Transferability of ISSR, SCoT and SSR Markers for Chrysanthemum × Morifolium Ramat and Genetic Relationships Among Commercial Russian Cultivars
Abstract
:1. Introduction
2. Results
2.1. Transferability and Discriminating Power of SCoT, ISSR, and SSR Markers for Chrysanthemum
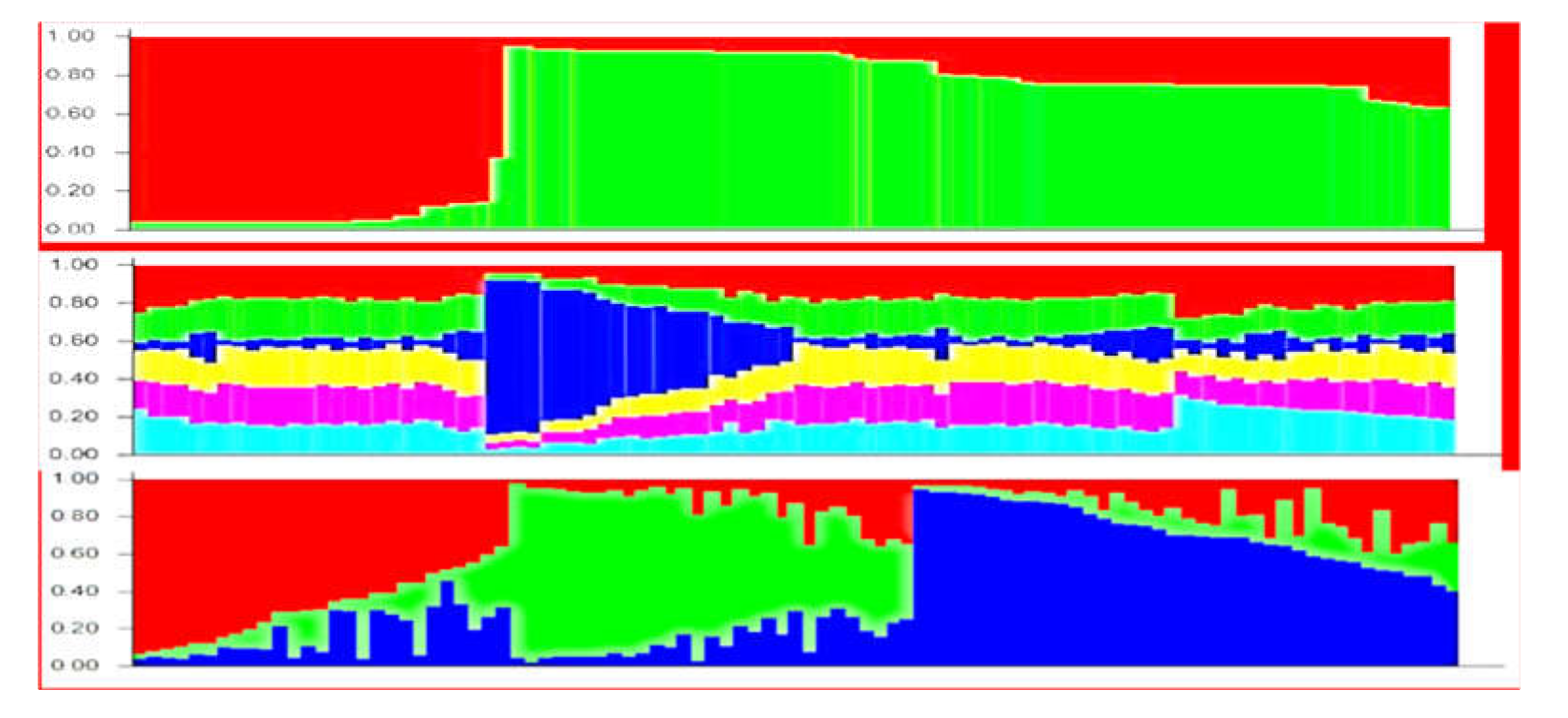
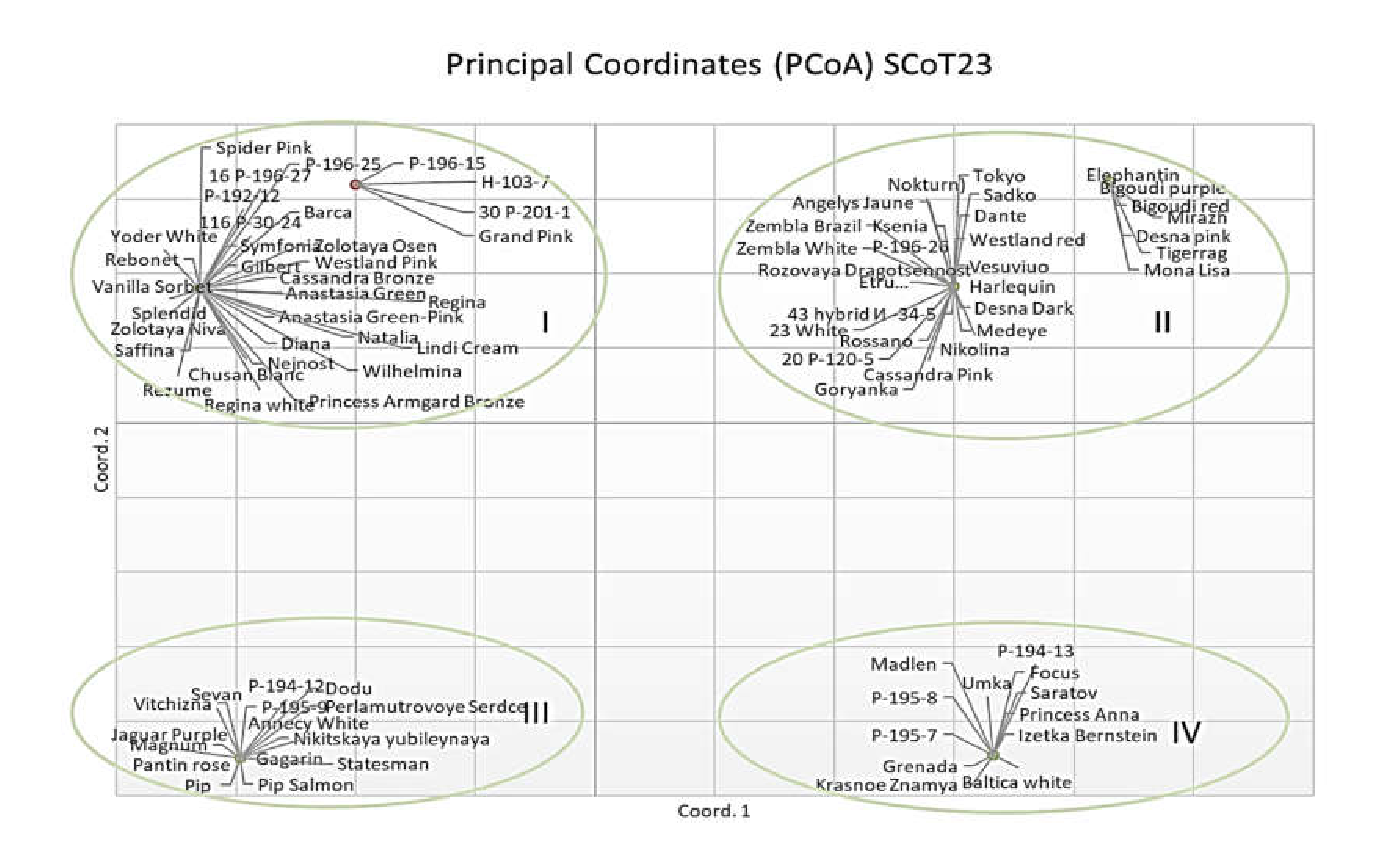
2.2. Genetic Structureof Germplasm Collection Based on SSR, ISSR, and SCoT Polymorphisms
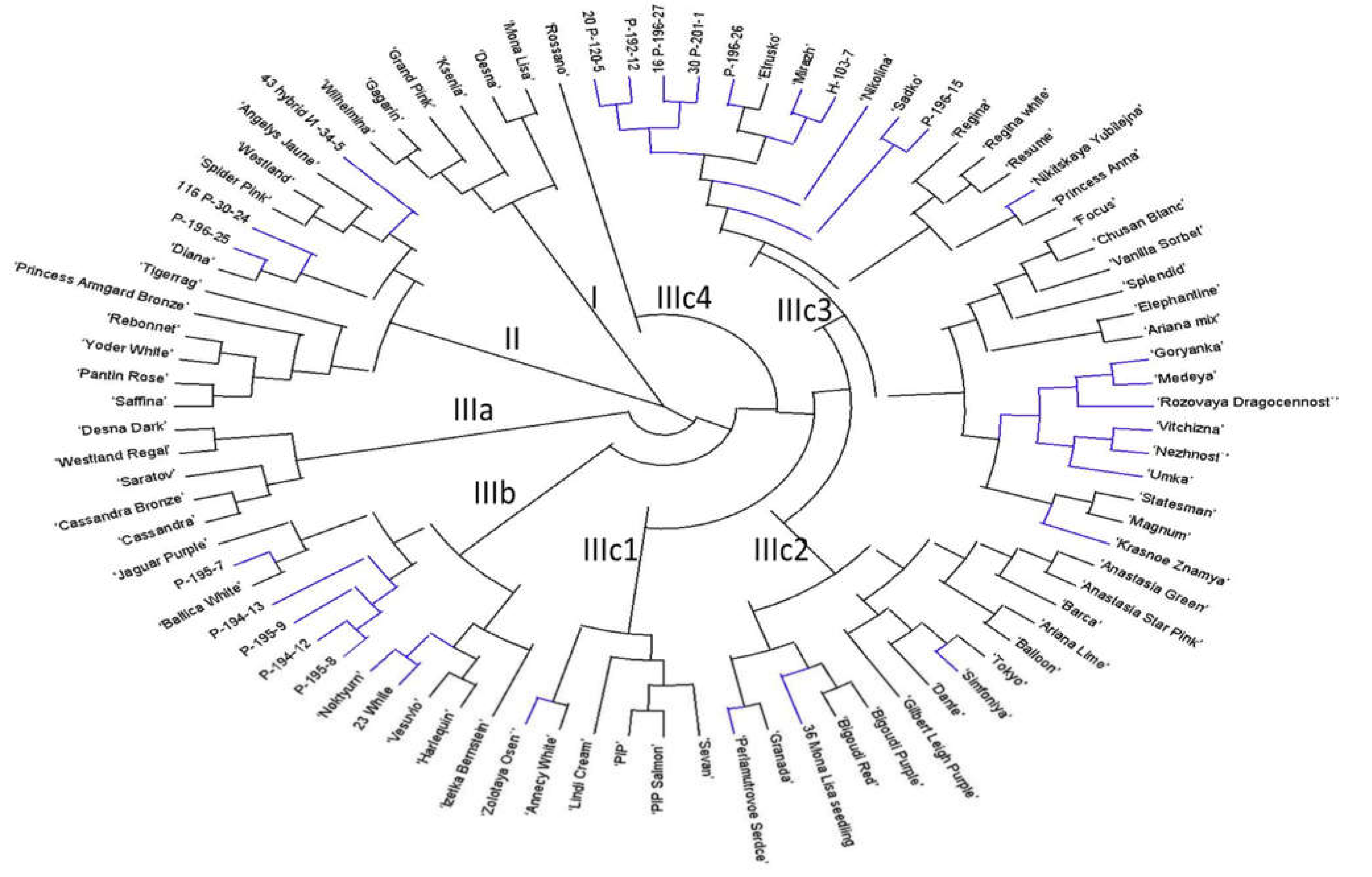
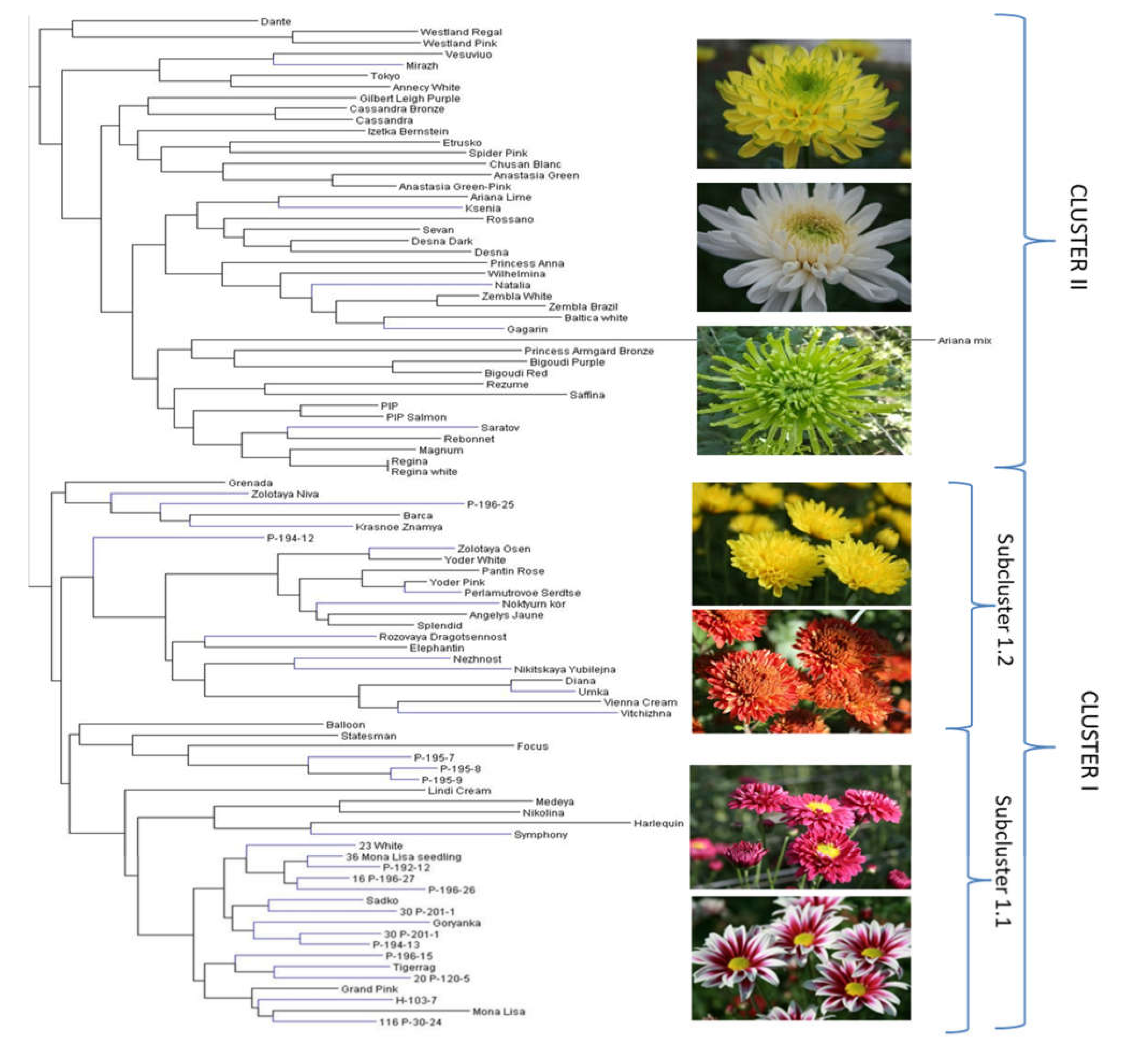
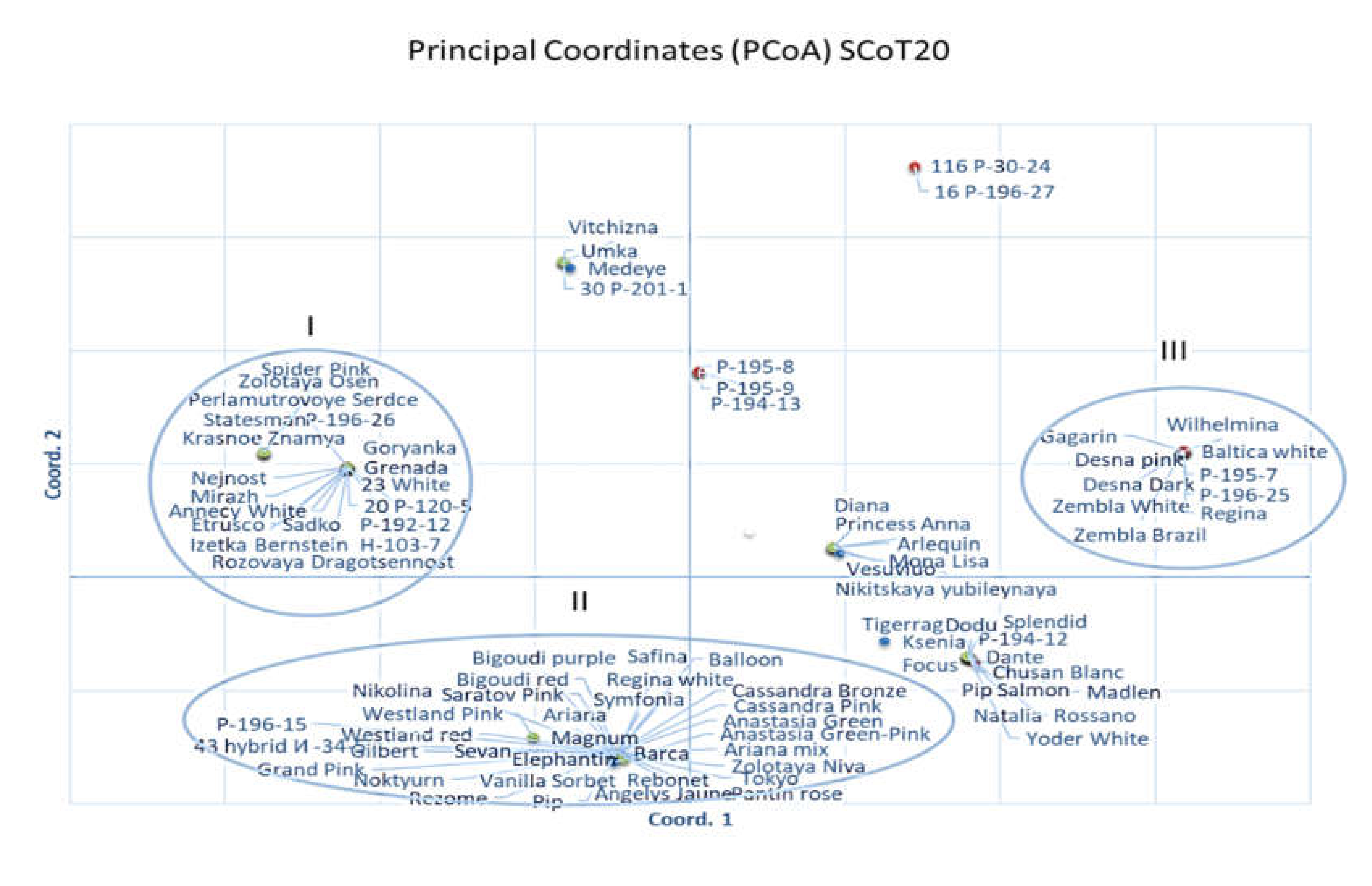
2.3. Genetic Diversity and Relationships and Correspondence with Phenotypical Traits
3. Discussion
3.1. Transferability and Discriminating Power of SCoT, ISSR, and SSR Markers for Chrysanthemum
3.2. Genetic Structure of Germplasm Collection Based on SSR, ISSR, and SCoT Polymorphisms
3.3. Genetic Diversity and Relationships and Correspondence with Phenotypical Traits
4. Materials and Methods
4.1. The Plant Material and DNA Extraction
4.2. Phenotypical Evaluation of Collection
