Temporal Evolution of the Gravitaxis of Euglena gracilis from a Single Cell
Abstract
1. Introduction
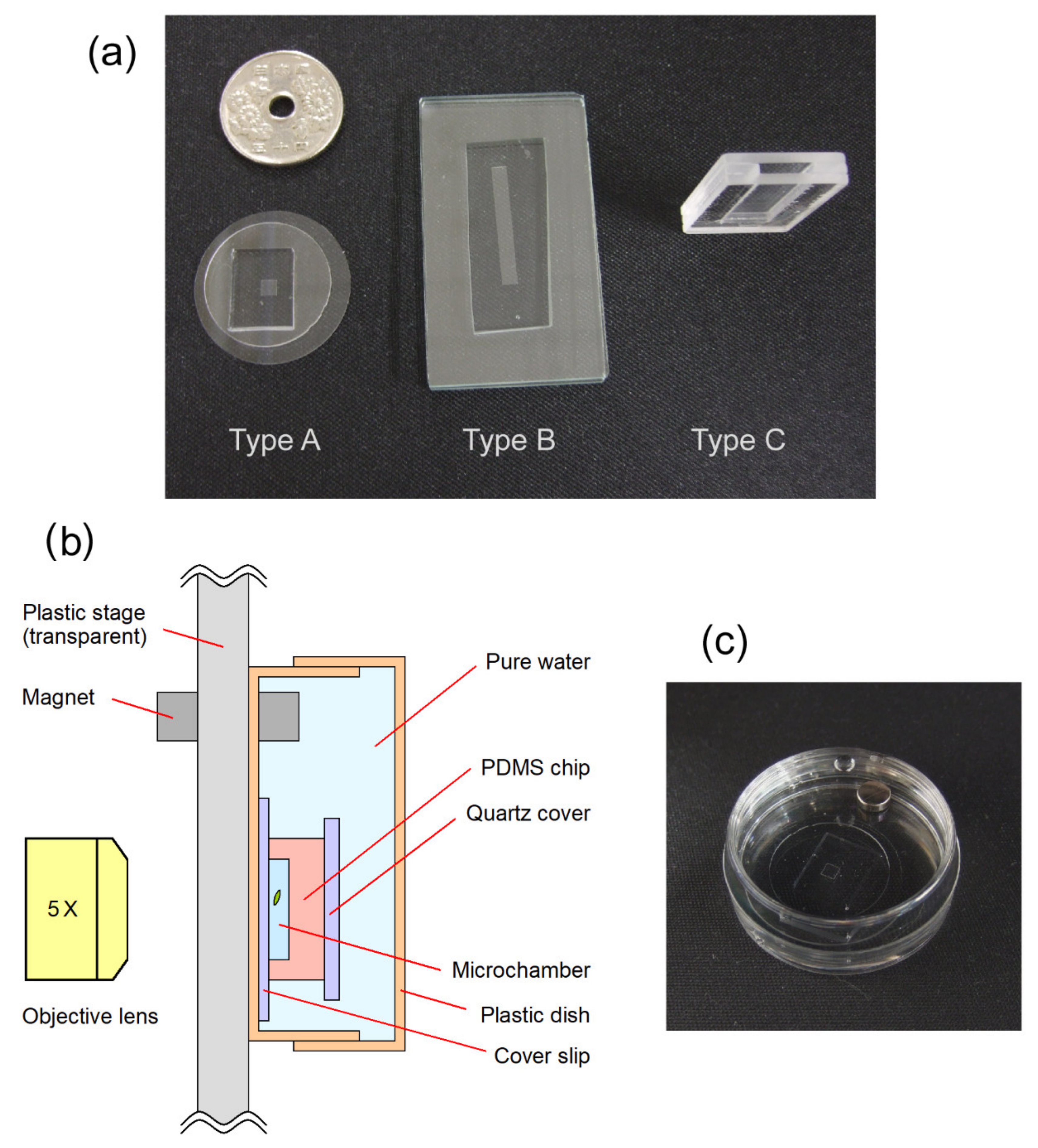
2. Materials and Methods
3. Results
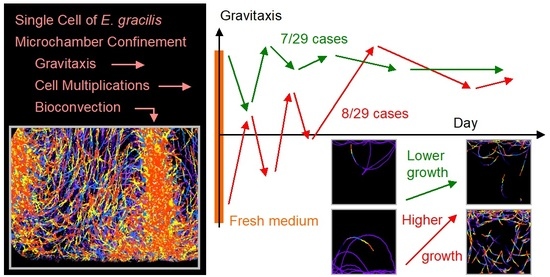
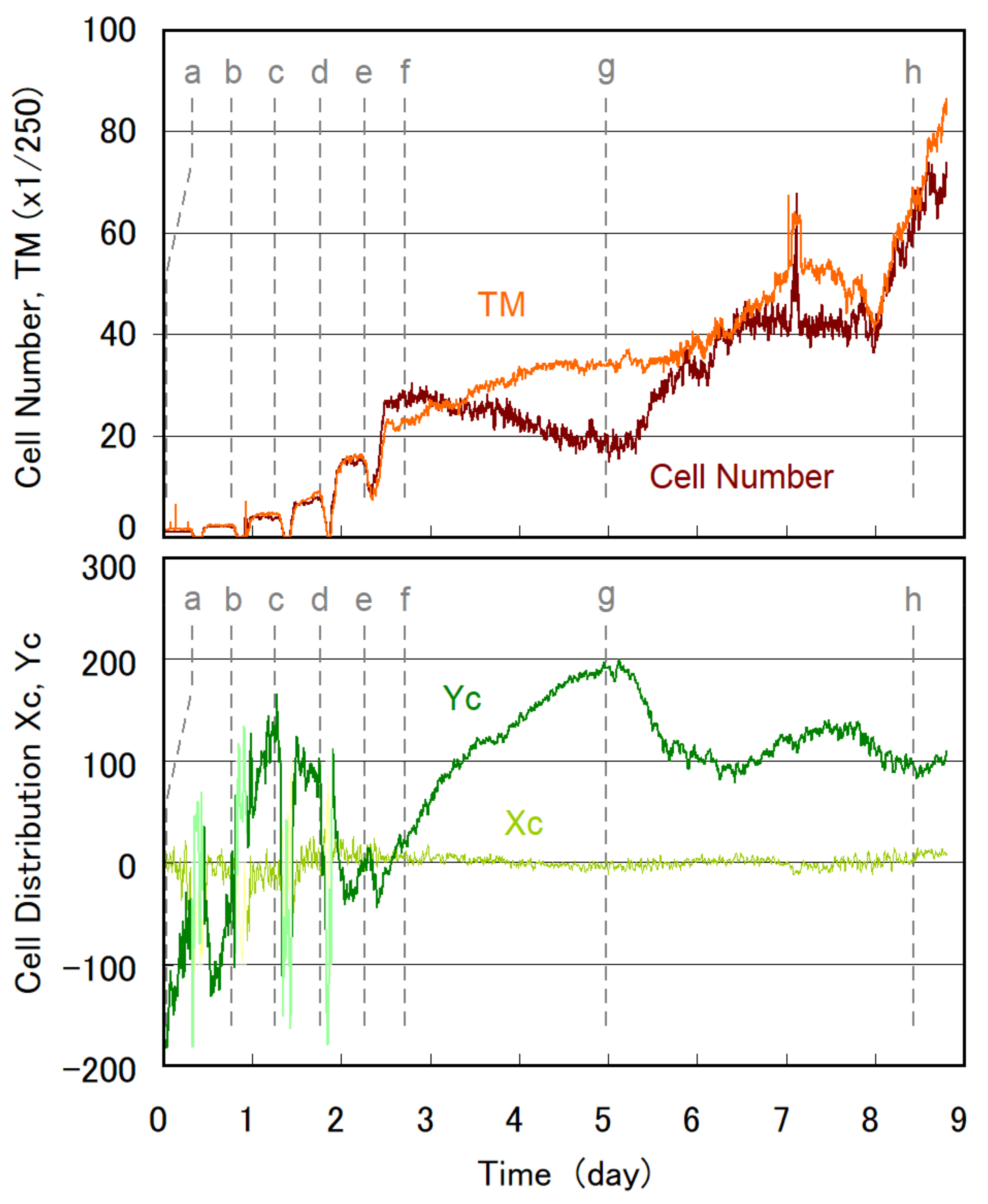
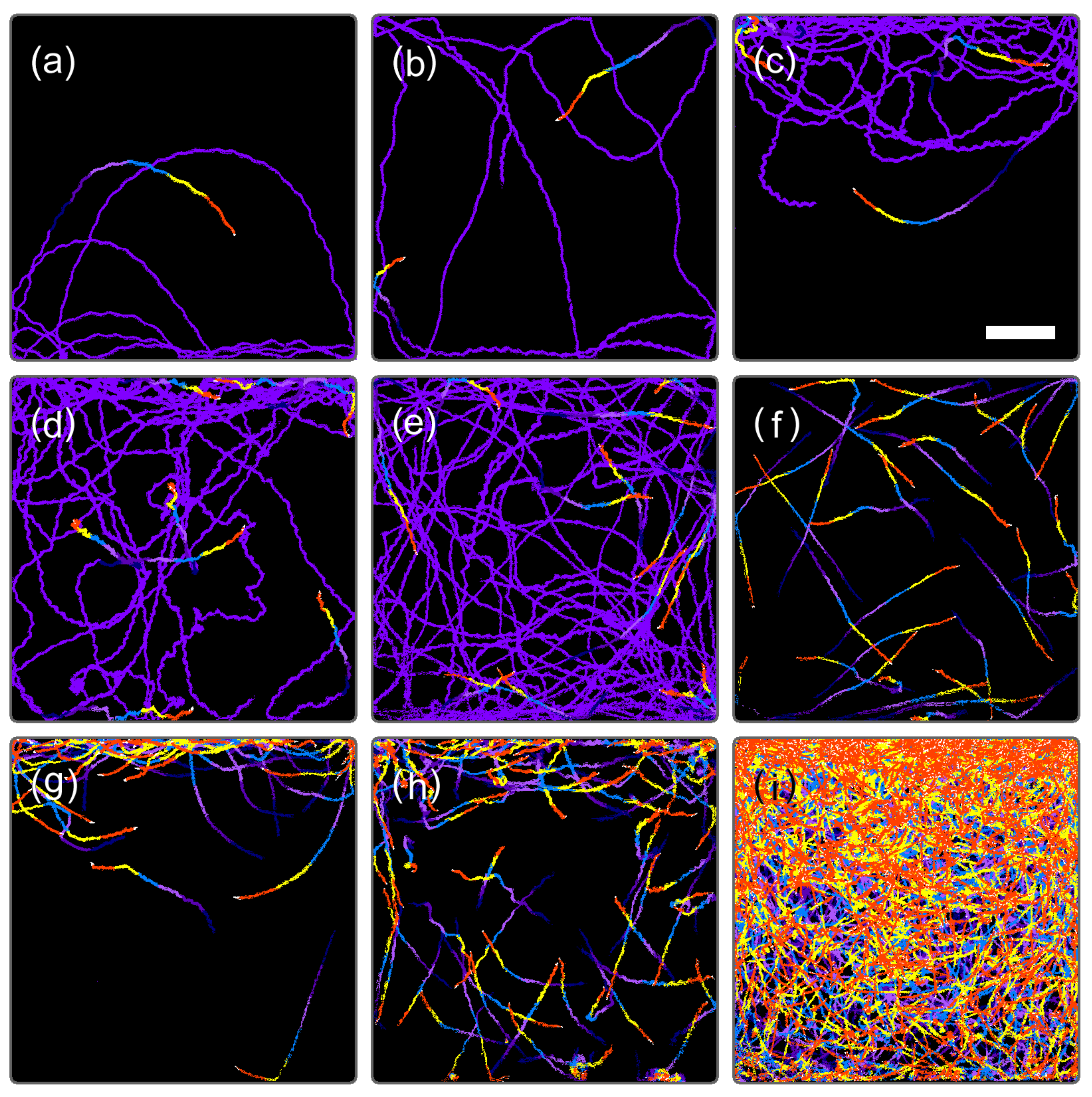
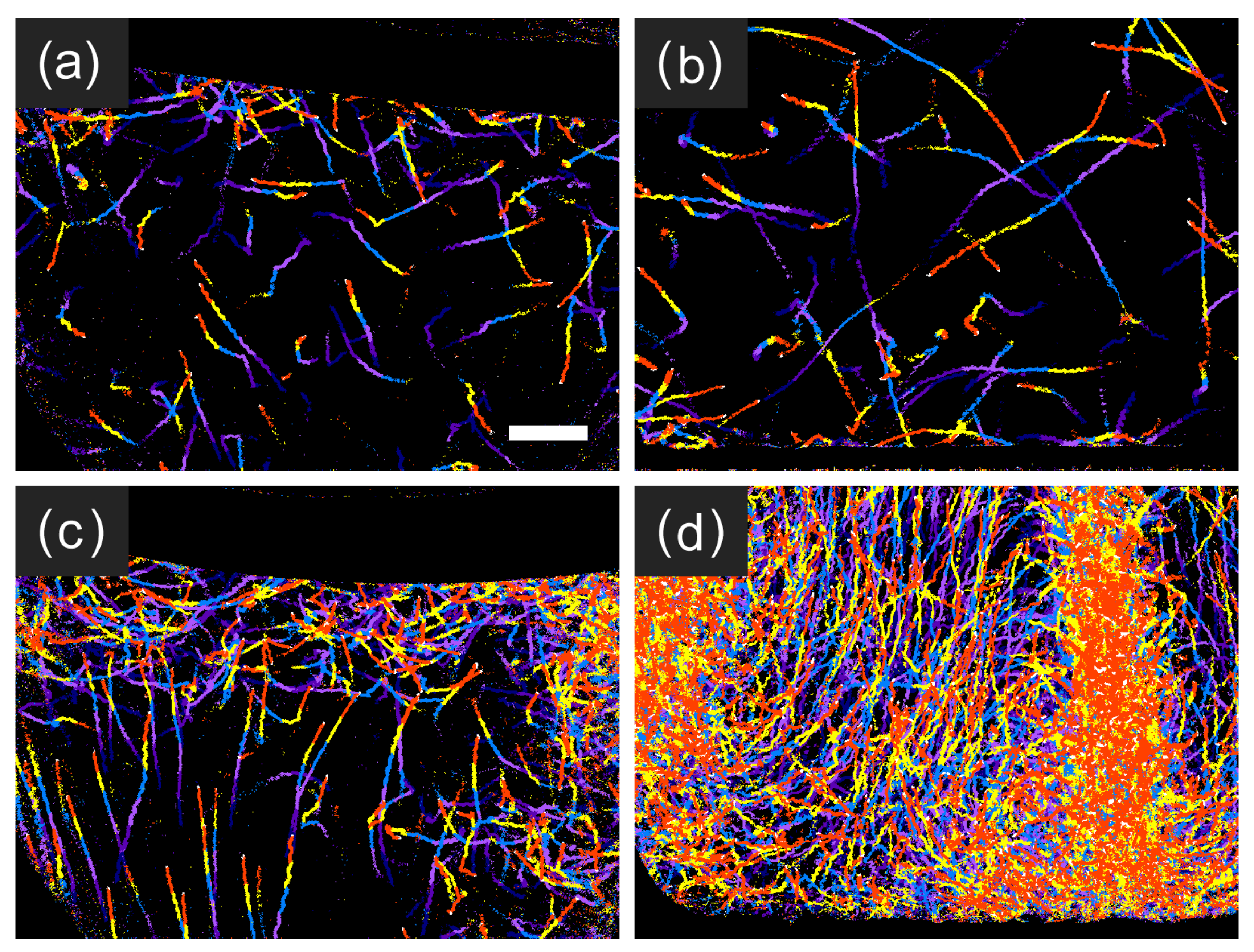
3.1. Temporal Evolution of Cell Multiplication and Gravitaxis
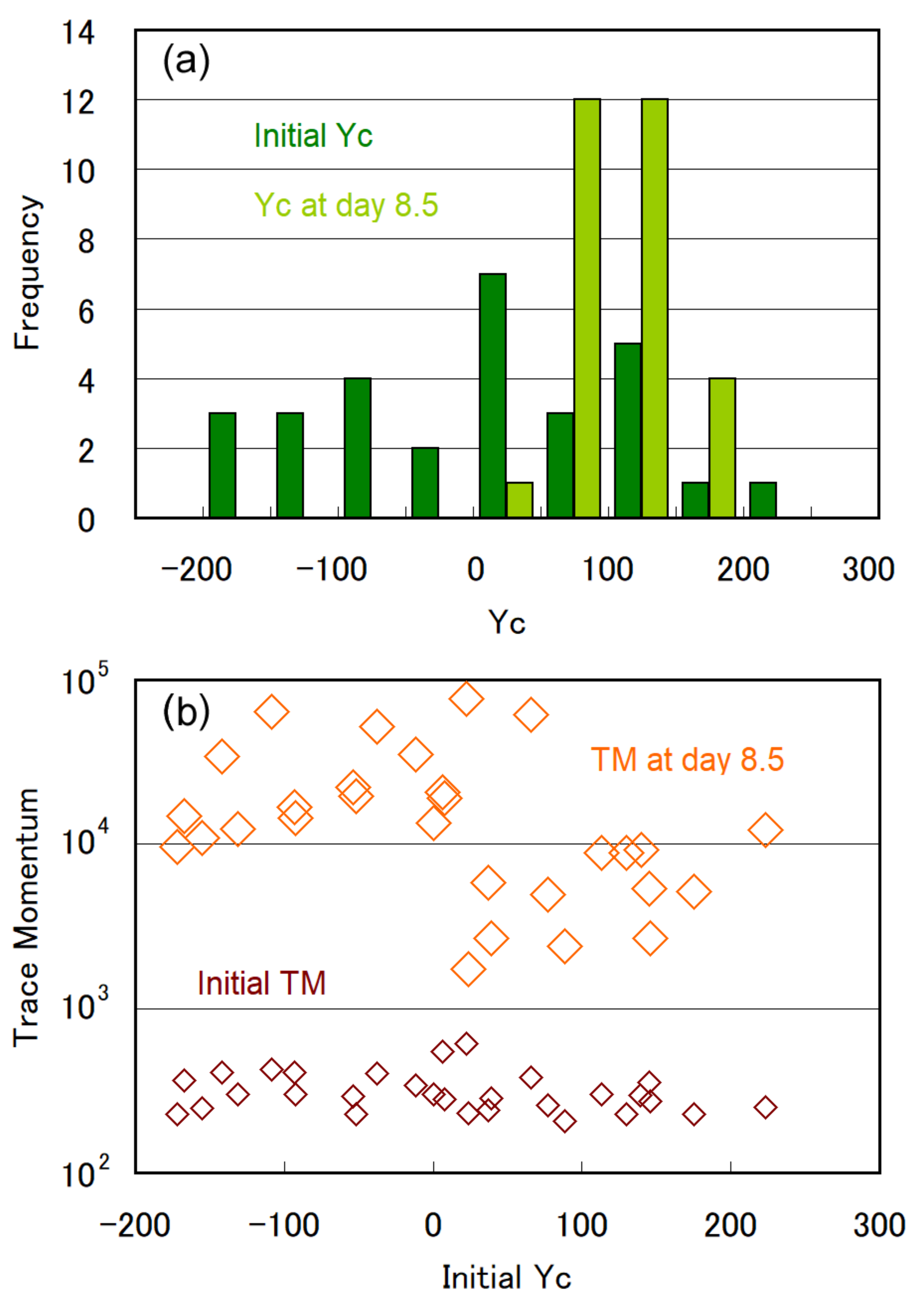
3.2. Trends of Gravitaxis and Cell Multiplication
3.3. Specific Observation for Top and Bottom Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Machemer, H.; Bräucker, R. Gravireception and Graviresponses in Ciliates. Acta Protozool. 1992, 31, 185–214. [Google Scholar] [PubMed]
- Hemmersbach, R.; Volkmann, D.; Häder, D.-P. Graviorientation in Protists and Plants. J. Plant Physiol. 1999, 154, 1–15. [Google Scholar] [CrossRef]
- Braun, M.; Böhmer, M.; Häder, D.-P.; Hemmersbach, R.; Palme, K. Gravitational Biology I: Gravity Sensing and Graviorientation in Microorganisms and Plants; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Lebert, M.; Häder, D.-P. How Euglena Tells Up from Down. Nature 1996, 379, 590. [Google Scholar] [CrossRef]
