Root Foraging Precision of Pinus pumila (Pall.) Regel Subjected to Contrasting Light Spectra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Set-Up of Heterogeneous Condition
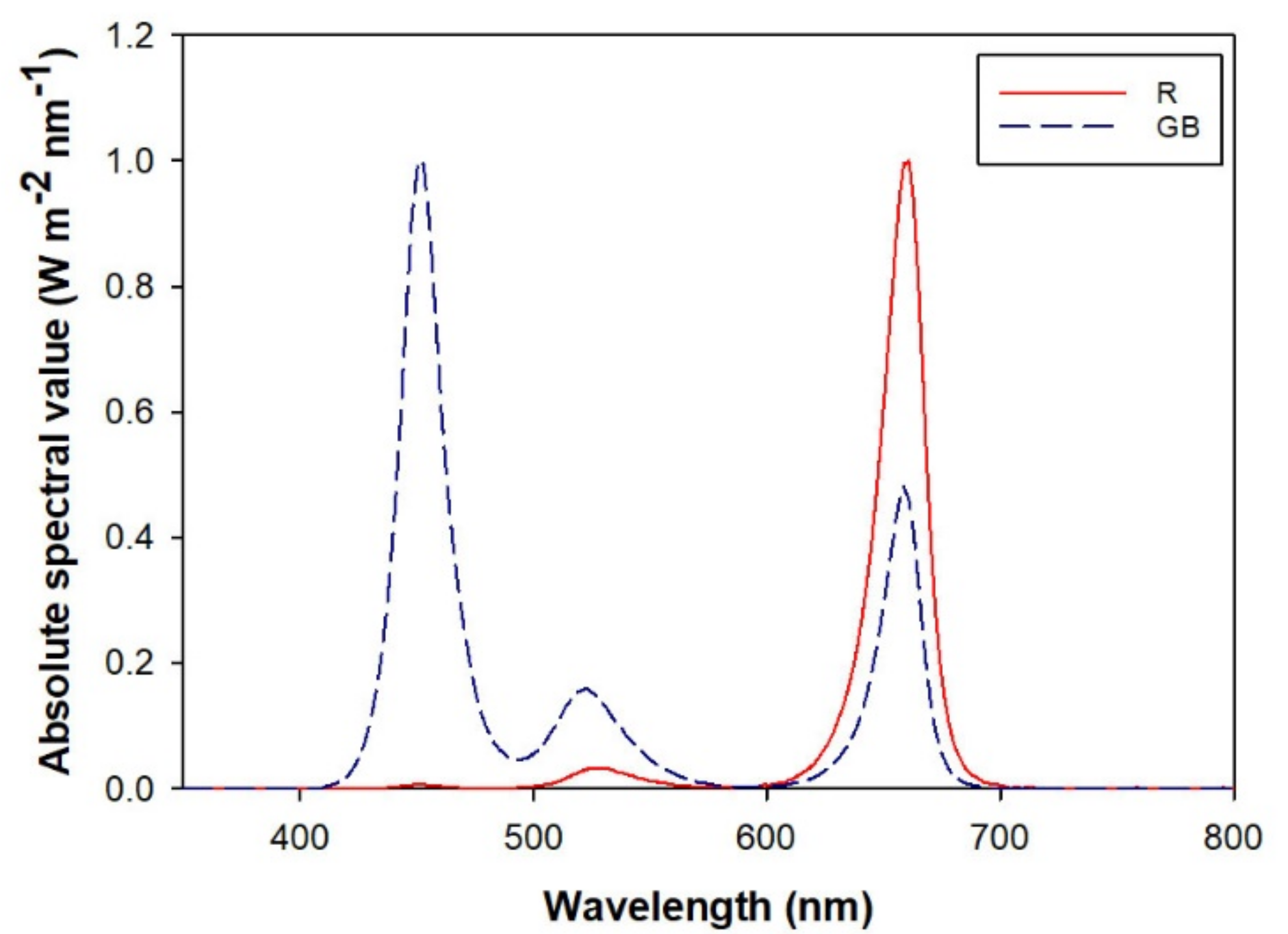
2.3. Light Spectra Treatment
2.4. Experiment Design and Arrangement
2.5. Sampling and Measurements
2.6. Variable Calculation and Statitstical Analysis
3. Results
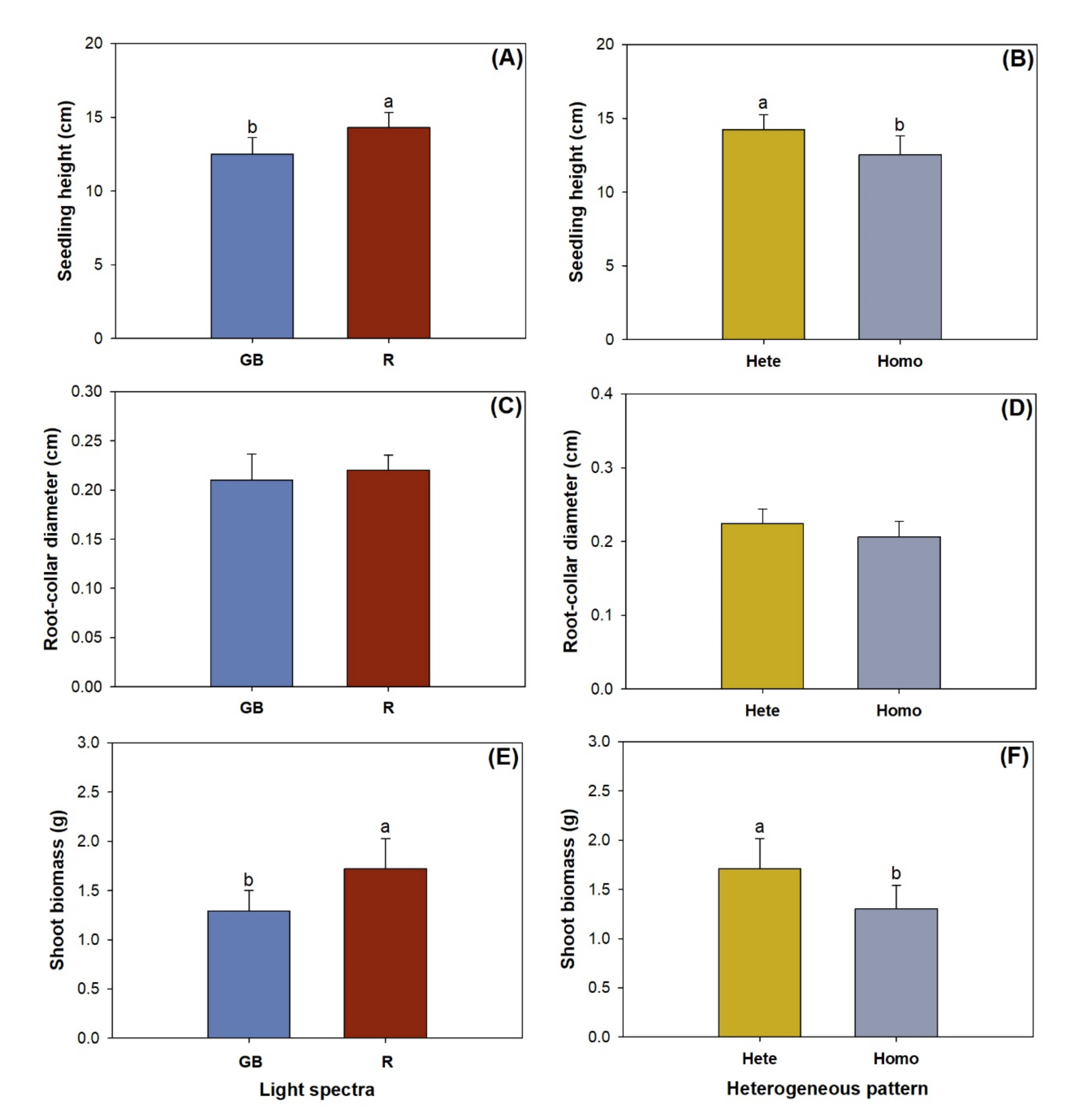
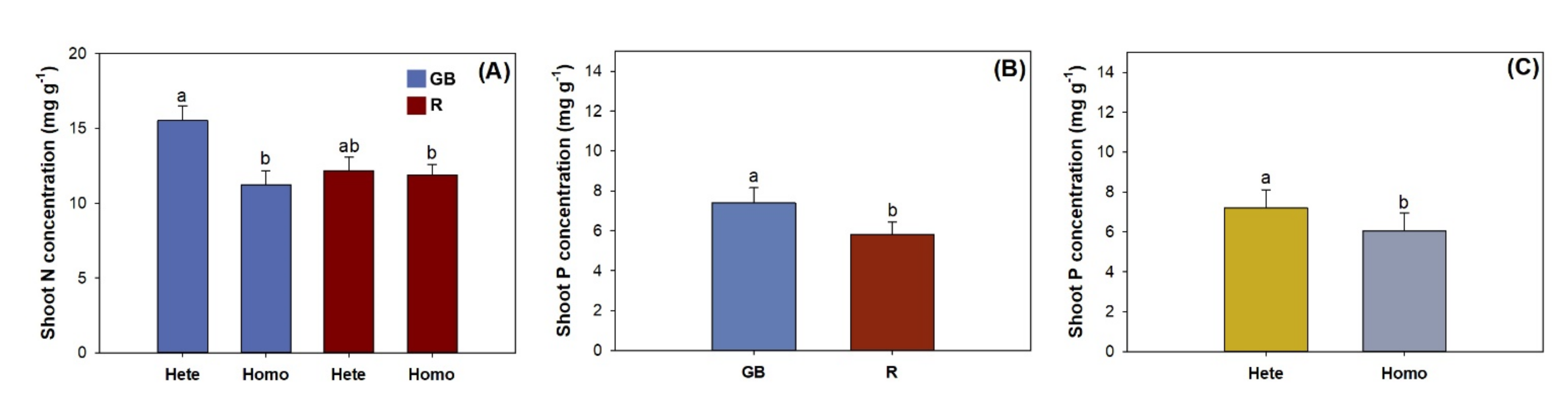
3.1. Shoot Growth, Biomass, and Nutirent Concentration
3.2. Foliar Variables
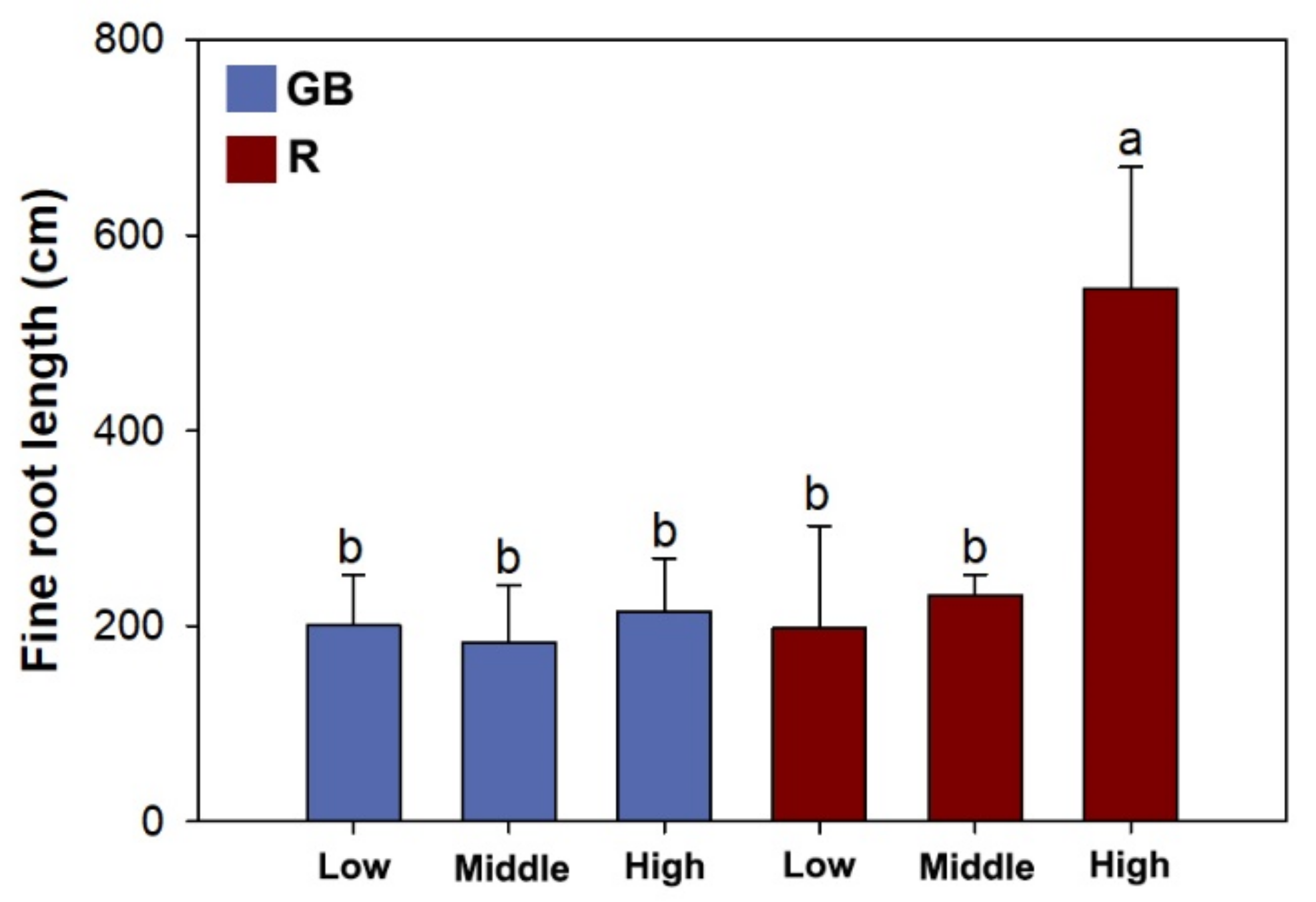
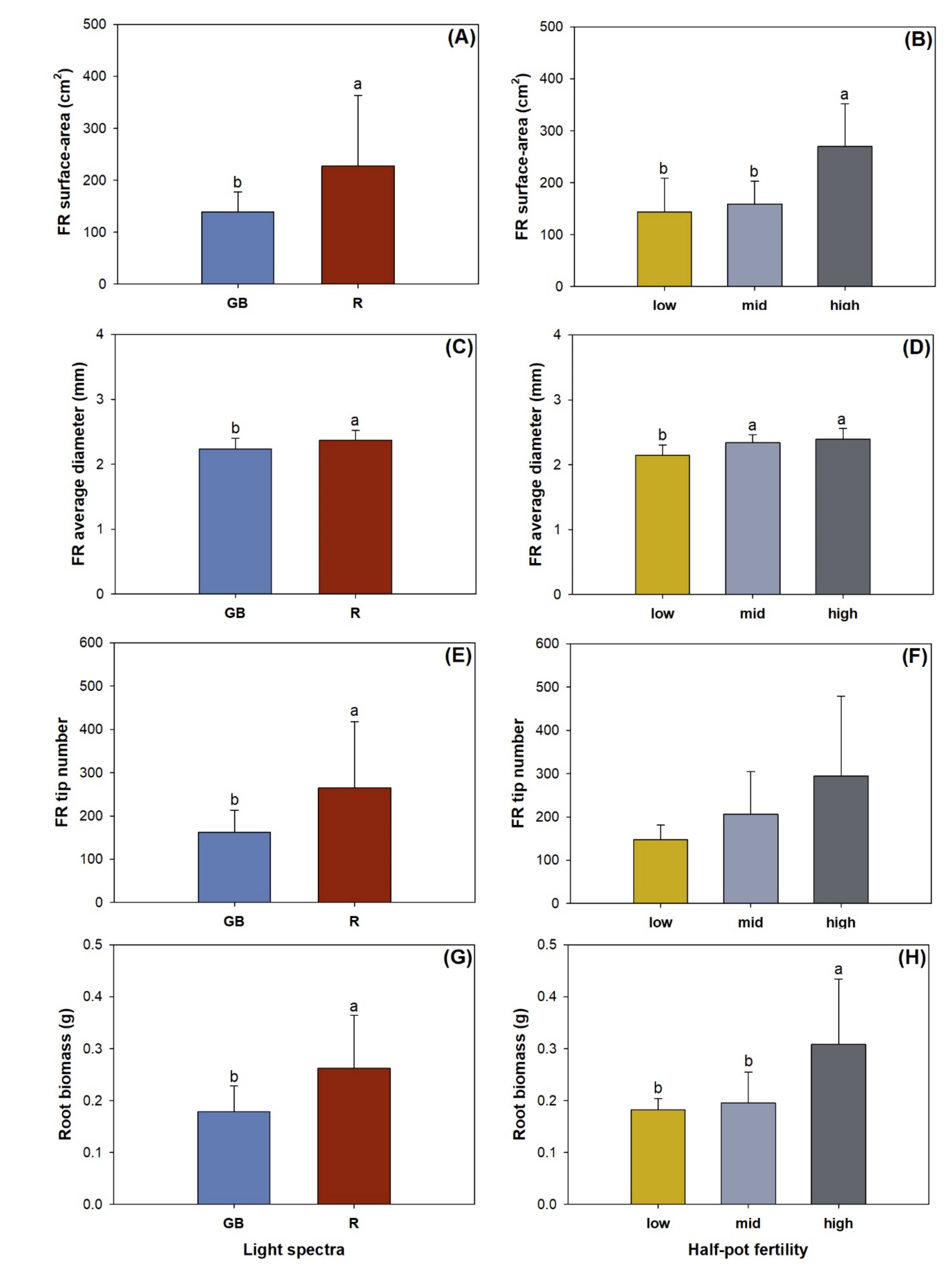
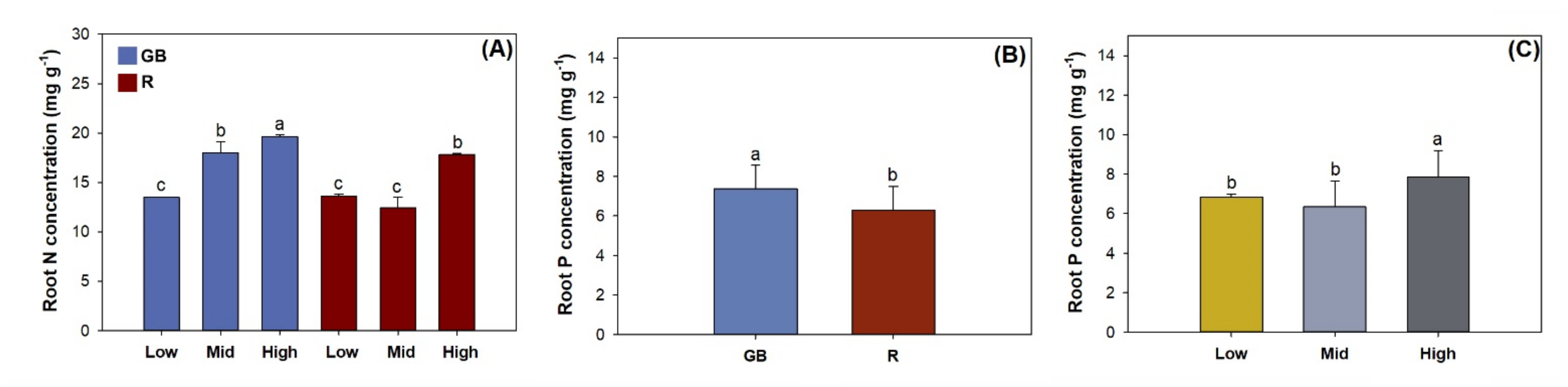
3.3. Fine Root Morphology, Biomass, and Nutrient Concentration
3.4. Root Foraging Precision
4. Discussion
4.1. Shoot Response
4.2. Nutrient Uptake and Allocation
4.3. Root Foraging Precision
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muscarella, R.; Uriarte, M.; Erickson, D.L.; Swenson, N.G.; Kress, W.J.; Zimmerman, J.K. Variation of tropical forest assembly processes across regional environmental gradients. Perspect. Plant Ecol. Evol. Syst. 2016, 23, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Burton, J.I.; Mladenoff, D.J.; Forrester, J.A.; Clayton, M.K. Effects of forest canopy gaps on the ground-layer plant community depend on deer: Evidence from a controlled experiment. J. Veg. Sci. 2021, 32. [Google Scholar] [CrossRef]
