Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus esculentus Genotypes
Abstract
:1. Introduction
2. Results
2.1. Effect of Hydropriming Duration on Seed Germination
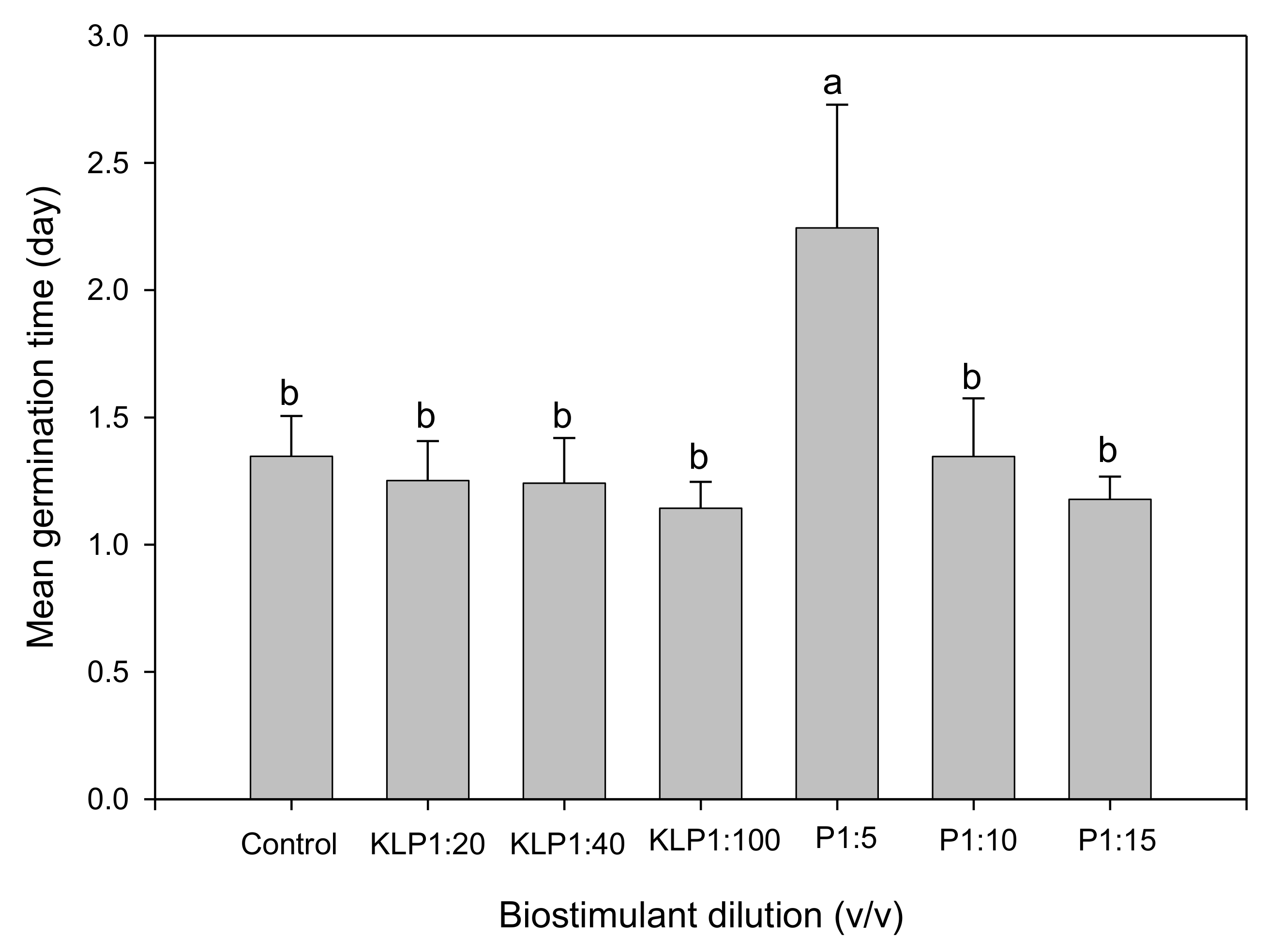
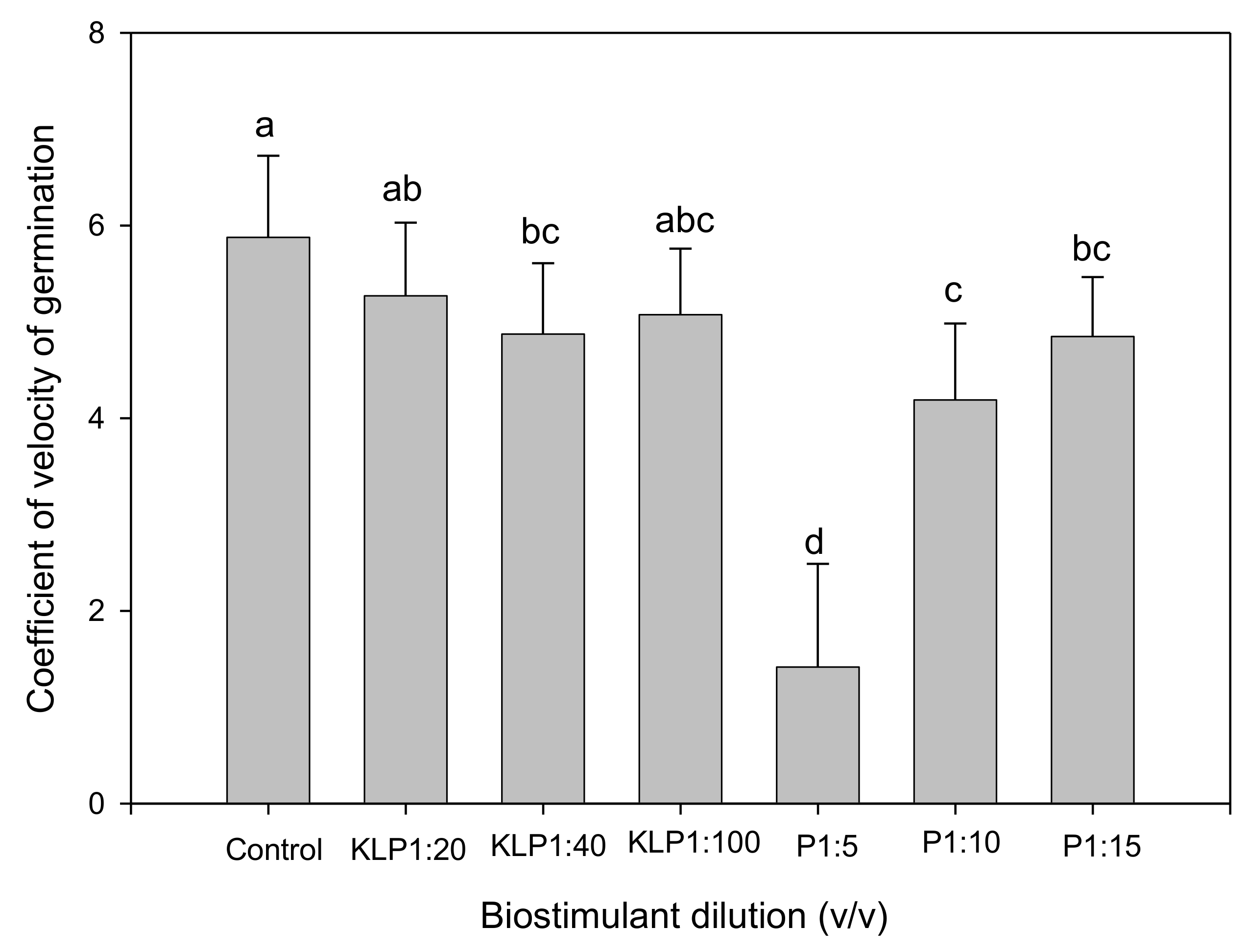
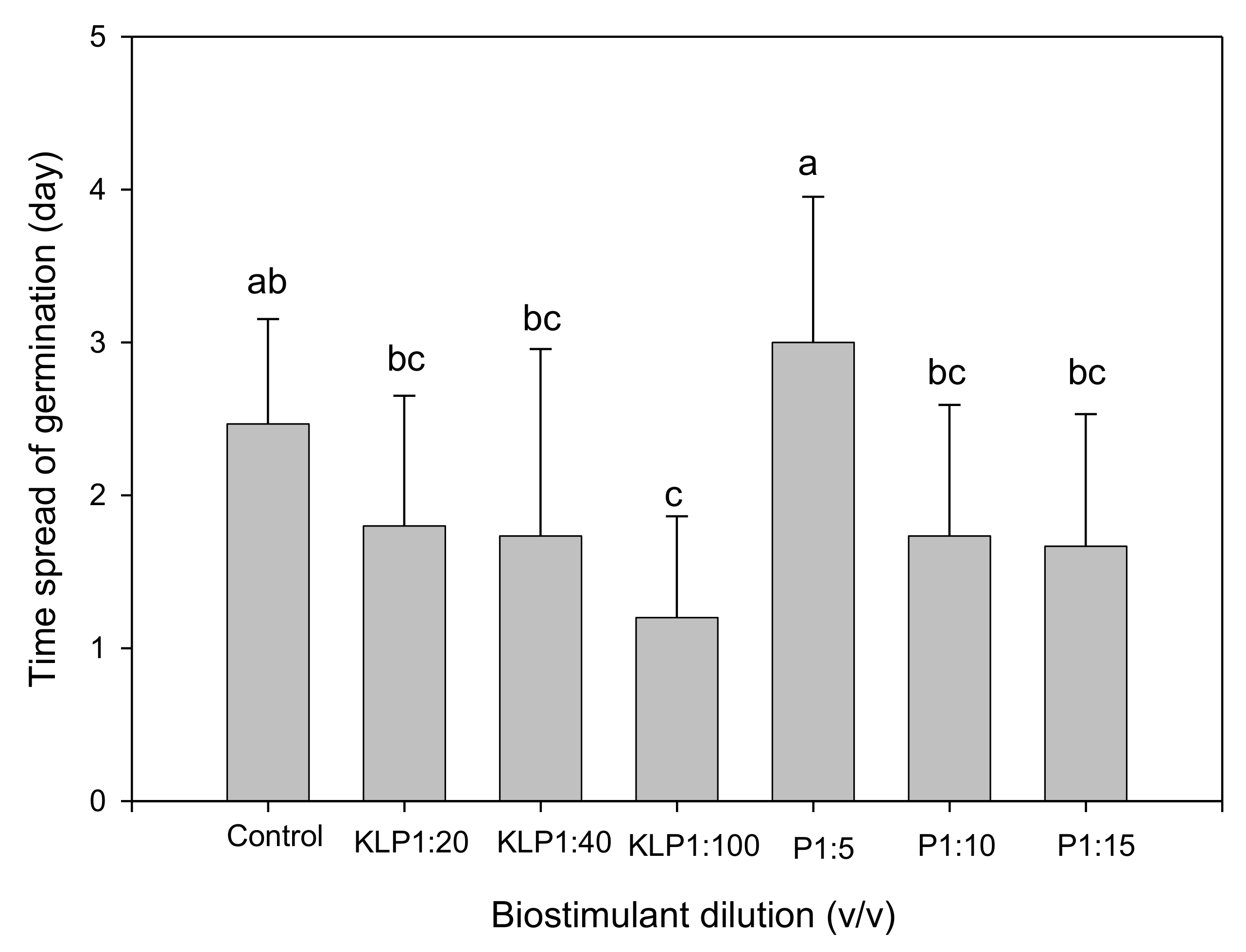
2.2. Effect of Biopriming Treatments on Seed Germination of Abelmoschus esculentus Genotypes
3. Discussion
4. Materials and Methods
4.1. Source of Biostimulants and Seeds
4.2. Hydropriming Duration Determination
- f = seeds germinated on day x,
- N = number of seeds germinated each day,
- T = number of days from seeding corresponding to N,
- G1 = germination percentage on the first day after sowing,
- G2 = germination percentage on the second day after sowing,
- n1, n2 … n10 = number of germinated seeds on the first, second and subsequent days until the 10th day; 10, 9… and 1 are weights given to the number of germinated seeds on the first, second, and subsequent days, respectively.