- Richter, P.; Ntefidou, M.; Streb, C.; Lebert, M.; Häder, D.-P. Cellular Perception and Transduction Mechanisms of Gravity in Unicellular Organisms. Curr. Topics Plant Biol. 2002, 3, 143–154. [Google Scholar]
- Fukui, K.; Asai, H. Negative Geotactic Behavior of Paramecium Caudatum is Completely Described by the Mechanism of Buoyancy-Oriented Upward Swimming. Biophys. J. 1985, 47, 479–482. [Google Scholar] [CrossRef]
- Häder, D.-P.; Hemmersbach, R.; Lebert, M. Gravity and the Behavior of Unicellular Organisms; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Ruangsomboon, S.; Sornchai, P.; Prachom, N. Enhanced Hydrocarbon Production and Improved Biodiesel Qualities of Botryococcus Braunii KMITL 5 by Vitamins Thiamine, Biotin and Cobalamin Supplementation. Algal Res. 2018, 29, 159–169. [Google Scholar] [CrossRef]
- Muramatsu, S.; Atsuji, K.; Yamada, K.; Ozasa, K.; Suzuki, H.; Takeuchi, T.; Hashimoto-Marukawa, Y.; Kazama, Y.; Abe, T.; Suzuki, K.; et al. Isolation and Characterization of a Motility-Defective Mutant of Euglena Gracilis. PeerJ Life Environ. 2020, 8, e10002. [Google Scholar] [CrossRef]
