Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens
Abstract
:1. Introduction
2. Results
2.1. Seedling Height and Biomass
2.2. Chlorophyll (a, b, and Total) and Carotenoids
2.3. Sugar Content
2.4. Nitrate Content
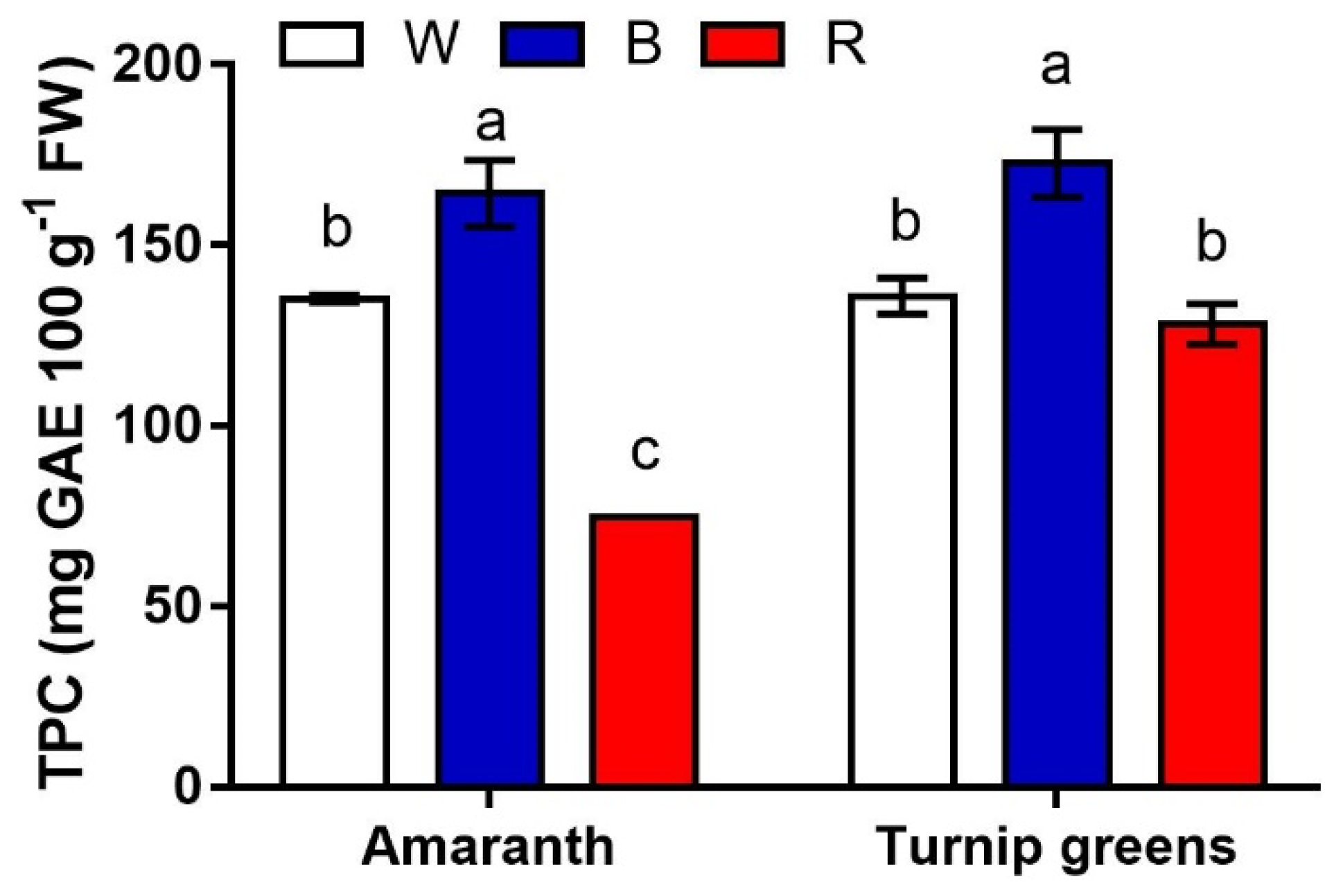
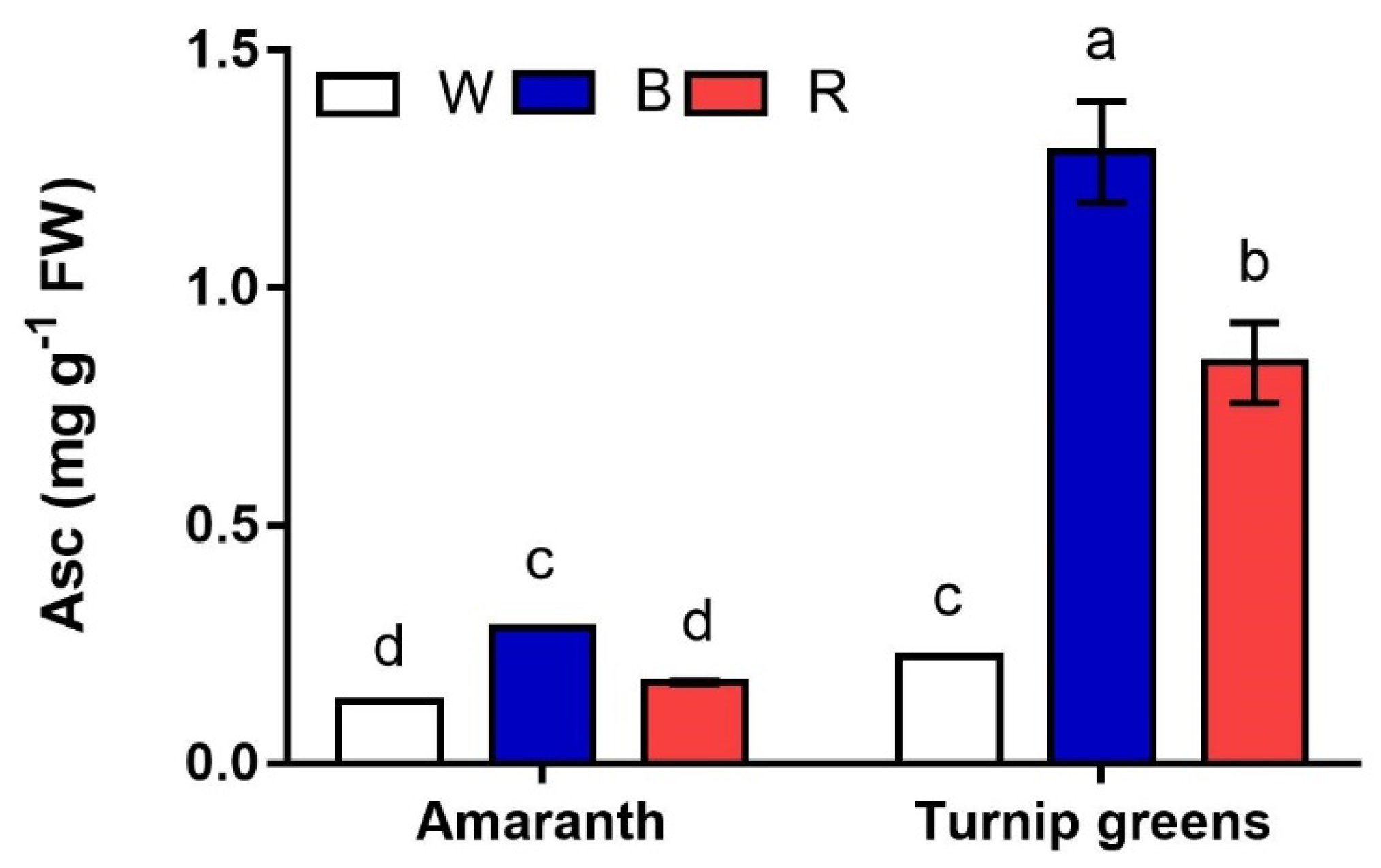
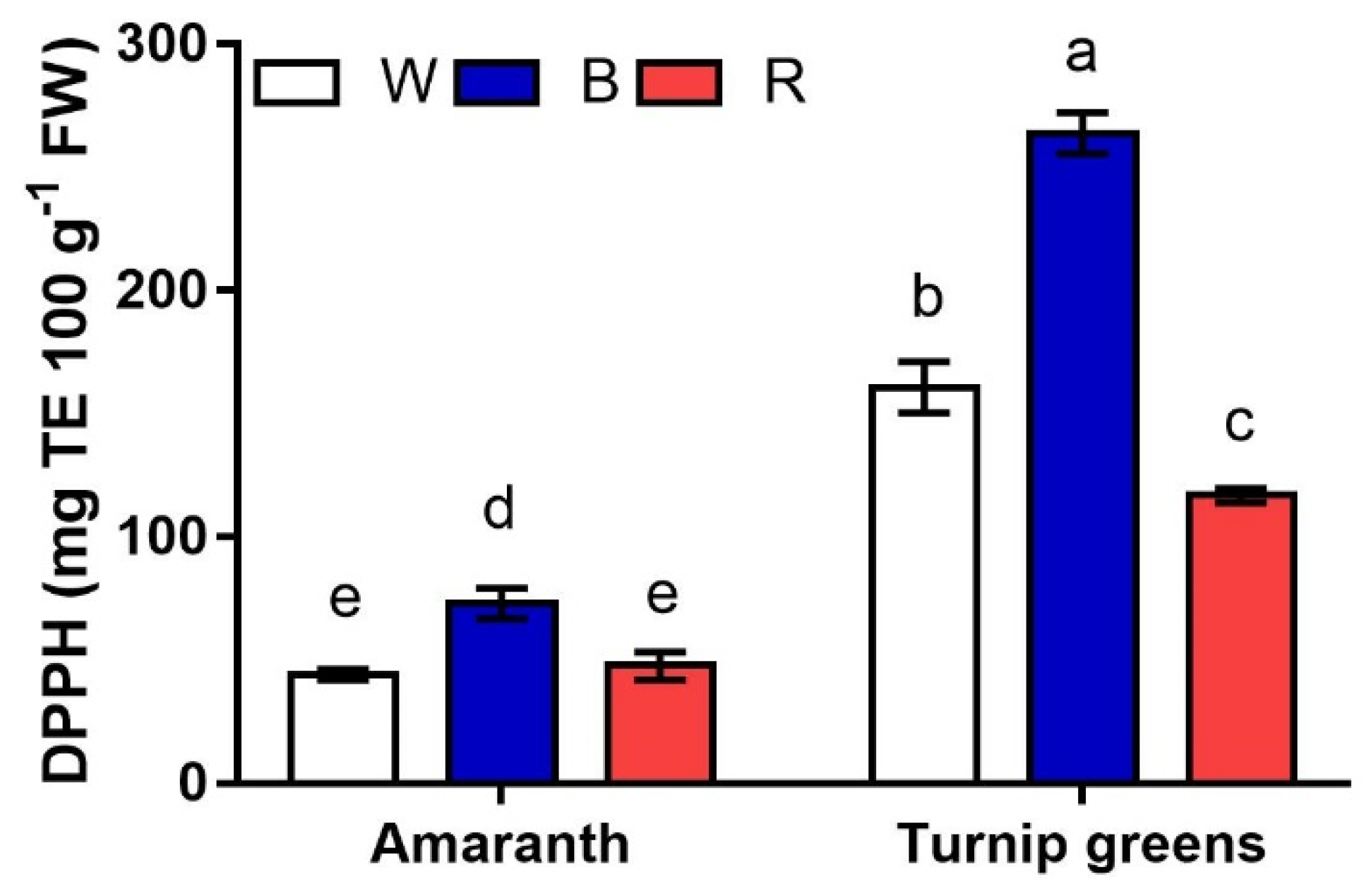
2.5. Antioxidants and Antioxidant Activity
2.6. Mineral Composition
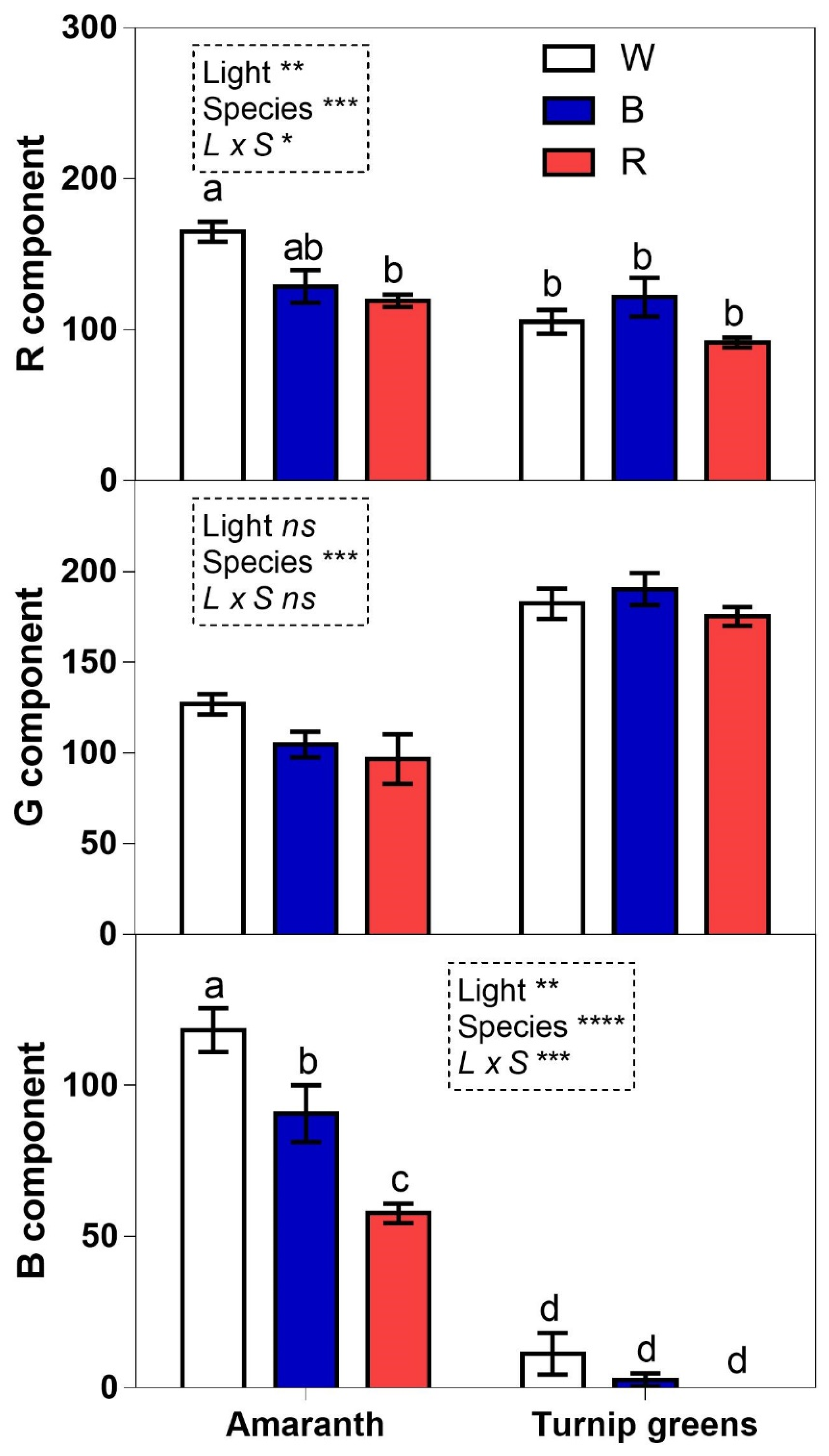
2.7. RGB Color Analysis
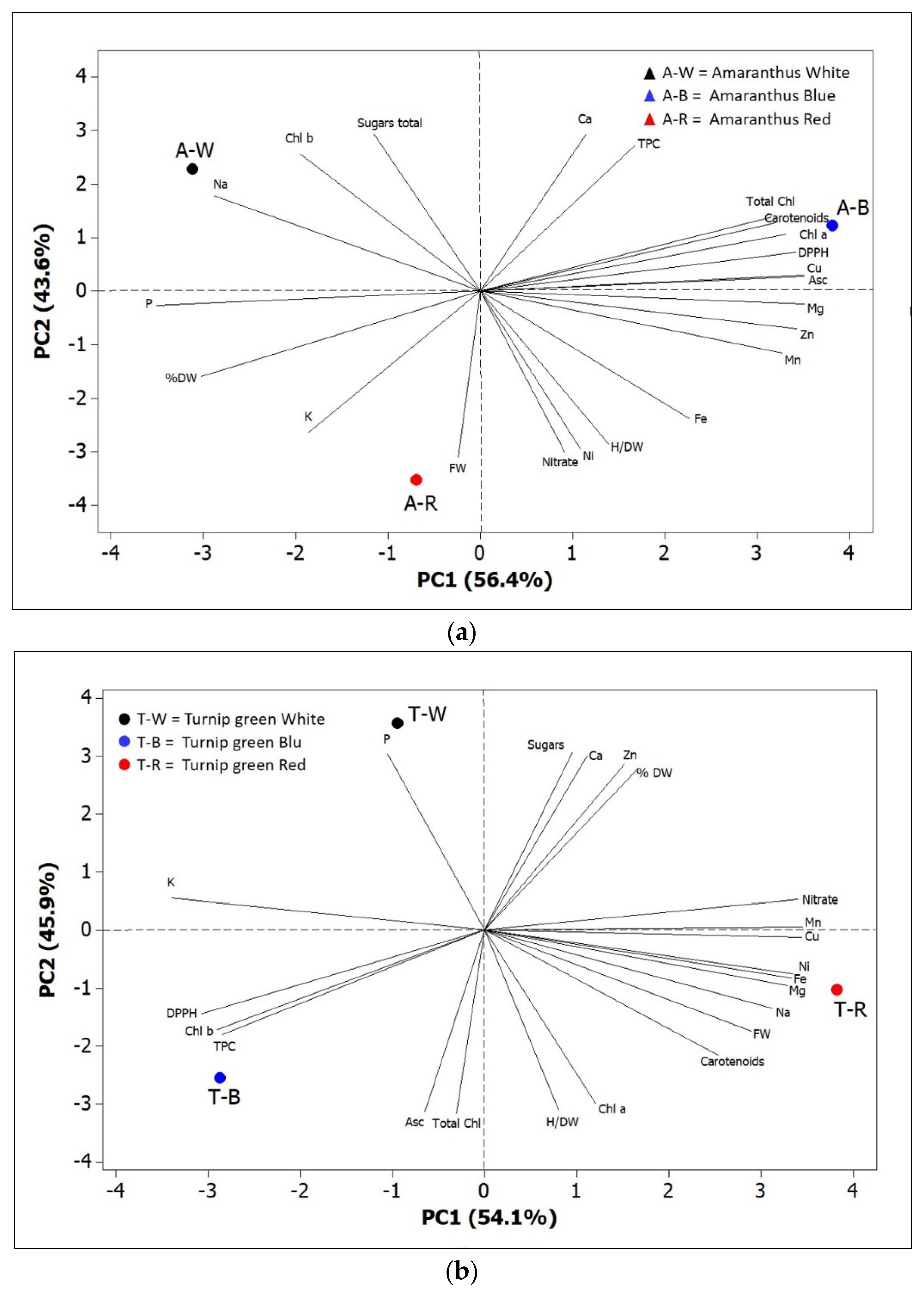
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Measurement and Data Collection of Growth Parameters
4.3. Chlorophyll and Carotenoid Pigments
4.4. Total Sugars
4.5. Nitrate Concentrations
4.6. Ascorbic Acid Analysis
4.7. Total Phenolic Compounds and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical-Scavenging Activity
4.8. Meso and Micro Elements
4.9. RGB Color Analysis
4.10. Statistical Analysis
5. Conclusions
- -
- blue light was particularly effective in enhancing the growth and nutritional characteristics (particularly antioxidant activity) of the two studied microgreens as compared to the more traditionally used white light;
- -
- red light seemed to be more effective than white light in promoting fresh biomass accumulation and hypocotyl growth. However, its effects on nutraceutical characteristics were quite different for the two genotypes, since it did not influence those of turnip greens but worsened those of amaranth (see nitrates, nickel, and total polyphenol contents) as compared to the other lights;
- -