- Matsuo, T.; Martinez-Ramos, M.; Bongers, F.; Van der Sande, M.T.; Poorter, L. Forest structure drives changes in light heterogeneity during tropical secondary forest succession. J. Ecol. 2021. [Google Scholar] [CrossRef]
- Meng, F.Q.; Zhang, G.F.; Li, X.C.; Niklas, K.J.; Sun, S.C. Growth synchrony between leaves and stems during twig development differs among plant functional types of subtropical rainforest woody species. Tree Physiol. 2015, 35, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonohat, G.; Balandier, P.; Ruchaud, F. Predicting solar radiation tranmittance in the understory of even-aged coniferous stands in temperate forests. Ann. For. Sci. 2004, 61, 629–641. [Google Scholar] [CrossRef] [Green Version]
- Capers, R.S.; Chazdon, R.L. Rapid assessment of understory light availability in a wet tropical forest. Agric. For. Meteorol. 2004, 123, 177–185. [Google Scholar] [CrossRef]
- Wei, H.X.; Chen, X.; Chen, G.S.; Zhao, H.T. Foliar nutrient and carbohydrate in Aralia elata can be modified by understory light quality in forests with different structures at Northeast China. Ann. For. Res. 2019, 62, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.X.; Zhao, H.T.; Chen, X.; He, X.Y. Secondary metabolites, carbohydrate accumulation, and nutrient uptake in Aralia elata (Miq.) seem seedlings exposed to shoot cutting and different LED spectra. Acta Physiol. Plant. 2020, 42, 162. [Google Scholar] [CrossRef]