- Zhou, Y.; Obenour, D.R.; Scavia, D.; Johengen, T.H.; Michalak, A.M. Spatial and Temporal Trends in Lake Erie Hypoxia, 1987–2007. Environ. Sci. Technol. 2013, 47, 899–905. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Johnson, L.T.; Sharpley, A.N.; Smith, D.R.; Baker, D.B.; Bruulsema, T.W.; Confesor, R. Increased Soluble Phosphorus Loads to Lake Erie: Unintended Consequences of Conservation Practices? J. Environ. Quality 2017, 46, 123–132. [Google Scholar] [CrossRef]
- Buetow, D.E. The biology of Euglena; Academic Press: New York, NY, USA, 1968; Volume 1. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Chapter 4-Anatomy of Euglena Gracilis. Handbook of Algal Science, Technology, and Medicine; Academic Press: New York, NY, USA, 2020; Volume 70, pp. 61–70. [Google Scholar]
- Stallwitz, E.; Häder, D.-P. Effects of Heavy Metals on Motility and Gravitactic Orientation of the Flagellate, Euglena Gracilis. Eur. J. Protistol. 1994, 30, 18–24. [Google Scholar] [CrossRef]
- Lebert, M.; Porst, M.; Häder, D.-P. Circadian Rhythm of Gravitaxis in Euglena Gracilis. J. Plant Physiol. 1999, 155, 344–349. [Google Scholar] [CrossRef]
- Häder, D.-P.; Hemmersbach, R. Gravitaxis in Euglena. In Euglena: Biochmistry, Cell and Molecular Biology; Schwartzbach, S.D., Sigeoka, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 237–266. [Google Scholar]