- the response to the spectral system is typically species-specific; for this reason, it is possible to adopt a specific light formula that allows maximizing both plant growth and nutritional quality, thereby enhancing the microgreen industry.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, J.M.; Cufr, C.A.; Denzel, M.A.; Neff, M.M. The Dof transcription factor OBP3 modulates phytochrome and cryptochrome signaling in arabidopsis. Plant Cell 2005, 17, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Balibrea, S.; A Moreno, D.; García-Viguera, C. Influence of light on health-promoting phytochemicals of broccoli sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Liu, X.; Chang, T.; Guo, S.; Xu, Z.; Li, J. Effect of different light quality of led on growth and photosynthetic character in cherry tomato seedling. Acta Hortic. 2011, 325–330. [Google Scholar] [CrossRef]
- Batista, D.; Felipe, S.H.S.; Silva, T.D.; De Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.; Ríos, A.M.R.; Faria, D.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? In Vitro Cell. Dev. Biol. Anim. 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Gupta, S.D. Light Emitting Diodes for Agriculture; Springer: Singapore, 2017; p. 334. [Google Scholar]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Bian, Z.-H.; Cheng, R.-F.; Yang, Q.-C.; Wang, J.; Lu, C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, J.; Zhang, Y.; Cai, X.; Gong, P.; Zhang, J.; Wang, T.; Li, H.; Ye, Z. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2010, 30, 389–398. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.-C. Red light is effective in reducing nitrate concentration in rocket by increasing nitrate reductase activity, and contributes to increased total glucosinolates content. Front. Plant Sci. 2020, 11, 604. [Google Scholar] [CrossRef]