- Kelly, J.W.G.; Landhausser, S.M.; Chow, P.S. The impact of light quality and quantity on root-to-shoot ratio and root carbon reserves in aspen seedling stock. New For. 2015, 46, 527–545. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Su, Y.; Tan, J.H.; Luo, X.T.; Li, J.Y.; He, Q. Spectral effect on growth, dry mass, physiology and nutrition in Bletilla striata seedlings: Individual changes and collaborated response. Int. J. Agric. Biol. 2020, 24, 125–132. [Google Scholar]
- Vollsnes, A.V.; Melo, T.B.; Futsaether, C.M. Photomorphogenesis and pigment induction in lentil seedling roots exposed to low light conditions. Plant Biol. 2012, 14, 467–474. [Google Scholar] [CrossRef]
- Klem, K.; Gargallo-Garriga, A.; Rattanapichai, W.; Oravec, M.; Holub, P.; Vesela, B.; Sardans, J.; Penuelas, J.; Urban, O. Distinct morphological, physiological, and biochemical responses to light quality in barley leaves and roots. Front. Plant Sci. 2019, 10, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagnoli, A.; Dumroese, R.K.; Terzaghi, M.; Pinto, J.R.; Fulgaro, N.; Scippa, G.S.; Chiatante, D. Tree seedling response to LED spectra: Implications for forest restoration. Plant Biosyst. 2018, 152, 515–523. [Google Scholar] [CrossRef]
- Guo, S.L.; Zhang, S.; Jia, L.W.; Xu, M.Y.; Wang, Z.Y. Root growth of Eleuthero (Eleutherococcus senticosus Rupr. & Maxim. Maxim.) seedlings cultured with chitosan oligosaccharide addition under different light spectra. Not. Bot. Horti Agrobot. Cluj Na. 2020, 48, 626–635. [Google Scholar]
