Physiological Responses and Gene Expression Patterns in Open-Pollinated Seedlings of a Pummelo-Mandarin Hybrid Rootstock Exposed to Salt Stress and Huanglongbing
Abstract
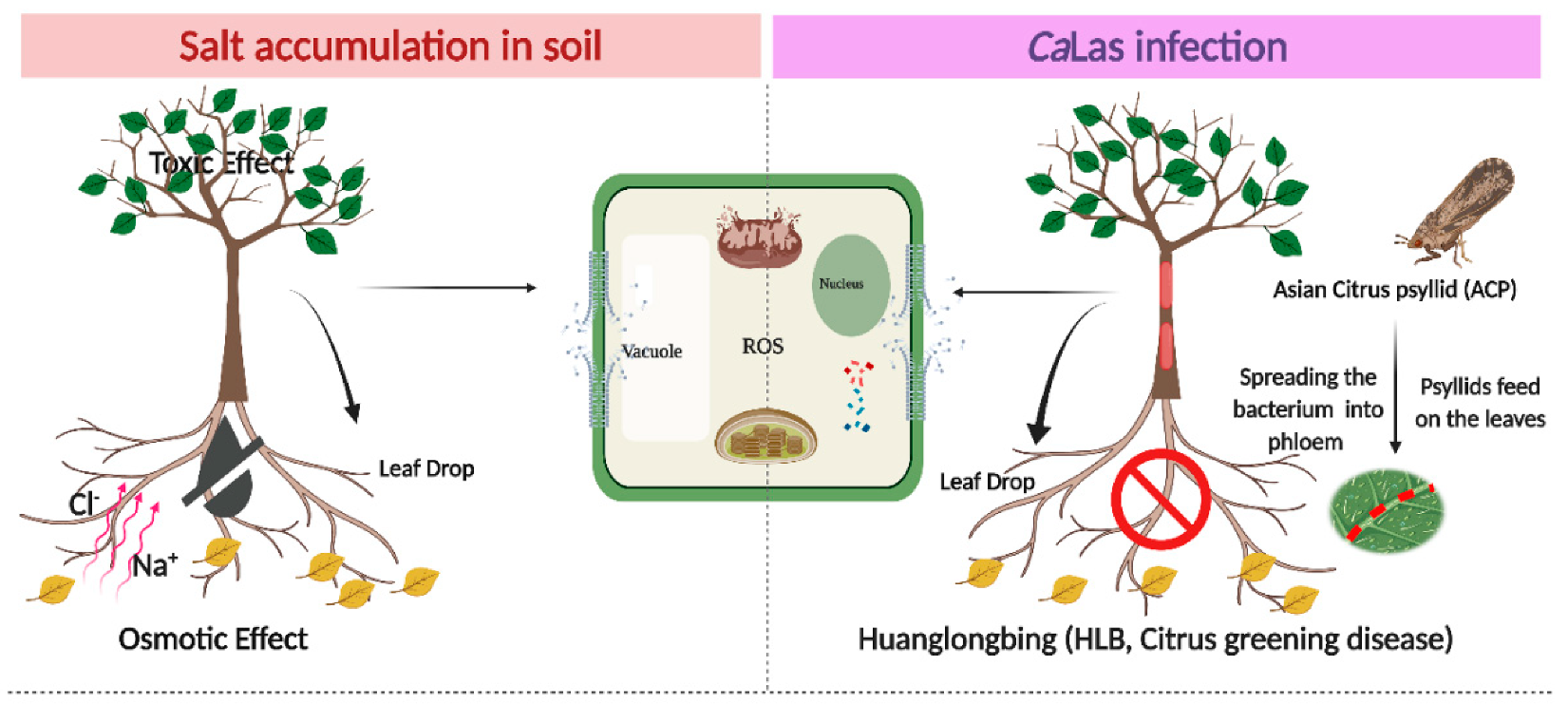
:1. Introduction
2. Results and Discussion
2.1. Simple Sequence Repeat (SSR) Marker Analysis
2.2. Effect of NaCl Treatment and CaLas on Total Chlorophyll and Starch Accumulation in Leaves
2.3. Effect of NaCl Treatment and CaLas on TPC, Proline and MDA Content
2.4. Effect of NaCl Treatment and CaLas Infection on Sodium and Chloride Content in Leaves and Roots
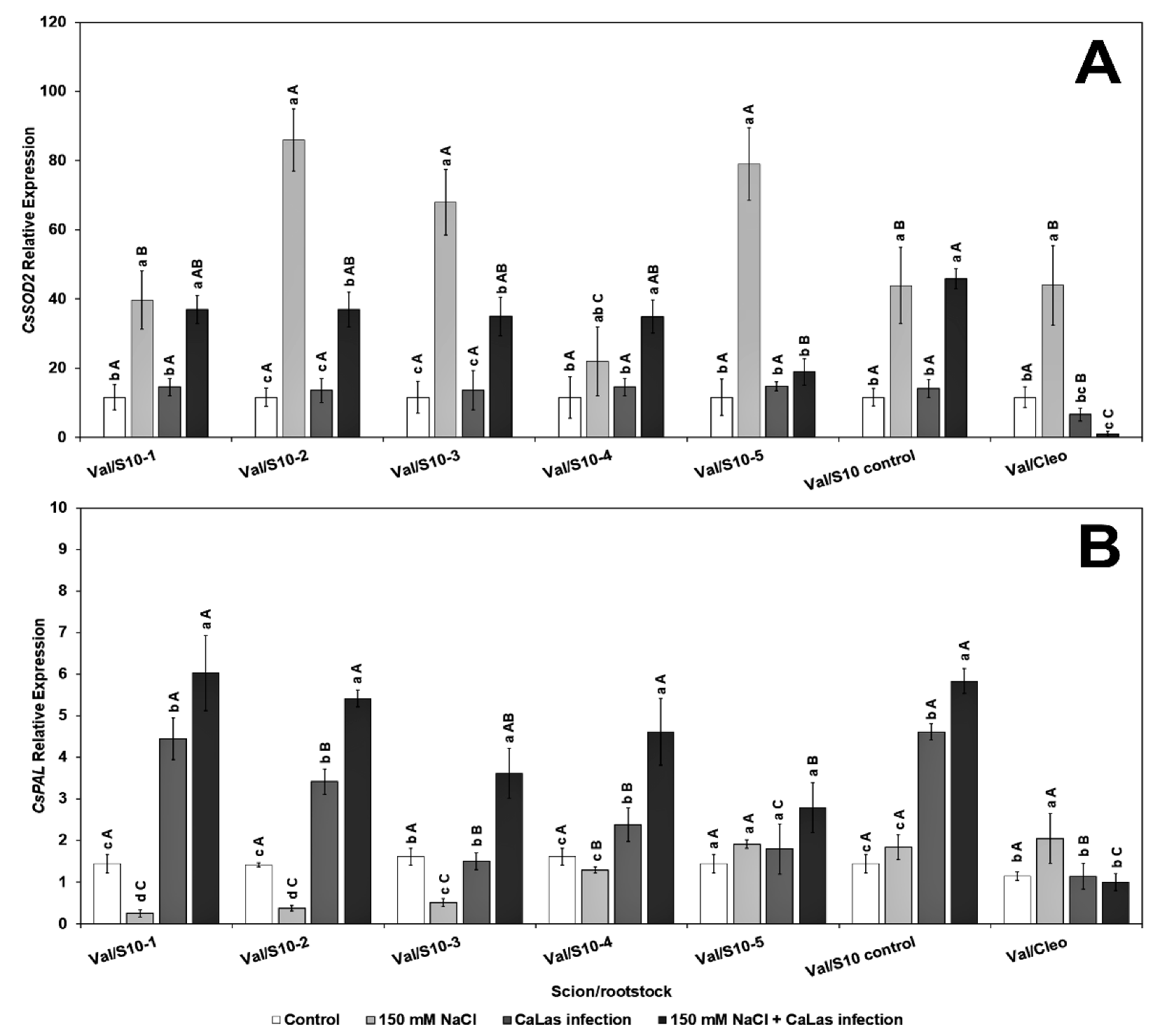
2.5. CaLas Diagnosis and Gene Expression Analysis
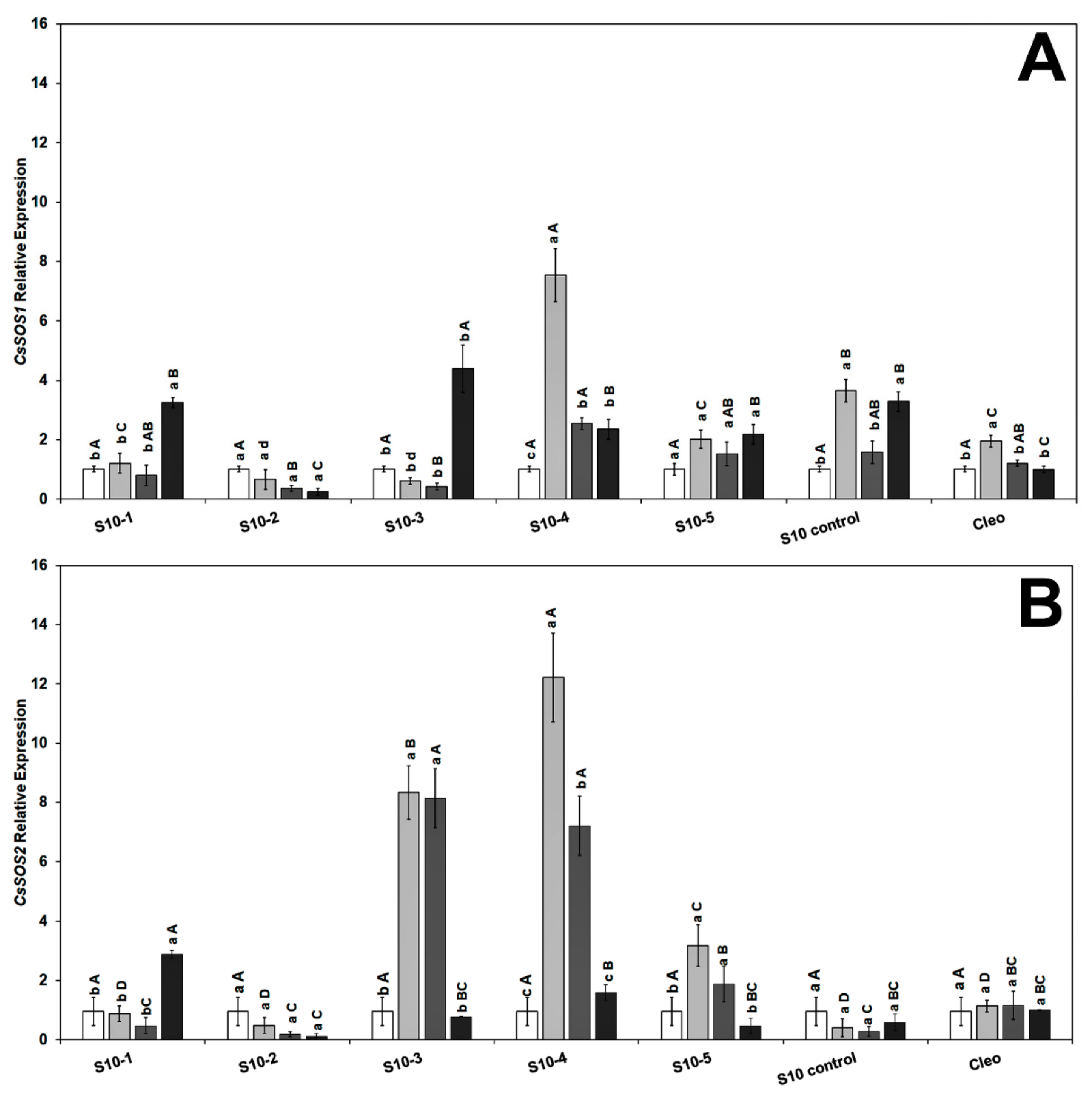
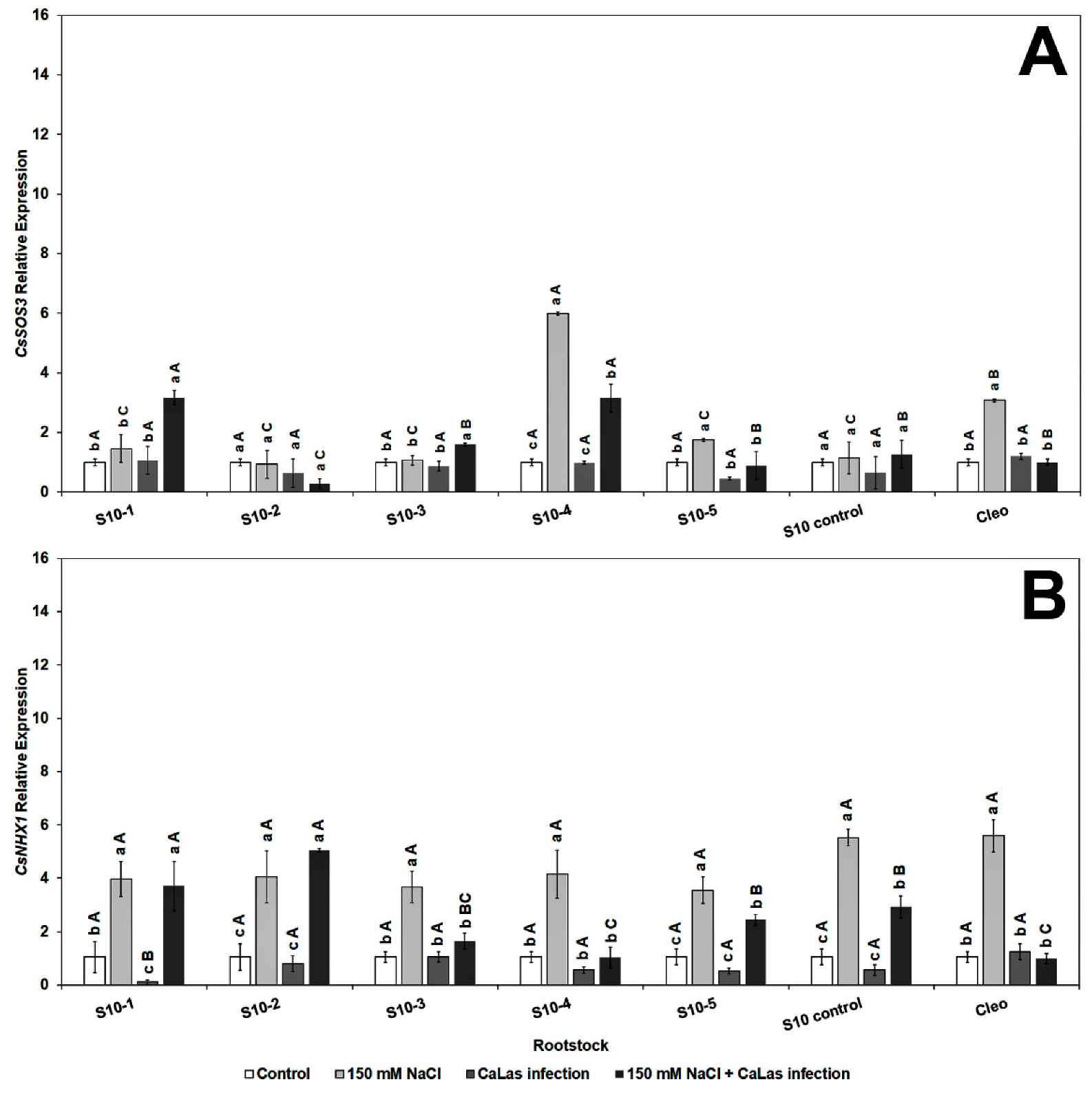
2.6. Gene Expression in the Citrus Scion and Rootstocks
3. Materials and Methods
3.1. Evaluation of NaCl Tolerance of S10 Lines
3.2. NaCl Treatment Application and Greenhouse Conditions
3.3. Simple Sequence Repeat (SSR) Marker Analysis
3.4. Physiological Variables Measurement
3.5. Sodium and Chloride Ion Analysis
3.6. CaLas Diagnosis and Gene Expression Analysis