- Shukla, M.R.; Singh, A.S.; Piunno, K.; Saxena, P.K.; Jones, A.M.P. Application of 3D printing to prototype and develop novel plant tissue culture systems. Plant Methods 2017, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Girardi, F.M.; Barra, M.B.; Zettler, C.G. Papillary thyroid carcinoma: Does the association with Hashimoto’s thyroiditis affect the clinicopathological characteristics of the disease? Braz. J. Otorhinolaryngol. 2015, 81, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Kalaitzoglou, P.; Van Ieperen, W.; Harbinson, J.; Van Der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-W.; Kang, P.; Park, H.; Oh, H.-Y.; Yang, J.-H.; Kim, Y.-H.; Kwon, S.-K. Synthesis and properties of blue-light-emitting anthracene derivative with diphenylamino-fluorene. Dye Pigment 2010, 85, 93–98. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Kwon, Y.; Sunesh, C.D.; Choe, Y. Light-emitting properties of cationic iridium complexes containing phenanthroline based ancillary ligand with blue-green and green emission colors. Opt. Mater. 2015, 39, 40–45. [Google Scholar] [CrossRef]
- Kyriacou, M.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Kyriacou, M.; Soteriou, G.A.; Colla, G.; Rouphael, Y. The occurrence of nitrate and nitrite in Mediterranean fresh salad vegetables and its modulation by preharvest practices and postharvest conditions. Food Chem. 2019, 285, 468–477. [Google Scholar] [CrossRef]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Ilakiya, T.; Parameswari, E.; Davamani, V.; Prakash, E. Microgreens combating malnutrition problem. Biot. Res. Today 2020, 2, 110–112. [Google Scholar]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Duchovskis, P. Red light-dose or wave-length-dependent photoresponse of antioxidants in herb microgreens. PLoS ONE 2016, 11, e0163405. [Google Scholar] [CrossRef]