- Zhao, J.; Chen, X.; Wei, H.X.; Lv, J.; Chen, C.; Liu, X.Y.; Wen, Q.; Jia, L.M. Nutrient uptake and utilization in Prince Rupprecht’s larch (Larix principis-rupprechtii Mayr.) seedlings exposed to a combination of light-emitting diode spectra and exponential fertilization. Soil Sci. Plant Nutr. 2019, 65, 358–368. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Xu, S.; Li, Y.; He, C. Nutrient assimilation and utilization in korean pine (Pinus koraiensis) seedlings exposed to exponential fertilization under contrasting spectra. Commun. Soil Sci. Plant Anal. 2020, 51, 2414–2428. [Google Scholar] [CrossRef]
- Smirnakou, S.; Ouzounis, T.; Radoglou, K. Effects of continuous spectrum LEDs used in indoor cultivation of two coniferous species Pinus sylvestris L. and Abies borisii-regis Mattf. Scand. J. For. Res. 2017, 32, 115–122. [Google Scholar] [CrossRef]
- Liu, P.; Cao, B.; Wang, Y.T.; Wei, Z.P.; Ye, J.F.; Wei, H.X. Spectral effect of streetlamps on urban trees: A simulated study on tissue water, nitrogen, and carbohydrate contents in maple and oak. PLoS ONE 2021, 16, e0248463. [Google Scholar]
- Li, X.W.; Chen, Q.X.; Lei, H.Q.; Wang, J.W.; Yang, S.; Wei, H.X. Nutrient uptake and utilization by fragrant rosewood (Dalbergia odorifera) seedlings cultured with oligosaccharide addition under different lighting spectra. Forests 2018, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.X.; Hauer, R.J.; Chen, G.S.; Chen, X.; He, X.Y. Growth, nutrient assimilation, and carbohydrate