4.3. Genetic Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, N.; Jędrzejczyk, I. Chrysanthemum plants regenerated from ovaries: A study on genetic and phenotypic variation. Turk. J. Bot. 2018, 42, 289–297. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.-J.; Chen, F.-D.; Fang, W.-M.; Teng, N.-J. Identification of Chrysanthemum (Chrysanthemum morifolium) self-incompatibility. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Younis, A.; Ryu, K.B.; Lim, K.-B.; Eun, C.-H.; Lee, J.; Sohn, S.-H.; Kwon, S.-J. Karyomorphological analysis of wild chrysanthemum boreale collected from four natural habitats in Korea. Flower Res. J. 2013, 21, 182–189. [Google Scholar] [CrossRef]
- Feng, S.; He, R.; Lu, J.; Jiang, M.; Shen, X.; Jiang, Y.; Wang, Z.; Wang, H. Development of SSR markers and assessment of genetic diversity in medicinal Chrysanthemum morifolium Cultivars. Front. Genet. 2016, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Mokhno, V.S.; Bratukhina, E.V.; Yakushina, L.G. Chrysanthemum breeding in the humid subtropics of the Krasnodar region. Subtrop. Ornam. Hortic. 2017, 63, 78–85. [Google Scholar]
- Zhang, Y.; Wang, C.; Ma, H.; Dai, S. Assessing the genetic diversity of chrysanthemum cultivars with microsatellites. J. Am. Soc. Hortic. Sci. 2013, 138, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.-S.; Guo, Q.-S.; Deng, Y.-M.; Guo, H.