- Craver, J.K.; Gerovac, J.R.; Lopez, R.G.; Kopsell, D.A. Light intensity and light quality from sole-source light-emitting diodes impact phytochemical concentrations within Brassica microgreens. J. Am. Soc. Hortic. Sci. 2017, 142, 3–12. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and red LED illumination improves growth and bioactive compounds contents in Acyanic and Cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of different ratios of blue and red LED light on Brassicaceae microgreens under a controlled environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Lee, S.-W.; Seo, J.M.; Lee, M.-K.; Chun, J.-H.; Antonisamy, P.; Arasu, M.V.; Suzuki, T.; Al-Dhabi, N.A.; Kim, S.-J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crops Prod. 2014, 54, 320–326. [Google Scholar] [CrossRef]
- Wu, M.-C.; Hou, C.-Y.; Jiang, C.-M.; Wang, Y.-T.; Wang, C.-Y.; Chen, H.-H.; Chang, H.-M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Laužikė, K.; Samuolienė, G. The distinct impact of multi-color LED light on nitrate, amino acid, soluble sugar and organic acid contents in red and green leaf lettuce cultivated in controlled environment. Food Chem. 2020, 310, 125799. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Jones-Baumgardt, C.; Zheng, Y. Responses of yield and appearance quality of four Brassicaceae microgreens to varied blue light proportion in red and blue light-emitting diodes lighting. Sci. Hortic. 2020, 259, 108857. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by led on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic. 2011, 179–184. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- European Food Safety Authority. Opinion of the scientific panel on contaminants in the food chain on a request for the European Commission to perform a scientific risk assessment on nitrate in vegetables. EFSA J. 2008, 689, 1–79. Available online: https://seguridadalimentaria.elika.eus/wp-content/uploads/articulos/Archivo291/CONTAM_NitratosVeg08.pdf (accessed on 15 June 2021).