4.3. Seed Priming with Biostimulants
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makena, I.M.; Matsiliza-Mlathi, B.; Kleynhans, R. Seed propagation and seed anatomy of three Eucomis species. Acta Hortic. 2018, 1204, 263–272. [Google Scholar] [CrossRef]
- Kader, M.A. A Comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar]
- Wang, W.-Q.; Liu, S.-J.; Song, S.-Q.; Møller, I.M. Proteomics of seed development, desiccation tolerance, germination and vigor. Plant Physiol. Biochem. 2015, 86, 1–15. [Google Scholar] [CrossRef]
- Wakjira, K.; Negash, L. Germination responses of Croton macrostachyus (Euphorbiaceae) to various physico-chemical pretreatment conditions. S. Afr. J. Bot. 2013, 87, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Zulfiqar, F. Effect of seed priming on horticultural crops. Sci. Hortic. 2021, 286, 110197. [Google Scholar] [CrossRef]
- Rifna, E.; Ramanan, K.R.; Mahendran, R. Emerging technology applications for improving seed germination. Trends Food Sci. Technol. 2019, 86, 95–108. [Google Scholar] [CrossRef]
- Khalaki, M.A.; Moameri, M.; Lajayer, B.A.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021, 93, 13–28. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Benhima, R.; Khouloud, M.; Boulif, R.; Douira, A.; Bamouh, A.; Kadmiri, I.M. Biostimulants derived from Moroccan seaweeds: Seed germination metabolomics and growth promotion of tomato plant. J. Plant Growth Regul. 2021, 40, 353–370. [Google Scholar] [CrossRef]
- Ngoroyemoto, N.; Gupta, S.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Effect of organic biostimulants on the growth and biochemical composition of Amaranthus hybridus L. S. Afr. J. Bot. 2019, 124, 87–93. [Google Scholar] [CrossRef]
- Tian, Y.; Guan, B.; Zhou, D.; Yu, J.; Li, G.; Lou, Y. Responses of seed germination, seedling growth, and seed yield traits to seed pretreatment in maize (Zea mays L.). Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Látr, A.; Rocha, I.; Freitas, H.; Vosátka, M.; Oliveira, R.S. Delivery of inoculum of Rhizophagus irregularis via seed coating in combination with Pseudomonas libanensis for cowpea production. Agronomy 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Amirkhani, M.; Mayton, H.S.; Netravali, A.N.; Taylor, A.G. A seed coating delivery system for bio-based biostimulants to enhance plant growth. Sustainability 2019, 11, 5304. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Stirk, W.A.; Rengasamy, K.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed: An overview. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Chichester, UK, 2020; pp. 33–55. [Google Scholar]
- El-Sheekh, M.M.; Ismail, M.M.; Hamouda, M.M. Influence of some brown seaweed extracts on germination and cytological responses of Trigonella foenum-graecum L. Biotechnol. Indian J. 2016, 12, 1–12. [Google Scholar]
- Carvalho, M.E.A.; Castro, P.R.C.; Novembre, A.D.C.; Chamma, H.M.C.P. Seaweed extract improves the vigor and provides the rapid emergence of dry bean seeds. Am. Eurasian J. Agric. Environ. Sci. 2013, 13, 1104–1107. [Google Scholar]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed biopriming with plant growth promoting rhizobacteria: A review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef]