3.7. Experimental Design and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, S.; Rohrig, E.; Solís, D.; Thomas, M.H. Citrus greening disease (Huanglongbing) in Florida: Economic impact, management and the potential for biological control. Agric. Res. 2016, 5, 109–118. [Google Scholar] [CrossRef]
- Yates, J.D.; Dewdney, M.M.; Brlansky, R. Exotic Citrus Diseases: Early Detection is the Solution to Protecting Florida Citrus. EDIS 2009, 3, 1–2. [Google Scholar]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- Vincent, C.; Morillon, R.; Arbona, V.; Gómez-Cadenas, A. Citrus in changing environments. In The Genus Citrus; Elsevier: Amsterdam, The Netherlands, 2020; pp. 271–289. [Google Scholar]
- Lloyd, J.; Syvertsen, J.; Kriedemann, P. Salinity Effects of Leaf Water Relations and Gas Exchange of ’Valencia’ Orange, Citrus sinensis (L.) Osbeck, on Rootstocks with Different Salt Exclusion Characteristics. Funct. Plant Biol. 1987, 14, 605–617. [Google Scholar] [CrossRef]
- Francois, L.; Clark, R. Salinity effects on yield and fruit quality of ‘Valencia’ orange. J. Am. Soc. Hortic. Sci. 1980, 105, 199–202. [Google Scholar]
- Grosser, J.W.; Omar, A.A.; Gmitter, J.A. Salinity tolerance of ‘Valencia’ orange trees on allotetraploid rootstocks. Proc. Fla. State Hortic. Soc. 2012, 125, 50–55. [Google Scholar]