- European Commission Commission. Regulation (EU) No 1258/2011 of 2 December 2011 amending regulation (EC) No. 1881/2006 as regards maximum levels for nitrates in foodstuffs. Off. J. Eur. Union 2011, 320, 15–17. [Google Scholar]
- Nam, T.G.; Kim, D.-O.; Eom, S.H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2017, 27, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Długosz-Grochowska, O.; Kołton, A.; Wojciechowska, R. Modifying folate and polyphenol concentrations in Lamb’s lettuce by the use of LED supplemental lighting during cultivation in greenhouses. J. Funct. Foods 2016, 26, 228–237. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Hu, T.; Zhang, S.; Zhang, Y.; Zhao, T.; Yu, H.; Kang, Y. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.; Sams, C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef] [Green Version]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, S.; Li, Z.; Niyogi, K.K.; Bassi, R.; Dall’Osto, L. The Arabidopsis szl1 mutant reveals a critical role of b-carotene in photosystem I photoprotection. Plant Physiol. 2012, 159, 1745–1758. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Duchovskis, P. Nutrient levels in Brassicaceae Microgreens increase under tailored light-emitting diode spectra. Front. Plant Sci. 2019, 10, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stange, C.; Flores, C. Carotenoids and photosynthesis regulation of carotenoid biosyntesis by photoreceptors. In Advances in Photosynthesis: Fundamental Aspects; InTech: Rijekia, Croatia, 2012. [Google Scholar] [CrossRef] [Green Version]
- Son, K.-H.; Oh, M.-M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Hosseini, A.; Mehrjerdi, M.Z.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Cocetta, G.; Rossoni, M.; Gardana, C.; Mignani, I.; Ferrante, A.; Spinardi, A. Methyl jasmonate affects phenolic metabolism and gene expression in blueberry (Vaccinium corymbosum). Physiol. Plant. 2014, 153, 269–283. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Janghel, E.; Gupta, V.; Rai, M.; Rai, J. Micro determination of ascorbic acid using methyl viologen. Talanta 2007, 72, 1013–1016. [Google Scholar] [CrossRef]