metabolism in Korean pine (Pinus koraiensis) seedlings in response to light spectra. Forests 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.X.; Chen, G.S.; Chen, X.; Zhao, H.T. Growth and nutrient uptake in Aralia elata seedlings exposed to exponential fertilization under different illumination spectra. Int. J. Agric. Biol. 2020, 23, 644–652. [Google Scholar]
- Smirnakou, S.; Ouzounis, T.; Radoglou, K.M. Continuous spectrum LEDs promote seedling quality traits and performance of Quercus ithaburensis var macrolepis. Front. Plant Sci. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.Y.; Zhou, B.Z.; Ge, X.G.; Cao, Y.H.; Brunner, I.; Shi, J.X.; Li, M.H. Species-specific responses of root morphology of three co-existing tree species to nutrient patches reflect their root foraging strategies. Front. Plant Sci. 2021, 11, 618222. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.X.; Guo, P.; Zheng, H.F.; He, X.Y.; Wang, P.J.; Ren, Z.B.; Zhai, C. Micro-scale heterogeneity in urban forest soils affects fine root foraging by ornamental seedlings of Buddhist pine and Northeast yew. Urban For. Urban Green. 2017, 28, 63–72. [Google Scholar] [CrossRef]
- Chen, W.L.; Koide, R.T.; Adams, T.S.; DeForest, J.L.; Cheng, L.; Eissenstat, D.M. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. USA 2016, 113, 8741–8746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, A. Plant nitrogen capture from organic matter as affected by spatial dispersion, interspecific competition and mycorrhizal colonization. New Phytol. 2003, 157, 303–314. [Google Scholar] [CrossRef]
