | 3.8c |
| Imazapic | 48 | 28b | 47b | 3.6b | 1.9b |
| Imazethapyr | 70 | 32b | 62c | 5.6c | 3.6c |
| Isoxaflutole | 75 | 8a | 40b | 0.4a | 1.2b |
| Metribuzin | 360 | 30b | 57c | 5.9c | 1.2b |
| Pendimethalin | 1000 | 0a | 0a | 0.0a | 0.0a |
| Prosulfocarb + S-metolachlor | 2300 | 7a | 0a | 0.1a | 0.0a |
| Pyroxasulfone | 100 | 2a | 2a | 0.02a | 0.03a |
| S-metolachlor | 1440 | 0a | 0a | 0.0a | 0.0a |
| Terbuthylazine | 752 | 35b | 67d | 6.6 | 3.8c |
| Triallate | 800 | 27b | 32b | 4.14b | 1.2b |
| Trifluralin | 600 | 7a | 5a | 1.3a | 0.04a |
| LSD (0.05) | - | 12 | 18 | 1.3 | 0.9 |
| Population | Gmax | Grate | x50 | R2 |
|---|---|---|---|---|
| Plant mortality (%) | ||||
| FTR3 | 6420 (102) | −117 (88) | −531 (135) | 0.95 |
| FTR8 | 108 (54) | −78 (42) | 74 (9) | 0.94 |
| FTR9 | 19,516 (187) | −152 (67) | −886 (184) | 0.73 |
| FTR11 | 108 (42) | −47(23) | 56 (44) | 0.96 |
| Biomass reduction (%) | ||||
| FTR3 | 4 (0.2) | −8 (1.3) | 33 (2) | 0.99 |
| FTR8 | 5 (1.4) | −31 (9) | 29 (20) | 0.99 |
| FTR9 | 4 (0) | −8 (0) | 33 (0) | 0.99 |
| FTR11 | 4 (0.3) | −14 (2) | 34 (3) | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahajan, G.; Chauhan, B.S. Evaluation of Preemergent Herbicides for Chloris virgata Control in Mungbean. Plants 2021, 10, 1632. https://doi.org/10.3390/plants10081632
Mahajan G, Chauhan BS. Evaluation of Preemergent Herbicides for Chloris virgata Control in Mungbean. Plants. 2021; 10(8):1632. https://doi.org/10.3390/plants10081632
Chicago/Turabian StyleMahajan, Gulshan, and Bhagirath S. Chauhan. 2021. "Evaluation of Preemergent Herbicides for Chloris virgata Control in Mungbean" Plants 10, no. 8: 1632. https://doi.org/10.3390/plants10081632
APA StyleMahajan, G., & Chauhan, B. S. (2021). Evaluation of Preemergent Herbicides for Chloris virgata Control in Mungbean. Plants, 10(8), 1632. https://doi.org/10.3390/plants10081632