- Hussein, K.A.; Joo, J.H. Plant growth-promoting rhizobacteria improved salinity tolerance of Lactuca sativa and Raphanus sativus. J. Microbiol. Biotechnol. 2018, 28, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Prathibha, K.S.; Siddalingeshwara, K.G. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescence as rhizobacteria on seed quality of sorghum. Int. J. Microbiol. Appl. Sci. 2013, 2, 11–18. [Google Scholar]
- Islam, M.T. Phytochemical information and pharmacological activities of okra (Abelmoschus esculentus): A literature-based review. Phytother. Res. 2019, 33, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mkhabela, S.S.; Shimelis, H.; Gerrano, A.S.; Mashilo, J. Phenotypic and genotypic divergence in okra [Abelmoschus esculentus (L.) Moench] and implications for drought tolerance breeding: A review. S. Afr. J. Bot. 2021. [Google Scholar] [CrossRef]
- Benchasri, S. Okra (Abelmoschus esculentus (L.) Moench) as a valuable vegetable of the world. Ratar. Povrt. 2012, 49, 105–112. [Google Scholar]
- Sharma, A.; Rathore, S.; Srinivasan, K.; Tyagi, R. Comparison of various seed priming methods for seed germination, seedling vigour and fruit yield in okra (Abelmoschus esculentus L. Moench). Sci. Hortic. 2014, 165, 75–81. [Google Scholar] [CrossRef]
- Sarma, B.; Gogoi, N. Germination and seedling growth of okra (Abelmoschus esculentus L.) as influenced by organic amendments. Cogent Food Agric. 2015, 1. [Google Scholar] [CrossRef]
- Zodape, S.; Kawarkhe, V.; Patolia, J.; Warade, A. Effect of liquid seaweed fertilizer on yield and quality of okra (Abelmoschus esculentus L.). J. Sci. Ind. Res. 2008, 67, 1115–1117. [Google Scholar]
- Sasikala, M.; Indumathi, E.; Radhika, S.; Sasireka, R. Effect of seaweed extract (Sargassum tenerrimum) on seed germination and growth of tomato plant (Solanum lycopersicum). Int. J. ChemTech Res. 2016, 9, 285–293. [Google Scholar]
- Michalak, I.; Dmytryk, A.; Schroeder, G.; Chojnacka, K. The application of homogenate and filtrate from Baltic seaweeds in seedling growth tests. Appl. Sci. 2017, 7, 230. [Google Scholar] [CrossRef]
- Demir, N.; Dural, B.; Yildirim, K. Effect of seaweed suspensions on seed germination of tomato, pepper and aubergine. J. Biol. Sci. 2006, 6, 1130–1133. [Google Scholar]
- Hidangmayum, A.; Sharma, R. Effect of different concentration of commercial seaweed liquid extract of Ascophylum nodosum on germination of onion (Allium cepa L.). Int. J. Sci. Res. 2017, 6, 1488–1491. [Google Scholar]
- Altindal, D. Effect of seaweed extracts (SE) applications on seed germination characteristics of wheat in salinity conditions. Int. J. Agric. For. Life Sci. 2019, 3, 115–120. [Google Scholar]
- MangMang, J.; Deaker, R.; Rogers, G. Early seedling growth response of lettuce, tomato and cucumber to Azospirillum brasilense inoculated by soaking and drenching. Hortic. Sci. 2016, 42, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Stamenov, D.; Djuric, S.; Jafari, T.H. Effect of PGPR on the germination and early development of onion (Allium cepa). In Proceedings of the ISER 137th International Conference, Paris, France, 7 December 2018; pp. 6–9. [Google Scholar]