-P. A comparative analysis of genetic diversity in medicinal Chrysanthemum morifolium based on morphology, ISSR and SRAP markers. Biochem. Syst. Ecol. 2010, 38, 1160–1169. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.; Chen, F.; Fang, W.; Li, F. A preliminary genetic linkage map of chrysanthemum (Chrysanthemum morifolium) cultivars using RAPD, ISSR and AFLP markers. Sci. Hortic. 2010, 125, 422–428. [Google Scholar] [CrossRef]
- Li, P.; Zhang, F.; Chen, S.; Jiangshuo, S.; Wang, H.; Su, J.; Fang, W.; Guan, Z.; Chen, F. Genetic diversity, population structure and association analysis in cut chrysanthemum (Chrysanthemum morifolium Ramat.). Mol. Genet. Genom. 2016, 291, 1117–1125. [Google Scholar] [CrossRef]
- Luo, C.; Chen, D.; Cheng, X.; Liu, H.; Li, Y.; Huang, C. SSR analysis of genetic relationship and classification in chrysanthemum germplasm collection. Hortic. Plant J. 2018, 4, 73–82. [Google Scholar] [CrossRef]
- Kobeissi, B.; Saidi, A.; Kobeissi, A.; Shafie, M. Applicability of SCoT and SSR Molecular Markers for Genetic Diversity Analysis in Chrysanthemum morifolium Genotypes. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2019, 89, 1067–1077. [Google Scholar] [CrossRef]