- Grieve, A.; Prior, L.; Bevington, K. Long-term effects of saline irrigation water on growth, yield, and fruit quality of ‘Valencia’ orange trees. Aust. J. Agric. Res. 2007, 58, 342–348. [Google Scholar] [CrossRef]
- Murkute, A.A.; Sharma, S.; Singh, S. Citrus in Terms of Soil and Water Salinity: A Review. J. Sci. Ind. Res. 2005, 64, 393–402. [Google Scholar]
- Munns, R.; Goyal, S.; Passioura, J. Salinity Stress and Its Mitigation; University of California: Davis, CA, USA, 2005. [Google Scholar]
- Vincent, C.; Rowland, D.; Schaffer, B.; Bassil, E.; Racette, K.; Zurweller, B. Primed acclimation: A physiological process offers a strategy for more resilient and irrigation-efficient crop production. Plant Sci. 2020, 295, 110240. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, L.M.; Dutt, M.; Vincent, C.I.; Grosser, J.W. Salinity-induced physiological responses of three putative salt tolerant citrus rootstocks. Horticulturae 2020, 6, 90. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Espinoza-Núñez, E.; Junior, J.P.; Mourão Filho, F.A.; Machado, E.C. Citrus rootstocks for improving the horticultural performance and physiological responses under constraining environments. In Improvement of Crops in the Era of Climatic Changes; Springer: New York, NY, USA, 2014; pp. 1–37. [Google Scholar]