- Sarić-Krsmanović, M.; Božić, D.; Radivojević, L.; Umiljendić, J.G.; Šantrić, L.; Vrbničanin, S. Effects of plant growth promoting rhizobacteria (PGPR) and cover crops on seed germination and early establishment of field dodder (Cuscuta campestris Yunk.). J. Pestic. Phytomed. 2017, 32, 105–111. [Google Scholar] [CrossRef]
- Nehra, V.; Saharan, B.S.; Choudhary, M.I. Evaluation of Brevibacillus brevis as a potential plant growth promoting rhizobacteria for cotton (Gossypium hirsutum) crop. SpringerPlus 2016, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Panobianco, M.; Viera, R.D. Electrical conductivity of soybean soaked seeds. Pesqui. Agropecu. Bras. 1996, 31, 621–627. [Google Scholar]
- Babiker, W.A.; Abdelmula, A.A.; Eldessougi, H.I.; Gasim, S.E.M. The effect of location, sowing date and genotype on seed quality traits in bread wheat (Triticum aestevium). Asian J. Plant Sci. Res. 2017, 7, 24–28. [Google Scholar]
- Khayamim, S.; Afshari, R.T.; YSadeghian, S.; Poustini, K.; Rouzbeh, F.; Abbasi, Z. Seed germination, plant establishment, and yield of sugar beet genotypes under salinity stress. J. Agric. Sci. Technol. 2014, 16, 779–790. [Google Scholar]
- Islam, A.K.M.A.; Anuar, N.; Yaakob, Z. Effect of genotypes and pre sowing treatments on seed germination behaviour of Jatropha. Asian J. Plant Sci. 2009, 8, 433–439. [Google Scholar] [CrossRef]
- Glass, G.V.; Peckham, P.D.; Sanders, J.R. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev. Educ. Res. 1972, 42, 237–288. [Google Scholar] [CrossRef]
- Snedecor, G.; Cochran, W. Statistical Methods, 7th ed.; The Iowa State University Press: Iowa City, IA, USA, 1980. [Google Scholar]

| Hydropriming Duration (h) | FGP (%) | MGT (day) | GI | CVG | GRI (%/day) | TSG (day) |
|---|---|---|---|---|---|---|
| 0 | 37.33 ± 7.06 b | 3.73 ± 0.20 a | 58.67 ± 11.84 c | 3.45 ± 1.09 b | 12.25 ± 2.70 c | 4.00 ± 0.58 |
| 6 | 62.67 ± 3.53 a | 3.61 ± 0.24 a | 99.67 ± 2.40 b | 9.05 ± 1.57 a | 19.25 ± 0.40 bc | 4.67 ± 0.88 |
| 12 | 60.00 ± 2.31 a | 2.66 ± 0.19 b | 110.00 ± 3.60 b | 6.04 ± 0.82 ab | 26.20 ± 1.89 b | 3.00 ± 0.58 |
| 24 | 72.00 ± 4.00 a | 2.52 ± 0.09 b | 134.67 ± 8.29 a | 8.20 ± 0.84 a | 38.09 ± 5.65 a | 5.00 ± 1.00 |
| LSD (p ≤ 0.05) | 14.91 | 0.6243 | 24.60 | 3.655 | 10.68 | ns |
| Source of Variation | df | MS | |||||
|---|---|---|---|---|---|---|---|
| FGP | MGT | GI | CVG | GRI | TSG | ||
| Genotype (G) | 4 | 358.75 *** | 0.33 ns | 6189.80 *** | 0.87 ns | 1019.47 *** | 13.32 *** |
| Biostimulant treatment (B) | 6 | 5963.53 *** | 2.20 *** | 75,466.80 *** | 31.69 *** | 6540.91 *** | 5.32 * |
| G × B | 24 | 333.31 *** | 0.18 ns | 4142.60 *** | 2.76 ns | 327.56 *** | 1.63 ns |
| Residual | 70 | 65.98 | 0.15 | 757.90 | 1.68 | 77.26 | 1.99 |
| Total | 104 | ||||||
| Genotype | Treatment | FGP (%) | GI | GRI (%/day) |
|---|---|---|---|---|
| VI037996 | Control | 69.33 ± 7.06 g–j | 235.00 ± 22.94 g–k | 57.93 ± 4.56 klm |
| KLP 1:20 | 74.67 ± 1.33 e–i | 250.30 ± 7.22 f–j | 60.95 ± 2.74 i–m | |
| KLP 1:40 | 78.67 ± 3.53 c–h | 266.30 ± 7.22 d–h | 68.43 ± 2.35 g–l | |
| KLP 1:100 | 82.67 ± 3.53 a–f | 286.30 ± 10.81 a–f | 76.67 ± 3.71 b–h | |