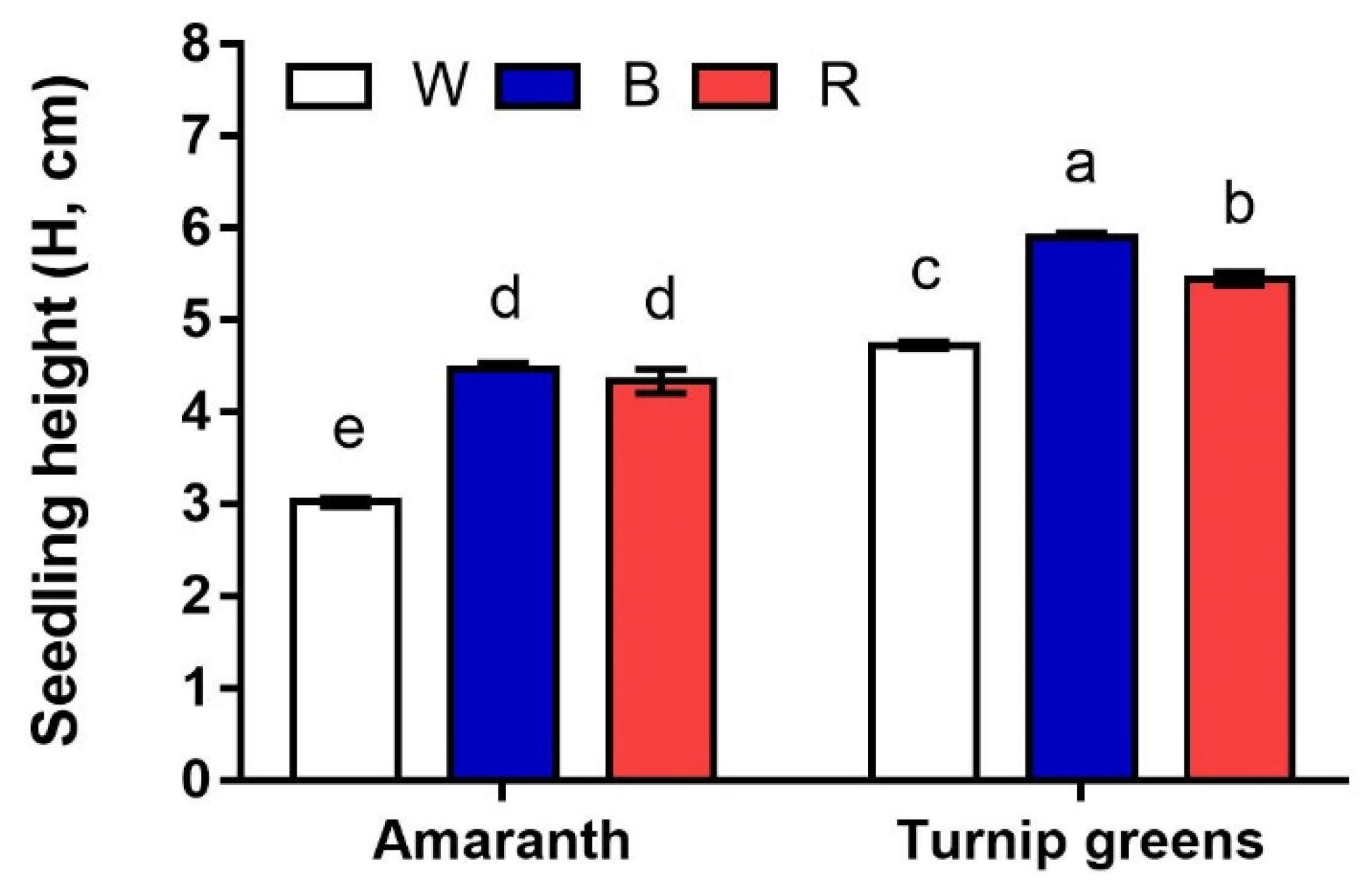
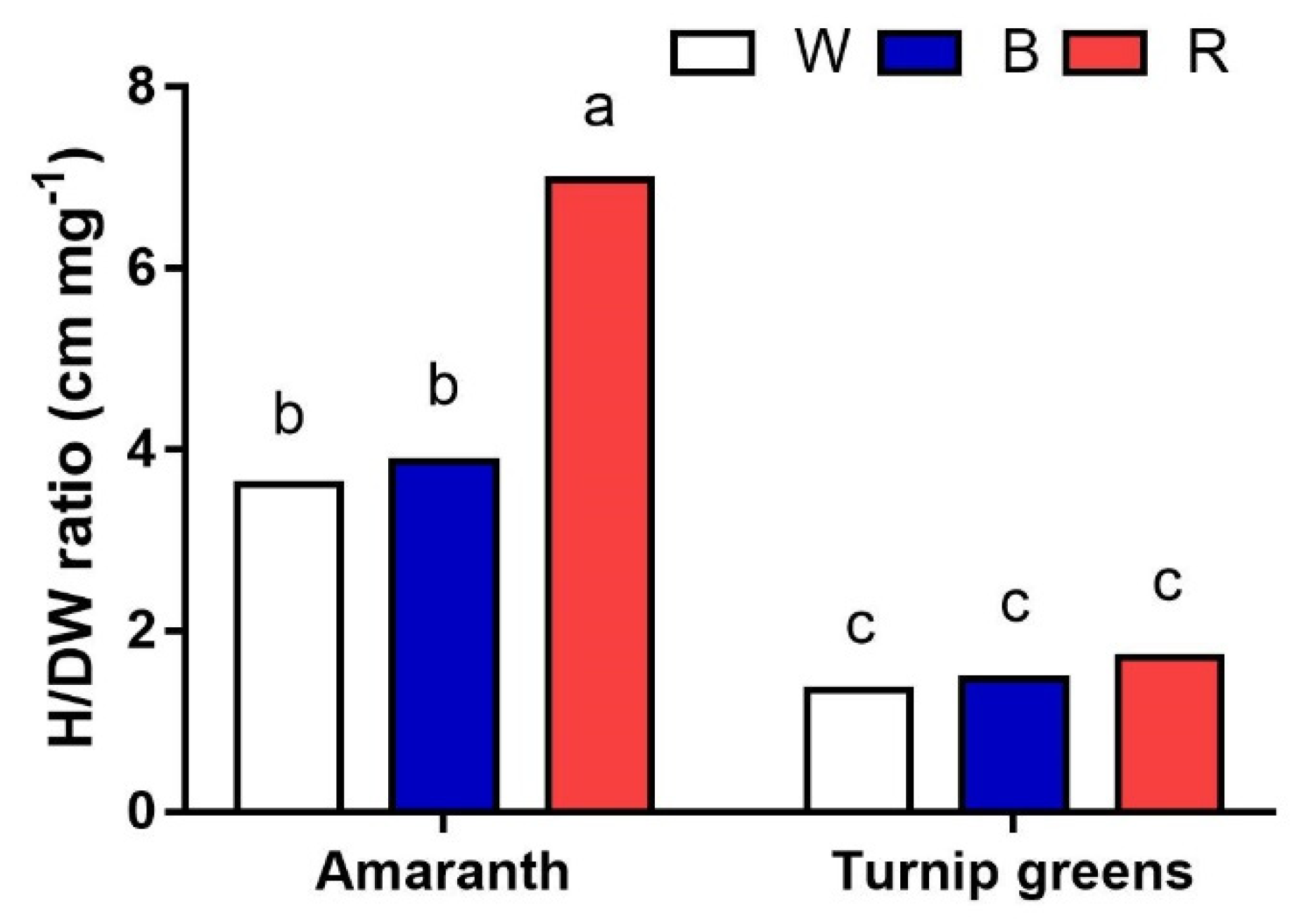
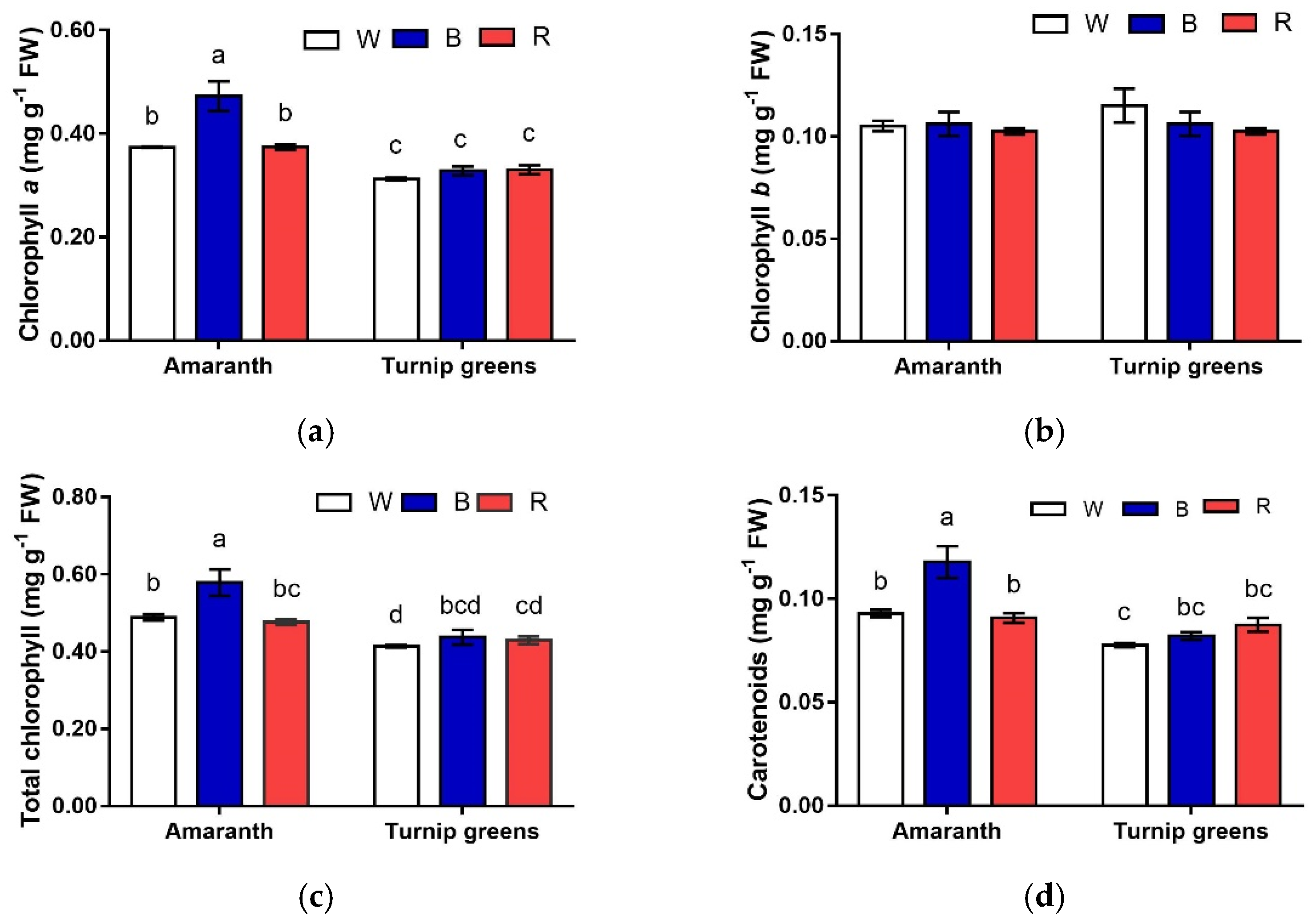
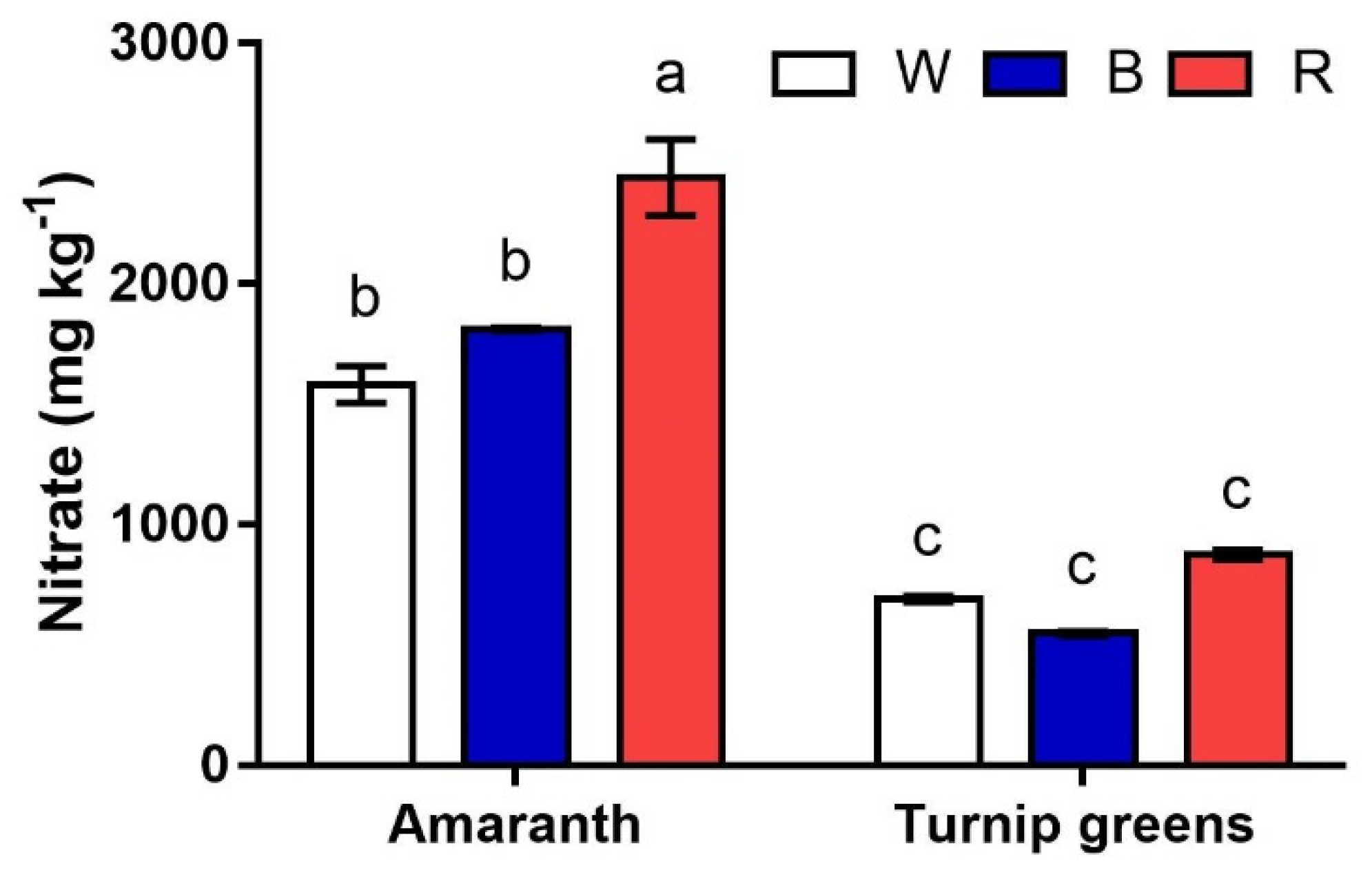
| Seedling Height (H, cm) | Fresh Biomass (FW, mg·Plant−1) | Dry Biomass (DW, %) | ||
|---|---|---|---|---|
| Species (S) | Amaranth | 3.9 ± 0.2 b | 18.5 ± 1.8 b | 5.4 ± 0.4 |
| Turnip greens | 5.4 ± 0.2 a | 66.4 ± 2.8 a | 5.2 ± 0.3 | |
| LED treatments (L) | W | 3.9 ± 0.4 c | 37.1 ± 9.9 b | 5.9 ± 0.3 a |
| B | 5.2 ± 0.4 a | 50.1 ± 11.8 a | 5.9 ± 0.3 a | |
| R | 4.9 ± 0.2 b | 40.2 ± 10.8 b | 4.2 ± 0.1 b | |
| Significance | S | *** | *** | ns |
| L | *** | *** | *** | |
| S × L | ** | ns | ns |
| Chl a (mg·g−1 FW) | Chl b (mg·g−1 FW) | Chl a/Chl b (mg·g−1 FW) | Total Chl (mg·g−1 FW) | Carotenoids (mg·g−1 FW) | Chl/Car (mg·g−1 FW) | ||
|---|---|---|---|---|---|---|---|
| Species (S) | Amaranth | 0.41 ± 0.0 a | 0.11 ± 0.0 a | 3.79 ± 0.18 a | 0.51 ± 0.0 a | 0.10 ± 0.00 a | 5.1 ± 0.1 |
| Turnip greens | 0.32 ± 0.0 b | 0.10 ± 0.0 b | 3.20 ± 0.20 b | 0.43 ± 0.0 b | 0.08 ± 0.00 b | 5.2 ± 0.1 | |
| LED treatment (L) | W | 0.34 ± 0.1 | 0.11 ± 0.0 | 3.18 ± 0.11 b | 0.45 ± 0.0 b | 0.08 ± 0.00 b | 5.3 ± 0.1 |
| B | 0.40 ± 0.0 | 0.11 ± 0.0 | 3.74 ± 0.33 a | 0.51 ± 0.0 a | 0.10 ± 0.01 a | 5.1 ± 0.2 | |
| R | 0.35 ± 0.0 | 0.10 ± 0.0 | 3.49 ± 0.08 ab | 0.45 ± 0.0 b | 0.09 ± 0.00 b | 5.1 ± 0.1 | |
| Significance | S | *** | *** | *** | *** | *** | ns |
| L | ns | ns | ** | ** | ** | ns | |
| S × L | *** | ns | ns | * | ** | ns |
| Total Sugars (mg·g−1 FW) | Nitrate (mg·kg−1) | ||
|---|---|---|---|
| Species (S) | Amaranth | 0.7 ± 0.0 b | 1990.9 ± 140.3 a |
| Turnip greens | 1.3 ± 0.0 a | 704.9 ± 48.0 b | |
| LED treatment (L) | W | 1.0 ± 0.4 | 1137.1 ± 202.5 b |