- Oustric, J.; Morillon, R.; Luro, F.; Herbette, S.; Lourkisti, R.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Carrizo citrange rootstock (Citrus sinensis Osb.× Poncirus trifoliata L. Raf.) enhances natural chilling stress tolerance of common clementine (Citrus clementina Hort. ex Tan). J. Plant Physiol. 2017, 214, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Graham, J. Root health in the age of HLB. Citrus Ind. 2015, 98, 14–18. [Google Scholar]
- Johnson, E.; Wu, J.; Bright, D.; Graham, J. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Gottwald, T.; Dixon, T. Florida Actions toward HLB Control. In Proceedings of the International Workshop on Citrus Greening, Ribeireo Preto, Brazil, 22 July 2006; pp. 14–21. [Google Scholar]
- Stover, E.; Inch, S.; Richardson, M.L.; Hall, D.G. Conventional citrus of some scion/rootstock combinations show field tolerance under high Huanglongbing disease pressure. HortScience 2016, 51, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Castle, W.S.; Bowman, K.D.; Grosser, J.W.; Ferrarezi, R.S.; Futch, S.H.; Rogers, S. Florida citrus rootstock selection guide. EDIS 2020, 1–4. Available online: http://www.crec.ifas.ufl.edu/extension/citrus_rootstock/templates/guide/explore.html (accessed on 2 July 2021).