- Mou, P.; Mitchell, R.J.; Jones, R.H. Root distribution of two tree species under a heterogeneous nutrient environment. J. Appl. Ecol. 1997, 34, 645–656. [Google Scholar] [CrossRef]
- Einsmann, J.C.; Jones, R.H.; Pu, M.; Mitchell, R.J. Nutrient foraging traits in 10 co-occurring plant species of contrasting life forms. J. Ecol. 1999, 87, 609–619. [Google Scholar] [CrossRef]
- Rajaniemi, T.K. Root foraging traits and competitive ability in heterogeneous soils. Oecologia 2007, 153, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Gazol, A.; Tamme, R.; Price, J.N.; Hiiesalu, I.; Laanisto, L.; Partel, M. A negative heterogeneity-diversity relationship found in experimental grassland communities. Oecologia 2013, 173, 545–555. [Google Scholar] [CrossRef]
- Chen, B.M.; Su, J.Q.; Liao, H.X.; Peng, S.L. A greater foraging scale, not a higher foraging precision, may facilitate invasion by exotic plants in nutrient-heterogeneous conditions. Ann. Bot. 2018, 121, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, S.; Ito, K. Vegetation dynamics of the Siberian dwarf pine (Pinus pumila Regel) in the Taisetsu mountain range, Hokkaido, Japan. Vegetatio 1984, 58, 105–113. [Google Scholar] [CrossRef]
- Wikipedia Contributors. Pinus pumila. Available online: https://en.wikipedia.org/w/index.php?title=Pinus_pumila&oldid=1022440608 (accessed on 10 May 2021).