- Cramer, M.; Myers, J. Growth and Photosynthetic Characteristics of Euglena Gracilis. Arch. Mikrobiol. 1952, 17, 384–402. [Google Scholar] [CrossRef]
- Ozasa, K.; Won, J.; Song, S.; Shinomura, T.; Maeda, M. Phototaxis and Photo-Shock Responses of Euglena Gracilis under Gravitaxis. Algal Res. 2019, 41, 101563. [Google Scholar] [CrossRef]
- Ozasa, K.; Lee, J.; Song, S.; Hara, M.; Maeda, M. Two-Dimensional Optical Feedback Control of Euglena Confined in Closed-Type Microfluidic Channels. LabChip 2011, 11, 1933–1940. [Google Scholar] [CrossRef]
- Ozasa, K.; Lee, J.; Song, S.; Hara, M.; Maeda, M. Gas/Liquid Sensing via Chemotaxis of Euglena Cells Confined in an Isolated Micro-Aquarium. LabChip 2013, 13, 4033–4039. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, K.; Lee, J.; Song, S.; Maeda, M. Real-Time Analysis of Chemotactic Motion of Euglena Cells Confined in a Microchip Toxicity Sensor. Key Eng. Mater. 2015, 644, 185–188. [Google Scholar]
- Gillott, M.A.; Triemer, R.E. The Ultrastructure of Cell Division in Euglena Gracilis. J. Cell Sci. 1978, 31, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.M.; Bouck, B.G. Synthesis and Mobilization of Flagellar Glycoprotein during Regeneration in Euglena. J. Cell Biol. 1982, 93, 432–441. [Google Scholar] [CrossRef]