- Song, X.; Zhao, X.; Fan, G.; Gao, K.; Dai, S.; Zhang, M.; Ma, C.; Wu, X. Genetic analysis of the corolla tube merged degree and the relative number of ray florets in chrysanthemum (Chrysanthemum × morifolium Ramat.). Sci. Hortic. 2018, 242, 214–224. [Google Scholar] [CrossRef]
- Kalia, R.K.; Rai, M.K.; Kalia, S.; Singh, R.; Dhawan, A.K. Microsatellite markers: An overview of the recent progress in plants. Euphytica 2010, 177, 309–334. [Google Scholar] [CrossRef]
- Wambulwa, M.C.; Meegahakumbura, M.K.; Chalo, R.; Kamunya, S.; Muchugi, A.; Xu, J.C.; Liu, J.; Li, D.Z.; Gao, L.M. Nuclear microsatellites reveal the genetic architecture and breeding history of tea germplasm of East Africa. Tree Genet. Genomes 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Reddy, M.P.; Sarla, N.; Siddiq, E. Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 2002, 128, 9–17. [Google Scholar] [CrossRef]
- Azhar, M.; Muhammad, H.A.; Siti, N.I.; Parween, K.S.A.S. Optimization of ISSR Markers for Molecular DNA Fingerprinting in Aquilaria sp; Nuclear Technical Convention: Bangi, Malaysia, 2013. [Google Scholar]
- Tsumura, Y.; Ohba, K.; Strauss, S.H. Diversity and inheritance of inter-simple sequence repeat polymorphisms in Douglas-fir (Pseudotsuga menziesii) and sugi (Cryptomeria japonica). Theor. Appl. Genet. 1996, 92, 40–45. [Google Scholar] [CrossRef]