| B | 1.0 ± 0.4 | 1247.1 ± 318.7 b | |
| R | 0.9 ± 0.4 | 1659.5 ± 357.7 a | |
| Significance | S | *** | *** |
| L | ns | *** | |
| S × L | Ns | *** |
| TPC (mg GAE·100 g−1 FW) | Asc (mg·g−1 FW) | DPPH (mg TE·100 g−1 FW) | ||
|---|---|---|---|---|
| Species (S) | Amaranth | 124.8 ± 13.5 b | 0.20 ± 0.0 b | 54.6 ± 5.2 b |
| Turnip greens | 145.6 ± 7.7 a | 0.78 ± 0.2 a | 180.5 ± 22.2 a | |
| LED treatment (L) | W | 135.6 ± 2.3 b | 0.79 ± 0.2 a | 102.4 ± 26.5 b |
| B | 168.6 ± 6.2 a | 0.20 ± 0.0 c | 168.5 ± 42.9 a | |
| R | 104.4 ± 12.2 c | 0.51 ± 0.1 b | 82.3 ± 15.7 c | |
| Significance | S | ** | *** | *** |
| L | *** | *** | *** | |
| S × L | ** | *** | ** |
| Na (g·kg−1 DW) | Mg (g·kg−1 DW) | K (g·kg−1 DW) | Ca (g·kg−1 DW) | Mn (mg·kg−1 DW) | Fe (mg·kg−1 DW) | Ni (mg·kg−1 DW) | Cu (mg·kg−1 DW) | Zn (mg·kg−1 DW) | P (g·kg−1 DW) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species (S) | Amaranth | 2.5 ± 0.1 b | 11.2 ± 0.3 a | 94.2 ± 1.7 a | 8.1 ± 0.2 b | 48.8 ± 1.4 b | 1.7 ± 0.2 | 9.5 ± 1.6 | 27.1 ± 1.3 a | 102.7 ± 1.5 a | 12.2 ± 0.2 a | |
| Turnip greens | 4.8 ± 0.1 a | 9.5 ± 1.1 b | 72.0 ± 1.4 b | 10.9 ± 0.2 a | 54.9 ± 4.5 a | 2.1 ± 0.5 | 8.2 ± 1.4 | 23.7 ± 1.7 b | 77.5 ± 2.0 b | 9.3 ± 0.1 b | ||
| p ≤ 0.001 | p ≤ 0.05 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.01 | p ≥ 0.05 | p ≥ 0.05 | p ≤ 0.05 | p ≤ 0.001 | p ≤ 0.001 | |||
| LED treatment (L) | W | 3.6 ± 0.4 | 8.9 ± 0.6 b | 83.9 ± 4.4 | 9.9 ± 0.7 a | 47.5 ± 1.7 b | 1.1 ± 0.0 b | 5.2 ± 0.8 b | 23.4 ± 1.4 | 91.7 ± 4.9 | 11.0 ± 0.7 a | |
| B | 3.5 ± 0.5 | 9.9 ± 1.1 b | 83.0 ± 3.7 | 9.4 ± 0.4 ab | 47.6 ± 2.5 b | 1.5 ± 0.2 b | 7.3 ± 1.2 b | 24.9 ± 2.5 | 88.0 ± 7.2 | 10.4 ± 0.5 b | ||
| R | 3.7 ± 0.6 | 12.2 ± 0.8 a | 82.6 ± 7.2 | 9.2 ± 0.8 b | 60.4 ± 5.3 a | 3.0 ± 0.5 a | 14.1 ± 0.8 a | 27.8 ± 1.5 | 90.6 ± 5.7 | 10.8 ± 0.7 ab | ||
| p ≥ 0.05 | p ≤ 0.01 | p ≥ 0.05 | p ≤ 0.05 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 | p ≥ 0.05 | p ≥ 0.05 | p ≤ 0.05 | |||