- Okuda, M.; Sumida, A.; Ishii, H.; Vetrova, V.P.; Hara, T. Establishment and growth pattern of Pinus pumila under a forest canopy in central Kamchatka. Ecol. Res. 2008, 23, 831–840. [Google Scholar] [CrossRef]
- Takahashi, K. Effects of climatic conditions on shoot elongation of alpine dwarf pine (Pinus pumila) at its upper and lower altitudinal limits in central Japan. Arct. Antarct. Alp. Res. 2003, 35, 1–7. [Google Scholar] [CrossRef]
- Amagai, Y.; Kaneko, M.; Kudo, G. Habitat-specific responses of shoot growth and distribution of alpine dwarf-pine (Pinus pumila) to climate variation. Ecol. Res. 2015, 30, 969–977. [Google Scholar] [CrossRef]
- Sano, Y.; Matano, T.; Ujihara, A. Growth of Pinus pumila and climate fluctuation in Japan. Nature 1977, 266, 159–161. [Google Scholar] [CrossRef]
- Moskalyuk, T.A. On adaptations of trees and shrubs in the north of the Russian Far East. Russ. J. Ecol. 2008, 39, 73–82. [Google Scholar] [CrossRef]
- Takahashi, K.; Aoki, K. Effects of climatic conditions on annual shoot length and tree-ring width of alpine dwarf pine Pinus pumila in central Japan. J. Plant. Res. 2015, 128, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, T. Photosynthesis and respiration of Pinus pumila needles in relation to needle age and season. Ecol. Res. 1990, 5, 333–340. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Z.; Zhu, Y.; Wang, Y.; Li, H.; Yang, D.; Zhao, X. Spatial heterogeneity of soil nutrients after the establishment of caragana intermedia plantation on sand dunes in alpine sandy land of the Tibet plateau. PLoS ONE 2015, 10, e0124456. [Google Scholar] [CrossRef] [PubMed]
- Apostol, K.G.; Dumroese, R.K.; Pinto, J.R.; Davis, A.S. Response of conifer species from three latitudinal populations to light spectra generated by light-emitting diodes and high-pressure sodium lamps. 2015, 45, 1711–1719. Can. J. For. Res. 2015, 45, 1711–1719. [Google Scholar] [CrossRef]
- Li, H.S. Mechanism and technology for experiments. In Principles and Experimental Techniques of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Wei, H.X.; Zhao, H.T.; Chen, X. Foliar N:P Stoichiometry in Aralia elata distributed on different slope degrees. Not. Bot. Horti Agrobot. Cluj Na. 2019, 47, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Zhao, S.J.; Yang, J.M.; Meng, L.Q.; Luo, Y.Q.; Hong, B.; Cui, W.; Wang, M.H.; Liu, W.C. Growth, nutrient uptake, and foliar gas exchange in pepper cultured with un-composted fresh spent mushroom residue. Not. Bot. Horti Agrobot. Cluj Na. 2019, 47, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Riikonen, J. Pre-cultivation of Scots pine and Norway spruce transplant seedlings under four different light spectra did not affect their field performance. New For. 2016, 47, 607–619. [Google Scholar] [CrossRef]
- Riikonen, J.; Kettunen, N.; Gritsevich, M.; Hakala, T.; Sarkka, L.; Tahvonen, R. Growth and development of Norway spruce and Scots pine seedlings under different light spectra. Environ. Exp. Bot. 2016, 121, 112–120. [Google Scholar] [CrossRef]
- Yan, X.L.; Ma, X.Q. Responses of root morphology and seedling growth in three tree species to heterogeneous supplies of ammonium and nitrate. For. Ecol. Manag. 2021, 479, 118538. [Google Scholar] [CrossRef]
- Yan, X.L.; Wang, C.; Ma, X.Q.; Wu, P.F. Root morphology and seedling growth of three tree species in southern China in response to homogeneous and heterogeneous phosphorus supplies. Trees Struct. Funct. 2019, 33, 1283–1297. [Google Scholar] [CrossRef]
- Rees, R.; Robinson, B.H.; Evangelou, M.W.H.; Lehmann, E.; Schulin, R. Response of Populus tremula to heterogeneous B distributions in soil. Plant. Soil 2012, 358, 403–415. [Google Scholar] [CrossRef]