- Ćalović, M.; Chunxian, C.; Qibin, Y.; Vladimir, O.; Frederick, G.; Grosser, J. New Somatic Hybrid Mandarin Tetraploids Generated by Optimized Protoplast Fusion and Confirmed by Molecular Marker Analysis and Flow Cytometry. J. Am. Soc. Hortic. 2019, 144, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Grosser, J.W.; Gmitter, F.G. Protoplast fusion for production of tetraploids and triploids: Applications for scion and rootstock breeding in citrus. Plant Cell Tissue Organ Cult. 2011, 104, 343–357. [Google Scholar] [CrossRef]
- Grosser, J.; Gmitter, F.; Castle, W. Breeding citrus rootstocks to mitigate Huanglongbing (HLB, or citrus greening disease). Acta Hortic. 2014, 1127, 83–88. [Google Scholar] [CrossRef]
- Syvertsen, J.; Bandaranyayake, W. Salinity Tolerance of ‘Hamlin’Orange Trees on the Hybrid Rootstocks US-897 and x639 Is Greater than of Trees on Cleopatra Mandarin. Proc. Fla. State Hortic. Soc. 2012, 125, 56–60. [Google Scholar]
- Albrecht, U.; Bowman, K.D. Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco × Poncirus trifoliata L. Raf.) to Huanglongbing. HortScience 2011, 46, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Mantri, N.; Patade, V.; Pang, E. Recent advances in rapid and sensitive screening for abiotic stress tolerance. In Improvement of Crops in the Era of Climatic Changes; Springer: New York, NY, USA, 2014; pp. 37–47. [Google Scholar]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 89–100. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, L.M.; Vincent, C.I.; Grosser, J.W.; Dutt, M. The response of salt-stressed Valencia sweet orange (Citrus sinensis) to salicylic acid and methyl jasmonate treatments. Plant Physiol. Rep. 2021, 26, 137–151. [Google Scholar] [CrossRef]
- Qiu, W.; Soares, J.; Pang, Z.; Huang, Y.; Sun, Z.; Wang, N.; Grosser, J.; Dutt, M. Potential mechanisms of AtNPR1 mediated resistance against Huanglongbing (HLB) in citrus. Int. J. Mol. Sci. 2020, 21, 2009. [Google Scholar] [CrossRef] [Green Version]
- Seo, P.J.; Lee, A.K.; Xiang, F.; Park, C.M. Molecular and functional profiling of Arabidopsis pathogenesis-related genes: Insights into their roles in salt response of seed germination. Plant Cell Physiol. 2008, 49, 334–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The Salt Overly Sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassil, E.; Tajima, H.; Liang, Y.C.; Ohto, M.A.; Ushijima, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwald, E. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Datta, S.K.; Muthukrishnan, S. Pathogenesis-Related Proteins in Plants; CRC Press: Boca Raton, FL, USA, 1999; p. 288. [Google Scholar]
- Fraire-Velázquez, S.; Rodríguez-Guerra, R.; Sánchez-Calderón, L. Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives; Shanker, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 3–26. [Google Scholar]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Kumar, K.R.R.; Kumar, D.; Shukla, P.; Kirti, P. Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachis diogoi, a wild peanut. PLoS ONE 2013, 8, e83963. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, Z.Z.; Zhou, X.F.; Yin, H.B.; Li, X.; Xin, X.F.; Hong, X.H.; Zhu, J.K.; Gong, Z. Overexpression of SOS (Salt Overly Sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol. Plant 2009, 2, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Lyman, J.; Fleming, R.H. Composition of sea water. J. Mar. Res. 1940, 3, 134–146. [Google Scholar]
- Rao, M.N.; Soneji, J.R.; Chen, C.; Huang, S.; Gmitter, F.G. Characterization of zygotic and nucellar seedlings from sour orange-like citrus rootstock candidates using RAPD and EST-SSR markers. Tree Genet. Genomes 2008, 4, 113–124. [Google Scholar]
- Xiang, C.; Roose, M. Frequency and characteristics of nucellar and zygotic seedlings in 12 citrus rootstocks. Sci. Hortic. 1988, 37, 47–59. [Google Scholar] [CrossRef]
- Grosser, J.W.; Medina-Urrutia, V.; Ananthakrishnan, G.; Serrano, P. Building a replacement sour orange rootstock: Somatic hybridization of selected mandarin + pummelo combinations. J. Am. Soc. Hortic. Sci. 2004, 129, 530–534. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, A.; Pati, P.K.; Virk, G.; Nagpal, A. An efficient micropropagation protocol for Citrus jambhiri Lush. and assessment of clonal fidelity employing anatomical studies and RAPD markers. In Vitro Cell. Dev. Biol. Plant 2012, 48, 512–520. [Google Scholar]
- Achor, D.; Etxeberria, E.; Wang, N.; Folimonova, S.; Chung, K.; Albrigo, L. Sequence of anatomical symptom observations in citrus affected with huanglongbing disease. Plant Pathol. J. 2010, 9, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Thalmann, M.; Diana, S. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Diane, M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef]
