Interspecific Differences in Physiological and Biochemical Traits Drive the Water Stress Tolerance in Young Morus alba L. and Conocarpus erectus L. Saplings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Condition
2.2. Water Deficit Treatments
2.3. Growth and Biomass Traits Measurements
2.4. Chlorophyll a, b, Carotenoid Contents and Leaf Gas Exchange Measurements
2.5. Determination of Proline, Soluble Sugar, Total Phenolic Content and Total Soluble Protein
2.6. Determination of Malondialdehyde Contents (MDA) and Electrolyte Leakage (EL %)
2.7. Production of ROS and Antioxidant Enzyme Activity
2.8. Data Analysis
3. Results
3.1. Effect of Soil Water Deficit on Growth and Dry Weight Production
3.2. Effect of Soil Water Deficit on Chlorophyll a, b and Carotenoid Contents
3.3. Effect of Soil Water Deficit on Production of Proline, Soluble Sugar, Total Phenolic Content and Soluble Protein
3.4. Effect of Soil Water Deficit on Gas Exchange Attributes
3.5. Effect of Soil Water Deficit on the Production of Reactive Oxygen Species, Malondialdehyde (MDA) and Electrolyte Leakage
3.6. Effect of Soil Water Deficit on the Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R. Plant adaptations to salt and water stress: Differences and commonalities. Adv. Bot. Res. 2011, 57, 1–32. [Google Scholar]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakistan Economic Survey. Agricultural Statistics. 2020. Available online: http://www.finance.gov.pk/survey/chapters_19/2-Agriculture.pdf (accessed on 25 March 2021).
- Rahut, D.B.; Ali, A.; Imtiaz, M.; Mottaleb, K.A.; Erenstein, O. Impact of irrigation water scarcity on rural household food security and income in Pakistan. Water Supply 2016, 16, 675–683. [Google Scholar] [CrossRef]
- Qureshi, A.S.; McCornick, P.G.; Sarwar, A.; Sharma, B.R. Challenges and prospects of sustainable groundwater management in the Indus Basin, Pakistan. Water Resour. Manag. 2010, 24, 1551–1569. [Google Scholar] [CrossRef] [Green Version]
- Salinger, M.J. Climate variability and change: Past, present and future. An overview. Clim. Chang. 2005, 70, 9–29. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; Van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Shah, T.; Singh, O.P.; Mukherji, A. Some aspects of South Asia’s groundwater irrigation economy: Analyses from a survey in India, Pakistan, Nepal Terai and Bangladesh. J. Hydrogeol. 2006, 14, 286–309. [Google Scholar] [CrossRef]