- Okuwa-Hayashi, H.; Inui, H.; Inagaki, J.; Nakazawa, M.; Ebara, S.; Sakamoto, T.; Nakano, Y. Effect of Nicotinamide on the Flagellar Detachment and Regeneration of Euglena. Vitam. Japan 2019, 93, 115–122. [Google Scholar]
- Hill, N.A.; Pedley, T.J. Bioconvection. Fluid Dyn. Res. 2005, 37, 1–20. [Google Scholar] [CrossRef]
- Bearon, R.N.; Grünbaum, D. Bioconvection in a Stratified Environment: Experiments and Theory. Phys. Fluids 2006, 18, 127102. [Google Scholar] [CrossRef]
- Bees, M.A. Advances in Bioconvection. Ann. Rev. Fluid Mechan. 2020, 52, 449–476. [Google Scholar] [CrossRef]
- Ozasa, K.; Lee, J.; Song, S.; Maeda, M. Transient Freezing Behavior in Photophobic Responses of Euglena Gracilis Investigated in a Microfluidic Device. Plant Cell Physiol. 2014, 55, 1704–1712. [Google Scholar] [CrossRef]
- Kato, S.; Ozasa, K.; Maeda, M.; Tanno, Y.; Tamaki, S.; Higuchi-Takeuchi, M.; Numata, K.; Kodama, Y.; Sato, M.; Toyooka, K.; et al. Carotenoids are Essential for Light Perception by the Eyespot Apparatus to Initiate the Phototactic Movement of Euglena Gracilis. Plant J. 2020, 101, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Tanno, Y.; Kato, S.; Ozasa, K.; Wakazaki, M.; Sato, M.; Toyooka, K.; Maoka, T.; Ishikawa, T.; Maeda, M.; et