- Luz, G.C.; Strioto, D.K.; Mangolin, C.A.; Machado, M.D.F.P. ISSR markers to assess genetic diversity of cultivated populations from artificial selection of Stevia rebaudiana (Bert. ) Bertoni. Breed. Sci. 2020, 70, 508–514. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Etminan, A.; Pour-Aboughadareh, A.; Mohammadi, R.; Ahmadi-Rad, A.; Noori, A.; Mahdavian, Z.; Moradi, Z. Applicability of start codon targeted (SCoT) and inter-simple sequence repeat (ISSR) markers for genetic diversity analysis in durum wheat genotypes. Biotechnol. Biotechnol. Equip. 2016, 30, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Chen, F.; Chen, S.; Teng, N.; Fang, W. Intergeneric hybridization and relationship of genera within the tribe Anthemideae Cass. (I. Dendranthema crassum (kitam.) kitam. × Crossostephium chinense (L.) Makino). Euphytica 2009, 169, 133–140. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Chen, F.; Li, J.; Fang, W. Cytogenetic study of three edible chrysanthemum cultivars. Генетика 2011, 47, 176–181. [Google Scholar] [CrossRef]
- Barkley, N.A.; Krueger, R.R.; Federici, C.T.; Roose, M.L. What phylogeny and gene genealogy analyses reveal about homoplasy in citrus microsatellite alleles. Plant Syst. Evol. 2009, 282, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Šarhanová, P.; Pfanzelt, S.; Brandt, R.; Himmelbach, A.; Blattner, F.R. SSR-seq: Genotyping of microsatellites using next-generation sequencing reveals higher level of polymorphism as compared to traditional fragment size scoring. Ecol. Evol. 2018, 8, 10817–10833. [Google Scholar] [CrossRef]
- Kumar, J.; Agrawal, V. Assessment of genetic diversity, population structure and sex identification in dioecious crop, Trichosanthes dioica employing ISSR, SCoT and SRAP markers. Heliyon 2019, 5, e01346. [Google Scholar] [CrossRef] [Green Version]
- Chesnokov, Y.; Artemyeva, A. Evaluation of the measure of polymorphism. Sel’skokhozyaistvennaya Biol. 2015, 50, 571–578. [Google Scholar] [CrossRef]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; Rabaiolli, S.M.D.S.; Stefanel, C.M.; Lemos, S.C.M.; Silveira, R.L.R.; Buuron, S.K.; Dos Santos, R.S.M.; Moro, S.C. Determining the Polymorphism Information Content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef]