| PGPR 1:5 | 64.00 ± 6.11 ij | 214.70 ± 16.48 ijk | 50.04 ± 2.07 m | |
| PGPR 1:10 | 72.00 ± 4.62 f–j | 248.30 ± 14.77 f–j | 65.56 ± 2.26 h–l | |
| PGPR 1:15 | 72.00 ± 2.31 f–j | 249.30 ± 7.79 f–j | 67.11 ± 1.46 h–l | |
| VI046567 | Control | 94.67 ± 5.33 a | 326.70 ± 19.34 a | 88.22 ± 6.88 abc |
| KLP 1:20 | 90.67 ± 3.53 abc | 315.70 ± 12.81 abc | 88.78 ± 3.91 ab | |
| KLP 1:40 | 82.67 ± 1.33 a–f | 288.00 ± 4.16 a–f | 81.60 ± 1.22 a–g | |
| KLP 1:100 | 88.00 ± 4.00 a–d | 304.70 ± 17.33 a–d | 83.56 ± 8.44 a–f | |
| PGPR 1:5 | 17.33 ± 1.33 k | 54.70 ± 4.91 l | 12.00 ± 1.39 n | |
| PGPR 1:10 | 78.67 ± 3.53 c–h | 270.70 ± 10.68 d–h | 71.11 ± 3.49 f–l | |
| PGPR 1:15 | 92.00 ± 2.31 ab | 320.30 ± 8.11 ab | 89.11 ± 2.48 ab | |
| VI055421 | Control | 77.33 ± 4.81 d–h | 259.30 ± 215.68 e–i | 65.87 ± 4.73 h–l |
| KLP 1:20 | 78.67 ± 9.61 c–h | 268.30 ± 30.56 d–h | 68.31 ± 6.22 g–l | |
| KLP 1:40 | 81.33 ± 1.33 b–g | 279.30 ± 6.01 b–g | 72.55 ± 5.12 d–j | |
| KLP 1:100 | 74.67 ± 2.67 e–i | 258.30 ± 8.33 e–i | 71.15 ± 1.82 f–l | |
| PGPR 1:5 | 25.33 ± 2.67 k | 78.70 ± 8.25 l | 13.53 ± 1.74 n | |
| PGPR 1:10 | 61.33 ± 7.06 j | 199.70 ± 25.83 k | 46.89 ± 9.32 m | |
| PGPR 1:15 | 78.67 ± 7.42 c–h | 270.00 ± 25.94 d–h | 71.82 ± 8.70 e–k | |
| VI050956 | Control | 94.67 ± 2.67 a | 330.00 ± 9.02 a | 92.44 ± 2.70 a |
| KLP 1:20 | 86.67 ± 3.53 a–e | 303.00 ± 12.29 a–e | 86.00 ± 3.46 a–e | |
| KLP 1:40 | 88.00 ± 2.31 a–d | 307.30 ± 8.11 a–d | 86.67 ± 2.67 a–d | |
| KLP 1:100 | 85.33 ± 1.33 a–e | 298.30 ± 4.84 a–e | 84.67 ± 1.77 a–f | |
| PGPR 1:5 | 16.00 ± 6.11 k | 51.00 ± 21.28 l | 9.04 ± 4.57 n | |
| PGPR 1:10 | 80.00 ± 4.62 b–g | 277.00 ± 16.19 b–g | 74.00 ± 5.03 c–i | |
| PGPR 1:15 | 82.67 ± 7.06 a–f | 286.70 ± 26.77 a–f | 77.33 ± 11.62 b–h | |
| VI033796 | Control | 82.67 ± 1.33 a–f | 279.70 ± 5.92 b–g | 68.67 ± 4.70 g–l |
| KLP 1:20 | 80.00 ± 0.00 b–g | 275.00 ± 2.00 c–g | 70.89 ± 3.11 f–l | |
| KLP 1:40 | 66.67 ± 2.67 hij | 226.70 ± 12.87 h–k | 59.05 ± 6.48 j–m | |
| KLP 1:100 | 89.33 ± 2.67 a–d | 307.30 ± 8.19 a–d | 79.56 ± 5.01 a–h | |
| PGPR 1:5 | 21.33 ± 5.33 k | 66.70 ± 12.68 l | 14.67 ± 2.15 n | |
| PGPR 1:10 | 61.33 ± 11.39 j | 212.70 ± 39.40 jk | 57.33 ± 10.48 lm | |
| PGPR 1:15 | 80.00 ± 2.31 b–g | 275.00 ± 7.23 c–g | 72.67 ± 2.52 d–j | |
| LSD (p ≤ 0.05) | 13.23 | 44.83 | 14.31 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus esculentus Genotypes. Plants 2021, 10, 1327. https://doi.org/10.3390/plants10071327
Makhaye G, Aremu AO, Gerrano AS, Tesfay S, Du Plooy CP, Amoo SO. Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus esculentus Genotypes. Plants. 2021; 10(7):1327. https://doi.org/10.3390/plants10071327
Chicago/Turabian StyleMakhaye, Gugulethu, Adeyemi O. Aremu, Abe Shegro Gerrano, Samson Tesfay, Christian P. Du Plooy, and Stephen O. Amoo. 2021. "Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus esculentus Genotypes" Plants 10, no. 7: 1327. https://doi.org/10.3390/plants10071327
APA StyleMakhaye, G., Aremu, A. O., Gerrano, A. S., Tesfay, S., Du Plooy, C. P., & Amoo, S. O. (2021). Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus esculentus Genotypes. Plants, 10(7), 1327. https://doi.org/10.3390/plants10071327