al. Carotenoid Accumulation in the Eyespot Apparatus Required for Phototaxis is Independent of Chloroplast Development in Euglena Gracilis. Plant Sci. 2020, 298, 110564. [Google Scholar] [CrossRef]
- Iseki, M.; Matsunaga, S.; Murakami, A.; Ohno, K.; Shiga, K.; Yoshida, K.; Sugai, M.; Takahashi, T.; Hori, T.; Watanabe, M. A Blue-Light Activated Adenylyl Cyclase Mediates Photoavoidance in Euglena gracilis. Nature 2002, 415, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Suzuki, T.; Watanabe, M.; Iseki, M. Kinetic Analysis of the Activation of Photoactivated Adenylyl Cyclase (PAC), a Blue-Light Receptor for Photomovements of Euglena. Photochem. Photobiol. Sci. 2005, 4, 727–731. [Google Scholar] [CrossRef][Green Version]
- Häder, D.-P.; Iseki, M. Photomovement in Euglena. In Euglena: Biochmistry, Cell and Molecular Biology; Schwartzbach, S.D., Shigeoka, S., Eds.; Springer: Berlin, Germany, 2017; pp. 207–235. [Google Scholar]
- Häder, D.-P.; Richter, P.R.; Schuster, M.; Daiker, V.; Lebert, M. Molecular Analysis of the Graviperception Signal Transduction in the Flagellate Euglena Gracilis: Involvement of a Transient Receptor Potential-Like Channel and a Calmodulin. Adv. Space Res. 2009, 43, 1179–1184. [Google Scholar] [CrossRef]