- Zafar, Z.; Rasheed, F.; Abdullah, M.; Salam, M.M.A.; Mohsin, M. Effects of water deficit on growth and physiology of Young Conocarpus erectus L. and Ficus benjamina L. saplings. Bangladesh J. Bot. 2019, 48, 1215–1221. [Google Scholar] [CrossRef]
- Sabir, M.A.; Rasheed, F.; Zafar, Z.; Khan, I.; Nawaz, M.F.; Haq, I.U.; Bilal, M. A consistent CO2 assimilation rate and an enhanced root development drives the tolerance mechanism in Ziziphus jujuba under soil water deficit. Arid Land Res. Manag. 2020, 34, 392–404. [Google Scholar] [CrossRef]
- Zafar, Z.; Rasheed, F.; Atif, R.M.; Maqsood, M.; Gailing, O. Salicylic Acid-Induced Morpho-Physiological and Biochemical Changes Triggered Water Deficit Tolerance in Syzygium cumini L. Saplings. Forests 2021, 12, 491. [Google Scholar] [CrossRef]
- Rasheed, F.; Gondal, A.; Kudus, K.A.; Zafar, Z.; Nawaz, M.F.; Khan, W.R.; Abdullah, M.; Ibrahim, F.H.; Depardieu, C.; Pazi, A.M.M.; et al. Effects of soil water deficit on three tree species of the arid environment: Variations in growth, physiology, and antioxidant enzyme activities. Sustainability 2021, 13, 3336. [Google Scholar] [CrossRef]
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2017, 90, 61–78. [Google Scholar] [CrossRef]
- Zarafshar, M.; Akbarinia, M.; Askari, H.; Hosseini, S.M.; Rahaie, M.; Struve, D.; Striker, G.G. Morphological, physiological and biochemical responses to soil water deficit in seedlings of three populations of wild pear (Pyrus boisseriana). Biotechnol. Agron. Soc. Environ. 2014, 18, 353–366. [Google Scholar]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Lopes, J.I.; Goncalves, B.C.; Ferreira, T.C.; Correia, C.M. Physiological behaviour, oxidative damage and antioxidative protection of olive trees grown under different irrigation regimes. Plant Soil 2007, 292, 1–12. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar]
- Laxa, M.; Michael, L.; Wilena, T.; Kamel, C.; Karl, J.D. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, C.B.; Rouina, B.; Sensoy, S.; Boukhris, M.; Abdallah, F.B. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Hessini, K.; Martinez, J.P.; Gandour, M.; Albouchi, A.; Soltani, A.; Abdelly, C. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ. Exp. Bot. 2009, 67, 312–319. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and proteomic responses of Mulberry Trees (Morus alba. L.) to combined salt and drought stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.R.; Chaitanya, K.V.; Jutur, P.P.; Sumithra, K. Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ. Exp. Bot. 2004, 52, 33–42. [Google Scholar] [CrossRef]