- Rouached, H. Red light means on for phosphorus. Nat. Plants 2018, 4, 983–984. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Yanagisawa, S. Effect of phytochrome-mediated red light signaling on phosphorus uptake and accumulation in rice. Soil Sci. Plant. Nutr. 2020, 66, 745–754. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kanno, S.; Mabuchi, A.; Monda, K.; Iba, K.; Yanagisawa, S. A phytochrome-B-mediated regulatory mechanism of phosphorus acquisition. Nat. Plants 2018, 4, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Mou, P.P.; Weiner, J.; Li, S. Contrasts between whole-plant and local nutrient levels determine root growth and death in Ailanthus altissima (Simaroubaceae). Am. J. Bot. 2014, 101, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mou, P.P.; Jones, R.H. Nutrient foraging via physiological and morphological plasticity in three plant species. Can. J. For. Res. 2006, 36, 164–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Ma, X.; Jin, G. Foraging ability and growth performance of four subtropical tree species in response to heterogeneous nutrient environments. J. For. Res. 2010, 15, 91–98. [Google Scholar] [CrossRef]
- Hamberg, L.; Velmala, S.M.; Sievanen, R.; Kalliokoski, T.; Pennanen, T. Early root growth and architecture of fast- and slow-growing Norway spruce (Picea abies) families differ-potential for functional adaptation. Tree Physiol. 2018, 38, 853–864. [Google Scholar] [CrossRef]
- Ward, D. Shade affects fine-root morphology in range-encroaching eastern redcedars (Juniperus virginiana) more than competition, soil fertility and pH. Pedobiologia 2021, 84, 150708. [Google Scholar] [CrossRef]

| Variables | Source of Variance | ||
|---|---|---|---|
| LS | HP | LS × HP | |
| Seedling height | 24.21 * 1 | 21.43 * | 0.07 |
| RCD 2 | 0.78 | 2.26 | 0.88 |
| Shoot biomass | 24.42 * | 21.49 * | 0.15 |
| R/S 3 | 0.59 | 0.04 | 0.07 |
| Shoot N concentration | 6.82 * | 19.42 * | 15.13 * |
| Shoot P concentration | 84.28 *** | 45.12 ** | 0.01 |
| Foliar chlorophyl-a | 0.56 | 19.33 * | 2.00 |
| Foliar chlorophyl-b | 6.72 * | 26.05 ** | 0.83 |
| Foliar carotenoid | 24.21 * | 21.43 * | 0.07 |
| Foliar protein | 16.75 * | 6.34 * | 3.61 |
| GS activity 4 | 10.43 * | 20.23 * | 0.04 |
| ACP activity 5 | 1.40 | 13.02 * | 1.32 |
| Variable | Light Spectra | Heterogeneous Pattern | ||
|---|---|---|---|---|
| GB 1 | R 2 | Hete 3 | Homo 4 | |
| Chlorophyl-a (mg g−1) | 1.70 ± 0.55a 5 | 1.59 ± 0.27a | 1.96 ± 0.33A | 1.32 ± 0.16B |
| Chlorophyl-b (mg g−1) | 1.81 ± 0.44a | 1.52 ± 0.26b | 1.95 ± 0.29A | 1.38 ± 0.18B |
| Carotenoid (mg g−1) | 12.48 ± 1.14b | 14.29 ± 1.04a | 14.24 ± 1.00A | 12.53 ± 1.27B |
| Protein (mg g−1) | 1.50 ± 0.32a | 1.05 ± 0.16b | 1.42 ± 0.40A | 1.14 ± 0.22B |
| GS 6 activity (µgNPP g−1 FW min−1) | 3.08 ± 0.48a | 2.55 ± 0.48b | 3.18 ± 0.37A | 2.44 ± 0.41B |
| ACP 7 activity (A mg−1 protein h−1) | 1.68 ± 0.54a | 1.90 ± 0.39a | 2.21 ± 0.28A | 1.46 ± 0.37B |
| Variables | Source of Variance | ||
|---|---|---|---|
| LS | HPF | LS × HPF | |
| FR 1 length | 7.88 * 2 | 8.11 * | 6.04 * |
| FR surface-area | 7.17 * | 4.63 * | 3.12 |
| FR diameter | 7.07 * | 6.43 * | 1.08 |
| FR tip number | 6.09 * | 3.20 | 1.55 |
| Biomass | 10.59 * | 7.97 * | 1.07 |
| N concentration | 85.68 *** | 59.69 *** | 25.60 *** |
| P concentration | 6.75 * | 4.45 * | 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Gao, J.; Zhao, Y.; Liu, J. Root Foraging Precision of Pinus pumila (Pall.) Regel Subjected to Contrasting Light Spectra. Plants 2021, 10, 1482. https://doi.org/10.3390/plants10071482
He C, Gao J, Zhao Y, Liu J. Root Foraging Precision of Pinus pumila (Pall.) Regel Subjected to Contrasting Light Spectra. Plants. 2021; 10(7):1482. https://doi.org/10.3390/plants10071482
Chicago/Turabian StyleHe, Chunxia, Jun Gao, Yan Zhao, and Jing Liu. 2021. "Root Foraging Precision of Pinus pumila (Pall.) Regel Subjected to Contrasting Light Spectra" Plants 10, no. 7: 1482. https://doi.org/10.3390/plants10071482
APA StyleHe, C., Gao, J., Zhao, Y., & Liu, J. (2021). Root Foraging Precision of Pinus pumila (Pall.) Regel Subjected to Contrasting Light Spectra. Plants, 10(7), 1482. https://doi.org/10.3390/plants10071482