- Nasir, A.; Bail, A.L.; Daiker, V.; Klima, J.; Richter, P.; Lebert, M. Identification of a Flagellar Protein Implicated in the Gravitaxis in the Flagellate Euglena Gracilis. Sci. Rep. 2018, 8, 7605. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozasa, K.; Kang, H.; Song, S.; Kato, S.; Shinomura, T.; Maeda, M. Temporal Evolution of the Gravitaxis of Euglena gracilis from a Single Cell. Plants 2021, 10, 1411. https://doi.org/10.3390/plants10071411
Ozasa K, Kang H, Song S, Kato S, Shinomura T, Maeda M. Temporal Evolution of the Gravitaxis of Euglena gracilis from a Single Cell. Plants. 2021; 10(7):1411. https://doi.org/10.3390/plants10071411
Chicago/Turabian StyleOzasa, Kazunari, Hyunwoong Kang, Simon Song, Shota Kato, Tomoko Shinomura, and Mizuo Maeda. 2021. "Temporal Evolution of the Gravitaxis of Euglena gracilis from a Single Cell" Plants 10, no. 7: 1411. https://doi.org/10.3390/plants10071411
APA StyleOzasa, K., Kang, H., Song, S., Kato, S., Shinomura, T., & Maeda, M. (2021). Temporal Evolution of the Gravitaxis of Euglena gracilis from a Single Cell. Plants, 10(7), 1411. https://doi.org/10.3390/plants10071411