- Dhanyalakshmi, K.H.; Nataraja, K.N. Mulberry (Morus spp.) has the features to treat as a potential perennial model system. Plant Signal. Behav. 2018, 13, 1491267. [Google Scholar] [CrossRef]
- Redha, A.; Al-Hasan, R.; Afzal, M. Synergistic and concentration-dependent toxicity of multiple heavy metals compared with single heavy metals in Conocarpus lancifolius. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Khalil, R.; Ali, Q.; Hafeez, M.M.; Malik, A. Phytochemical activities of Conocarpus erectus: An overview. Biol. Clin. Sci. Res. J. 2020, e008, 1–6. [Google Scholar]
- Rasheed, F.; Dreyer, E.; Richard, B.; Brignolas, F.; Brendel, O.; Le Thiec, D. Vapour pressure deficit during growth has little impact on genotypic differences of transpiration efficiency at leaf and whole plant level: An example from Populus nigra L. Plant. Cell Environ. 2015, 38, 670–684. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ainsworth, E.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Nayyar, H. Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ. Exp. Bot. 2003, 50, 253–264. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Ma, F.; Feng, F.; Shu, H. Responses of growth and antioxidant system root-zone hypoxia stress in two Malus species. Plant Soil 2009, 327, 95–105. [Google Scholar] [CrossRef]
- Bayer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Knörzer, O.C.; Burner, J.; Boger, P. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol. Plant. 1996, 97, 388–396. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar]
- Hu, L.; Wang, Z.; Huang, B. Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C3 perennial grass species. Physiol. Plant. 2010, 139, 93–106. [Google Scholar] [CrossRef]
- Rasheed, F.; Delagrange, S. Acclimation of Betula alleghaniensis Britton to moderate soil water deficit: Small morphological changes make for important consequences in crown display. Tree Physiol. 2016, 36, 1320–1329. [Google Scholar]
- Correia, B.; Pintó-Marijuan, M.; Neves, L.; Brossa, R.; Dias, M.C.; Costa, A.; Castro, B.B.; Araujo, C.; Santos, C.; Chaves, M.M.; et al. Water stress and recovery in the performance of two Eucalyptus globulus clones: Physiological and biochemical profiles. Physiol. Plant. 2014, 150, 580–592. [Google Scholar] [CrossRef]