- Thitisaksakul, M.; Maria, A.; Shaoyun, D.; Diane, B. Overexpression of GSK3-like Kinase 5 (OsGSK5) in rice (Oryza sativa) enhances salinity tolerance in part via preferential carbon allocation to root starch. Funct. Plant Biol. 2017, 44, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Kyoko, H.; Takashi, H.; Teruko, K.; Tadashi, I.; Naoko, F.; Yasunori, N.; Yoshiyuki, M.; Masaaki, Y.; Toshiaki, T. Common reed produces starch granules at the shoot base in response to salt stress. New Phytol. 2007, 176, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [Green Version]
- Levy, Y.; Syvertsen, J. Irrigation water quality and salinity effects in citrus trees. Hortic. Rev. 2004, 30, 37–82. [Google Scholar]
- McCollum, G.; Baldwin, E. Huanglongbing: Devastating disease of citrus. Hortic. Rev. 2017, 44, 315–361. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.M.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Luro, F.; Costantino, G.; Ollitrault, P.; Morillon, R. Physiological analysis of salt stress behaviour of citrus species and genera: Low chloride accumulation as an indicator of salt tolerance. S. Afr. J. Bot. 2012, 81, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Zrig, A.; Mohamed, H.B.; Tounekti, T.; Khemira, H.; Serrano, M.; Valero, D.; Vadel, A. Effect of rootstock on salinity tolerance of sweet almond (cv. Mazzetto). S. Afr. J. Bot. 2016, 102, 50–59. [Google Scholar] [CrossRef]
- Albrecht, U.; Fiehn, O.; Bowman, K.D. Metabolic variations in different citrus rootstock cultivars associated with different responses to Huanglongbing. Plant Physiol. Biochem. 2016, 107, 33–44. [Google Scholar] [CrossRef]
- Wang, N.; Trivedi, P. Citrus huanglongbing: A newly relevant disease presents unprecedented challenges. Phytopathology 2013, 103, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.S.; Heinen, J.L.; Holaday, A.S.; Burke, J.J.; Allen, R.D. Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA 1993, 90, 1629–1633. [Google Scholar] [CrossRef] [Green Version]
- Opdenakker, K.; Remans, T.; Keunen, E.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels. Environ. Exp. Bot. 2012, 83, 53–61. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Lafuente, M.T.; Zacarias, L.; Granell, A. Involvement of phenylalanine ammonia-lyase in the response of Fortune mandarin fruits to cold temperature. Physiol. Plant 2000, 108, 382–389. [Google Scholar] [CrossRef]
- Mrázová, A.; Belay, S.A.; Eliášová, A.; Perez-Delgado, C.; Kaducová, M.; Betti, M.; Vega, J.M.; Paľove-Balang, P. Expression, activity of phenylalanine-ammonia-lyase and accumulation of phenolic compounds in Lotus japonicus under salt stress. Biologia 2017, 72, 36–42. [Google Scholar] [CrossRef]
- Zou, X.; Bai, X.; Wen, Q.; Xie, Z.; Wu, L.; Peng, A.; He, Y.; Xu, L.; Chen, S. Comparative analysis of tolerant and susceptible citrus reveals the role of methyl salicylate signaling in the response to huanglongbing. J. Plant Growth Regul. 2019, 38, 1516–1528. [Google Scholar] [CrossRef]
- Wu, J.; Alférez, F.; Johnson, E.; Graham, J. Up-regulation of PR1 and less disruption of hormone and sucrose metabolism in roots is associated with lower susceptibility to ‘Candidatus Liberibacter asiaticus’. Plant Pathol. 2018, 67, 1426–1435. [Google Scholar] [CrossRef]
- Hong, J.K.; Hwang, B.K. Induction of enhanced disease resistance and oxidative stress tolerance by overexpression of pepper basic PR-1 gene in Arabidopsis. Physiol. Plant 2005, 124, 267–277. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Kumar, S.; Mishra, S.; Kobayashi, Y.; Panda, S.K.; Sahoo, L. Enhanced salinity tolerance in transgenic mungbean overexpressing Arabidopsis antiporter (NHX1) gene. Mol. Breed. 2016, 36, 144. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [Green Version]
- Halfter, U.; Ishitani, M.; Zhu, J.K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS. Proc. Natl. Acad. Sci. USA 2000, 97, 3735–3740. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Rosales, R.; Burns, J.K. Phytohormone changes and carbohydrate status in sweet orange fruit from Huanglongbing-infected trees. J. Plant Growth Regul. 2011, 30, 312–321. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol Vitic. 1965, 16, 144–158. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Anderson, D.; Henderson, L. Comparing sealed chamber digestion with other digestion methods used for plant tissue analysis. J. Agron. 1988, 80, 549–552. [Google Scholar] [CrossRef]
- Munter, R.; Halverson, T.; Anderson, R. Quality assurance for plant tissue analysis by ICP-AES [Inductively coupled plasma-atomic emission spectroscopy]. Commun. Soil Sci. Plant Anal. 1984, 15, 1285–1322. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, Y.; Hu, H.; Yuan, Q.; Peng, G.; Xia, Y. Development and application of molecular-based diagnosis for ‘Candidatus Liberibacter asiaticus’, the causal pathogen of citrus huanglongbing. Plant Pathol. 2006, 55, 630–638. [Google Scholar] [CrossRef]