| S × L | Amaranth | W | 2.7 ± 0.0 b | 10.2 ± 0.1 ab | 93.6 ± 1.6 a | 8.3 ± 0.3 c | 43.8 ± 0.5 b | 1.1 ± 0.1 c | 4.6 ± 2.1 | 24.8 ± 4.2 ab | 101.7 ± 6.5 | 12.6 ± 0.5 |
| B | 2.4 ± 0.1 b | 12.3 ± 0.3 a | 90.8 ± 3.8 a | 8.5 ± 0.4 c | 52.7 ± 0.7 b | 1.9 ± 0.1 b | 9.2 ± 2.2 | 30.2 ± 0.2 a | 103.7 ± 5.6 | 11.6 ± 0.3 | ||
| R | 2.4 ± 0.3 b | 11.1 ± 0.5 a | 98.2 ± 6.7 a | 7.5 ± 0.8 c | 49.9 ± 2.9 b | 2.1 ± 0.3 b | 14.5 ± 2.7 | 26.2 ± 4.2 ab | 102.8 ± 0.6 | 12.3 ± 0.5 | ||
| Turnip greens | W | 4.6 ± 0.0 a | 7.5 ± 0.2 b | 74.1 ± 0.1 b | 11.5 ± 0.5 a | 51.2 ± 2.0 b | 1.2 ± 0.1 c | 5.7 ± 1.8 | 22.0 ± 2.6 ab | 81.7 ± 4.4 | 9.4 ± 0.2 | |
| B | 4.7 ± 0.2 a | 7.7 ± 1.2 b | 75.1 ± 1.1 b | 10.2 ± 0.4 b | 42.6 ± 3.9 b | 1.2 ± 0.1 c | 5.3 ± 2.0 | 19.6 ± 3.4 b | 72.3 ± 3.1 | 9.3 ± 0.2 | ||
| R | 5.0 ± 0.1 a | 13.3 ± 2.6 a | 66.9 ± 2.3 b | 10.9 ± 0.3 ab | 70.9 ± 8.9 a | 3.9 ± 1.1 a | 13.6 ± 1.2 | 29.4 ± 3.0 a | 78.4 ± 6.3 | 9.3 ± 0.3 | ||
| p ≤ 0.05 | p ≤ 0.01 | p ≤ 0.01 | p ≤ 0.05 | p ≤ 0.001 | p ≤ 0.01 | p ≥ 0.05 | p ≤ 0.05 | p ≥ 0.05 | p ≥ 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, S.; Cavallaro, V.; Ferrante, A.; Romano, D.; Patané, C. Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens. Plants 2021, 10, 1584. https://doi.org/10.3390/plants10081584
Toscano S, Cavallaro V, Ferrante A, Romano D, Patané C. Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens. Plants. 2021; 10(8):1584. https://doi.org/10.3390/plants10081584
Chicago/Turabian StyleToscano, Stefania, Valeria Cavallaro, Antonio Ferrante, Daniela Romano, and Cristina Patané. 2021. "Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens" Plants 10, no. 8: 1584. https://doi.org/10.3390/plants10081584
APA StyleToscano, S., Cavallaro, V., Ferrante, A., Romano, D., & Patané, C. (2021). Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens. Plants, 10(8), 1584. https://doi.org/10.3390/plants10081584