- Chaves, M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Muller, B.; Pantin, F.; Genard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Hu, Y.; Du, X.; Li, T.; Tang, H.; Wu, J. Salicylic acid induces physiological and biochemical changes in Torreya grandis cv. Merrillii seedlings under drought stress. Trees 2014, 28, 961–970. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, M.; Mirás-Avalos, J.M.; Valsesia, P.; Lescourret, F.; Génard, M.; Davarynejad, G.H.; Bannayan, M.; Azizi, M.; Vercambre, G. Disentangling the Effects of Water Stress on Carbon Acquisition, Vegetative Growth, and Fruit Quality of Peach Trees by Means of the QualiTree Model. Front. Plant Sci. 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogeat-Triboulot, M.B.; Mikael, B.; Jenny, R.; Laurent, J.; Didier, L.T.; Payam, F.; Basia, V.; Erwin, W.; Kris, L.; Thomas, T.; et al. Gradual soil water depletion results in reversible changes of gene expression, protein profiles ecophysiology and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol. 2007, 143, 876–892. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Ribas-Carbo, M.; Bota, J.; Galmes, J.; Henkle, M.; Martinez-Canellas, S.; Medrano, H. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2006, 172, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hasan, S.A.; Fariduddin, Q.; Ahmad, A. Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J. Plant Interact. 2008, 3, 297–304. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Regier, N.; Streb, S.; Cocozza, C.; Schaub, M.; Cherubini, P.; Zeeman, S.C.; Rrey, B. Drought tolerance of two black poplar (Populus nigra L.) clones: Contribution of carbohydrates and oxidative stress defence. Plant Cell Environ. 2009, 32, 1724–1736. [Google Scholar] [CrossRef] [Green Version]
- White, D.A.; Turner, N.C.; Galbraith, J.H. Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiol. 2000, 20, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Ghorbani, A.; Razavi, S.M.; Omran, V.O.G.; Pirdashti, H. Piriformospora indica alleviates salinity by boosting redox poise and antioxidative potential of tomato. Russ. J. Plant Physiol. 2018, 65, 898–907. [Google Scholar] [CrossRef]