- Vincent, C.; Guha, A.; Killiny, N.; Diepenbrock, L. Understory Environment Promotes Photosynthetic Efficiency and Mitigates Severity and Function of an Introduced, Vectored Pathosystem: A Study of a Feral Citrus Population in Central Florida. Trees 2021, 1–15. [Google Scholar] [CrossRef]
- Gao, X.; Ren, Z.; Zhao, Y.; Zhang, H. Overexpression of SOD2 increases salt tolerance of Arabidopsis. Plant Physiol. 2003, 133, 1873–1881. [Google Scholar] [CrossRef] [Green Version]
- Gholizadeh, A.; Kohnehrouz, B.B. Activation of phenylalanine ammonia lyase as a key component of the antioxidative system of salt-challenged maize leaves. Braz. J. Plant Physiol. 2010, 22, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Barragán, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernández, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]

| Genotype a | CX6F04 | CX6F29 | CX6F18 | CX6F14 | CX0035 | CX6F18 | Origin |
|---|---|---|---|---|---|---|---|
| HBP | 100 b | 289 | 149/150 | 263/274 | 395 | 192 | Parent |
| SHEKWASHA | 106/113 | 269/274 | 150/156 | 274 | 429 | 197 | Parent |
| S10 | 100/106 | 289 | 149/150 | 263/274 | 395/429 | 192/197 | Parent |
| S10-line 1 | 106/113 | 289 | 150/156 | 274 | 395/429 | 192/197 | Zygotic |
| S10-line 2 | 113 | 289 | 149 | 263/274 | 395/429 | 192 | Zygotic |
| S10-line 3 | 106/113 | 269/274 | 149/150 | 263/274 | 395 | 192 | Zygotic |
| S10-line 4 | 113 | 274/289 | 150 | 263/274 | 395 | 197 | Zygotic |
| S10-line 5 | 106 | 289 | 149/150 | 263/274 | 395 | 192 | Zygotic |
| S10-line 6 (S10-control) c | 100/106 | 289 | 149/150 | 263/274 | 395/429 | 192/197 | Nucellar |
| Variables | NaCl Treatments | CaLas Infection | Scion/Rootstocks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Val/S10-1 | Val/S10-2 | Val/S10-3 | Val/S10-4 | Val/S10-5 | Val/S10-6 (S10-Control) | Val/Cleo | |||
| T Chl (mg−1 g FW) | Control | Control | 6.13 a B * | 6.68 a B | 7.23 a AB | 5.98 a B | 7.09 a AB | 9.10 a A | 7.22 a AB |
| 150 mM NaCl | Control | 5.93 a AB | 6.10 ab AB | 4.99 a B | 5.28 a AB | 6.14 ab AB | 6.78 b A | 5.62 b AB | |
| Control | CaLas infection | 4.41 ab B | 5.60 ab AB | 6.03 a A | 5.12 a AB | 6.01 ab A | 6.02 b B | 5.25 b AB | |
| 150 mM NaCl | CaLas infection | 3.73 b BC | 4.49 b ABC | 5.02 a ABC | 5.22 a AB | 4.77 b ABC | 5.91 b B | 3.01 c C | |
| Starch content (µg·mm−2) | Control | Control | 1.05 c A | 1.19 c A | 1.25 d A | 1.33 d A | 1.26 c A | 1.23 d A | 1.33 d A |
| 150 mM NaCl | Control | 7.41 b A | 6.53 b A | 6.70 c A | 6.53 c A | 7.30 b A | 6.67 c A | 6.27 c A | |
| Control | CaLas infection | 7.51 b A | 8.48 b A | 8.10 b A | 7.83 b A | 7.97 b A | 8.17 b A | 8.43 b A | |
| 150 mM NaCl | CaLas infection | 16.52 a B | 16.98 a B | 17.28 a AB | 16.89 a B | 17.27 a AB | 17.67 a AB | 18.28 a A | |
| Variables | NaCl Treatment | CaLas Infection | Scion/Rootstocks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Val/S10-1 | Val/S10-2 | Val/S10-3 | Val/S10-4 | Val/S10-5 | Val/S10-6 (S10-Control) | Val/Cleo | |||
| TPC (mg gallic acid g−1 FW) | Control | Control | 62.33 b A * | 60.37 b A | 60.00 ab AB | 53.00 b AB | 49.33 c B | 60.00 b AB | 57.00 b AB |
| 150 mM NaCl | Control | 41.00 c B | 45.00 c AB | 50.67 b A | 47.66 b AB | 51.33 bc A | 50.00 b A | 53.00 b A | |
| Control | CaLas infection | 58.67 b B | 61.33 b B | 60.00 ab B | 71.00 a A | 60.33 b B | 59.67 b B | 61.00 b B | |
| 150 mM NaCl | CaLas infection | 72.00 a CD | 73.67 a C | 66.67 a D | 72.66 a C | 74.67 a C | 90.33 a A | 80.33 a A | |
| Proline (µmol g−1 FW) | Control | Control | 46.00 c D | 52.67 c BC | 52.33 c C | 53.33 c ABC | 56.67 d AB | 53.67 a ABC | 57.00 c A |
| 150 mM NaCl | Control | 201.13a AB | 199.33 a AB | 221.33 a A | 174.33 a ABC | 157.00 a BC | 202.67a AB | 118.67 ab C | |
| Control | CaLas infection | 122.33 b A | 110.33 b B | 115.33 b AB | 114.67 b AB | 109.00 c B | 114.67c AB | 91.33 bc C | |
| 150 mM NaCl | CaLas infection | 227.00 a A | 113.67 b C | 121.67 b BC | 128.00 b BC | 141.66 b BC | 158.00b B | 142.00 a BC | |
| MDA in leaves (nmol−1 MDA eq. g FW) | Control | Control | 31.01 c B | 42.75 b A | 33.08 b B | 33.08 d B | 33.55 d B | 31.48 d B | 33.08 c B |
| 150 mM NaCl | Control | 61.41 b A | 38.21 b B | 63.14 a A | 56.28 c A | 57.88 c A | 49.48 c AB | 63.21 b A | |
| Control | CaLas infection | 63.65 b B | 88.49 a A | 77.55 a AB | 66.62 b AB | 71.28 b AB | 73.62 b AB | 86.35 a AB | |
| 150 mM NaCl | CaLas infection | 90.71 a ABC | 86.71 a ABC | 84.24 a BC | 76.51 a C | 93.77 a AB | 90.78 a AB | 96.51 a A | |