- Saheri, F.; Barzin, G.; Pishkar, L.; Boojar, M.M.A.; Babaeekhou, L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia 2020, 75, 2189–2200. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Jesus, C.; Meijón, M.; Monteiro, P.; Correia, B.; Amaral, J.; Escandón, M.; Cañal, J.M.; Pinto, G. Salicylic acid application modulates physiological and hormonal changes in Eucalyptus globulus under water deficit. Environ. Exp. Bot. 2015, 118, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Gondor, O.K.; Janda, T.; Soós, V.; Pál, M.; Majláth, I.; Adak, M.K.; Balázs, E.; Szalai, G. Salicylic acid induction of flavonoid biosynthesis pathways in wheat varies by treatment. Front. Plant Sci. 2016, 7, 1447. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Jafarnia, S.; Akbarinia, M.; Hosseinpour, B.; Modarres, S.A.M.; Salami, S.A. Effect of drought stress on some growth, morphological, physiological, and biochemical parameters of two different populations of Quercus brantii. iForest 2018, 11, 212–220. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crop. Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Turkan, I.; Bor, M.; Ozdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhang, J.L.; Liu, H.C.; Cao, K.F. Photosynthesis, non-photochemical pathways and activities of antioxidant enzymes in a resilient evergreen oak under different climatic conditions from a valley-savanna in Southwest China. Physiol. Plant. 2009, 135, 62–72. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
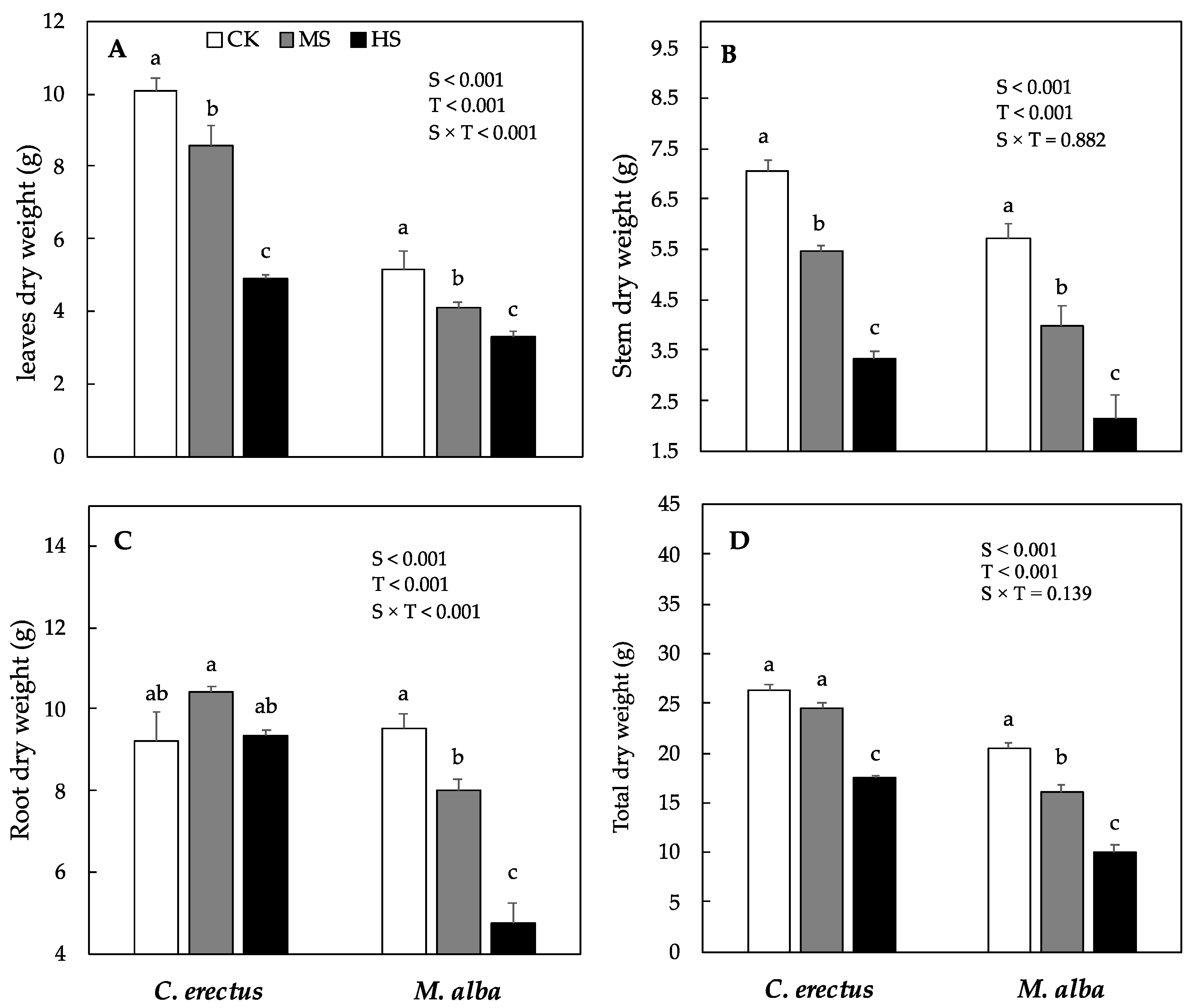

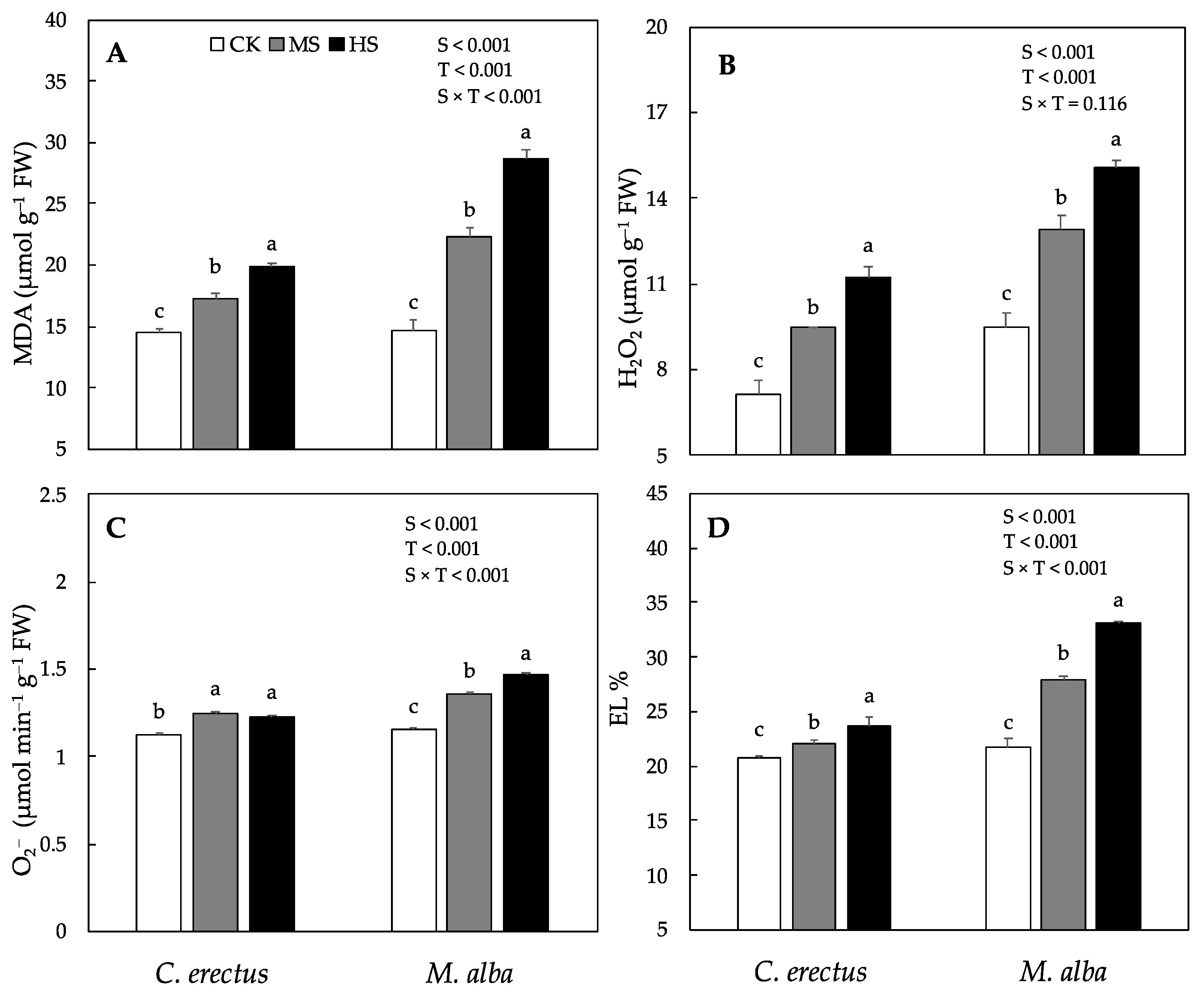

| Traits | Plant Height (cm) | Stem Diameter (mm) | R:S Ratio | |
|---|---|---|---|---|
| CK | 65.5 ± 1.85 a | 5.03 ± 0.22 ab | 0.58 ± 0.04 b | |
| C. erectus | MS | 68.8 ± 1.70 a | 5.32 ± 0.19 a | 0.74 ± 0.03 ab |
| HS | 56.3 ± 1.85 b | 3.73 ± 0.09 b | 1.13 ± 0.03 a | |
| CK | 62.9 ± 0.93 a | 4.67 ± 0.55 a | 0.88 ± 0.05 ab | |
| M. alba | MS | 49.7 ± 0.60 b | 4.58 ± 0.25 a | 0.99 ± 0.02 a |
| HS | 39.1 ± 1.70 c | 3.67 ± 0.30 b | 0.91 ± 0.22 ab | |
| S-effect | p < 0.001 | p = 0.532 | p = 0.143 | |
| T-effect | p < 0.001 | p < 0.001 | p = 0.014 | |
| S × T effect | p < 0.001 | p = 0.522 | p = 0.017 |
| Traits | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Carotenoid (mg g−1 FW) | Proline (μg g−1 FW) | Soluble Sugar (mg g−1 FW) | Soluble Protein (mg g−1 FW) | Total Phenolic Contents (mg g−1 FW) | |
|---|---|---|---|---|---|---|---|---|
| CK | 3.30 ± 0.07 a | 3.05 ± 0.02 a | 0.85 ± 0.01 a | 17.4 ± 0.63 c | 66.5 ± 0.69 c | 25.4 ± 0.49 c | 1.22 ± 0.02 c | |
| C. erectus | MS | 2.72 ± 0.05 b | 2.16 ± 0.04 b | 0.72 ± 0.01 b | 25.5 ± 0.94 b | 74.9 ± 1.12 b | 27.3 ± 0.30 b | 1.68 ± 0.05 b |
| HS | 1.83 ± 0.05 c | 1.70 ± 0.05 c | 0.62 ± 0.03 c | 29.9 ± 0.48 a | 91.8 ± 1.00 a | 30.4 ± 0.63 a | 2.13 ± 0.02 a | |
| CK | 2.53 ± 0.03 a | 2.18 ± 0.06 a | 0.80 ± 0.02 a | 17.4 ± 0.38 c | 71.6 ± 0.89 b | 25.4 ± 0.41 c | 1.39 ± 0.05 c | |
| M. alba | MS | 1.87 ± 0.02 b | 1.61 ± 0.15 b | 0.68 ± 0.00 b | 19.3 ± 0.44 b | 79.7 ± 0.42 a | 29.7 ± 0.54 b | 1.61 ± 0.01 b |
| HS | 1.01 ± 0.02 c | 0.98 ± 0.03 c | 0.61 ± 0.02 c | 23.3 ± 0.34 a | 81.1 ± 0.40 a | 33.4 ± 0.16 a | 1.90 ± 0.02 a | |
| S-effect | p < 0.001 | p < 0.001 | p = 0.029 | p < 0.001 | p = 0.708 | p < 0.001 | p = 0.132 | |
| T-effect | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| S × T effect | p = 0.714 | p = 0.131 | p = 0.545 | p = 0.004 | p < 0.001 | p = 0.072 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, Z.; Rasheed, F.; Ul Haq, A.; Ibrahim, F.H.; Afzal, S.; Nazre, M.; Akram, S.; Hussain, Z.; Kudus, K.A.; Mohsin, M.; et al. Interspecific Differences in Physiological and Biochemical Traits Drive the Water Stress Tolerance in Young Morus alba L. and Conocarpus erectus L. Saplings. Plants 2021, 10, 1615. https://doi.org/10.3390/plants10081615
Zafar Z, Rasheed F, Ul Haq A, Ibrahim FH, Afzal S, Nazre M, Akram S, Hussain Z, Kudus KA, Mohsin M, et al. Interspecific Differences in Physiological and Biochemical Traits Drive the Water Stress Tolerance in Young Morus alba L. and Conocarpus erectus L. Saplings. Plants. 2021; 10(8):1615. https://doi.org/10.3390/plants10081615
Chicago/Turabian StyleZafar, Zikria, Fahad Rasheed, Ahsan Ul Haq, Faridah Hanum Ibrahim, Shazia Afzal, Mohd Nazre, Seemab Akram, Zafar Hussain, Kamziah Abdul Kudus, Muhammad Mohsin, and et al. 2021. "Interspecific Differences in Physiological and Biochemical Traits Drive the Water Stress Tolerance in Young Morus alba L. and Conocarpus erectus L. Saplings" Plants 10, no. 8: 1615. https://doi.org/10.3390/plants10081615
APA StyleZafar, Z., Rasheed, F., Ul Haq, A., Ibrahim, F. H., Afzal, S., Nazre, M., Akram, S., Hussain, Z., Kudus, K. A., Mohsin, M., Qadeer, A., Raza, Z., & Khan, W. R. (2021). Interspecific Differences in Physiological and Biochemical Traits Drive the Water Stress Tolerance in Young Morus alba L. and Conocarpus erectus L. Saplings. Plants, 10(8), 1615. https://doi.org/10.3390/plants10081615