| MDA in roots (nmol−1 MDA eq. g FW) | Control | Control | 16.67 a A | 9.67 a BC | 7.27 c C | 7.27 b C | 7.20 c C | 7.80 b BC | 12.60 c AB |
| 150 mM NaCl | Control | 19.43 a B | 18.04 a B | 15.37 ab B | 31.5 a A | 16.63 b B | 23.97 a AB | 18.23 b B | |
| Control | CaLas infection | 11.41 a C | 12.54 a BC | 12.47 bc BC | 13.61b ABC | 14.07 b AB | 13.74 b AB | 15.74 bc A | |
| 150 mM NaCl | CaLas infection | 11.57 a C | 11.17 a C | 20.49 a BC | 30.89 a AB | 34.49 a A | 30.89 a AB | 33.89 a A | |
| Variables | NaCl Treatment | CaLas Infection | Scion/Rootstocks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Val/S10-1 | Val/S10-2 | Val/S10-3 | Val/S10-4 | Val/S10-5 | Val/S10-6 (S10-Control) | Val/Cleo | |||
| Na(L) (mM·L−1) | Control | Control | 37.57 c A * | 35.34 c A | 33.52 c A | 29.66 b A | 28.43 c A | 28.43 c A | 27.83 b A |
| 150 mM NaCl | Control | 184.37 a AB | 216.57 a A | 159.74 a ABC | 135.14 a BC | 160.68 a ABC | 94.43 b C | 159.80 a ABC | |
| Control | CaLas infection | 34.60 c A | 34.60 c A | 36.84 c A | 36.84 b A | 36.93 b A | 36.84 c A | 37.65 b A | |
| 150 mM NaCl | CaLas infection | 131.81 b AB | 81.17 b B | 80.19 b B | 105.73 a AB | 156.25 a A | 152.10 a AB | 174.58 a A | |
| CL(L) (mM·L−1) | Control | Control | 9.71 c A | 8.76 b A | 8.76 b A | 8.76 b A | 8.76 c A | 8.76 b A | 8.76 c A |
| 150 mM NaCl | Control | 23.71 b AB | 28.57 a AB | 40.00 a A | 34.47 a AB | 29.05 a AB | 29.71a AB | 38.09 a AB | |
| Control | CaLas infection | 9.71 c A | 10.00 b A | 9.62 b A | 9.81 b AB | 8.47 c B | 9.24 b AB | 9.24 c AB | |
| 150 mM NaCl | CaLas infection | 38.09 a A | 39.43 a A | 34.86 a A | 34.00 a A | 18.19 b B | 24.29 a B | 19.52 b B | |
| Variables | NaCl treatment | CaLas infection | Rootstocks | ||||||
| S10-1 | S10-2 | S10-3 | S10-4 | S10-5 | S10-control | Cleo | |||
| Na(R) (mM·L−1) | Control | Control | 73.92 c A * | 74.10 b A | 70.10 b AB | 71.46 c AB | 57.42 b AB | 64.03 b AB | 51.70 c B |
| 150 mM NaCl | Control | 195.44 b C | 263.22 c AB | 367.46 a A | 363.43 a A | 235.22 a BC | 325.00 a AB | 232.06 b BC | |
| Control | CaLas infection | 31.95 c A | 27.45 c A | 29.45 b A | 32.09 c A | 32.46 b A | 32.46 b A | 27.22 c A | |
| 150 mM NaCl | CaLas infection | 239.66 a B | 236.53 a B | 230.86 a B | 250.48 a B | 291.15 a AB | 272.59 a AB | 319.70 a A | |
| CL(R) (mM·L−1) | Control | Control | 6.44 b A | 6.50 b A | 6.53 c A | 6.48 c A | 6.49 b A | 6.51 b A | 6.48 b B |
| 150 mM NaCl | Control | 23.71 a A | 22.95 a A | 23.24 a A | 20.86 a A | 24.00 a A | 21.71 a A | 25.52 a A | |
| Control | CaLas infection | 7.05 b A | 7.14 b A | 6.29 c B | 6.00 c B | 6.00 b B | 6.29 b B | 5.81 b B | |
| 150 mM NaCl | CaLas infection | 22.95 a A | 23.14 a A | 16.86 b B | 13.05 b C | 21.90 a A | 22.86 a A | 18.00 b A | |
| Scion/Rootstock | Ct Value before NaCl Treatment | Ct Value after NaCl Treatment | |
|---|---|---|---|
| 0 mM NaCl | 150 mM NaCl | ||
| Val/S10—line 1 | 28.71± 2.65 ab * | 28.63 ± 0.43 ab | 29.86 ± 0.15 ab |
| Val/S10—line 2 | 25.30 ± 0.78 bc | 25.26 ± 0.08 bc | 25.48 ± 0.33 bc |
| Val/S10—line 3 | 27.77 ± 1.12 ab | 27.76 ± 0.03 ab | 27.88 ± 1.84 ab |
| Val/S10—line 4 | 30.46 ± 0.30 ab | 29.76 ± 0.21 ab | 28.48 ± 3.35 ab |
| Val/S10—line 5 | 31.45 ± 1.22 a | 30.15 ± 0.23 a | 28.27 ± 3.01 ab |
| Val/S10—line 6 (S10-control) | 27.72 ± 0.64 ab | 27.02 ± 0.25 b | 24.59 ± 1.42 bc |
| Val/Cleo | 26.87 ± 0.61 ab | 25.4 ± 0.55 e | 19.50 ± 0.99 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, L.M.; Huyck, P.J.; Vincent, C.I.; Gmitter, F.G., Jr.; Grosser, J.W.; Dutt, M. Physiological Responses and Gene Expression Patterns in Open-Pollinated Seedlings of a Pummelo-Mandarin Hybrid Rootstock Exposed to Salt Stress and Huanglongbing. Plants 2021, 10, 1439. https://doi.org/10.3390/plants10071439
Mahmoud LM, Huyck PJ, Vincent CI, Gmitter FG Jr., Grosser JW, Dutt M. Physiological Responses and Gene Expression Patterns in Open-Pollinated Seedlings of a Pummelo-Mandarin Hybrid Rootstock Exposed to Salt Stress and Huanglongbing. Plants. 2021; 10(7):1439. https://doi.org/10.3390/plants10071439
Chicago/Turabian StyleMahmoud, Lamiaa M., Patrick J. Huyck, Christopher I. Vincent, Frederick G. Gmitter, Jr., Jude W. Grosser, and Manjul Dutt. 2021. "Physiological Responses and Gene Expression Patterns in Open-Pollinated Seedlings of a Pummelo-Mandarin Hybrid Rootstock Exposed to Salt Stress and Huanglongbing" Plants 10, no. 7: 1439. https://doi.org/10.3390/plants10071439
APA StyleMahmoud, L. M., Huyck, P. J., Vincent, C. I., Gmitter, F. G., Jr., Grosser, J. W., & Dutt, M. (2021). Physiological Responses and Gene Expression Patterns in Open-Pollinated Seedlings of a Pummelo-Mandarin Hybrid Rootstock Exposed to Salt Stress and Huanglongbing. Plants, 10(7), 1439. https://doi.org/10.3390/plants10071439