- Ahmed, M.Z.S.; Masoud, I.M.; Zedan, S.Z.A. Genetic diversity and relationship among nine cultivated flax genotypes (Linum usitatissimum L.) based on SCoT Markers. Middle East J. Appl. Sci. 2018, 8, 1480–1490. [Google Scholar]
- Giachino, R.R.A. Investigation of the genetic variation of anise (Pimpinella anisum L.) using RAPD and ISSR markers. Genet. Resour. Crop. Evol. 2019, 67, 763–780. [Google Scholar] [CrossRef]
- Abdelaziz, S.M.; Medraoui, L.; Alami, M.; Pakhrou, O.; Makkaoui, M.; Boukhary, A.O.M.S.; Filali-Maltouf, A. Inter simple sequence repeat markers to assess genetic diversity of the desert date (Balanites aegyptiaca Del.) for Sahelian ecosystem restoration. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Ismail, N.A.; Rafii, M.Y.; Mahmud, T.M.M.; Hanafi, M.M.; Miah, G. Genetic diversity of torch ginger (Etlingera elatior) Germplasm Revealed by ISSR and SSR Markers. BioMed Res. Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilwari, A.; Sharma, R. Random amplified polymorphic DNA and inter simple sequence repeat markers reveals genetic diversity between micro propagated, wild and field cultivated genotypes of Gloriosa superba: An endangered medicinal plant. Mol. Biol. Rep. 2021, 48, 2437–2452. [Google Scholar] [CrossRef]
- Sharma, V.; Thakur, M. Applicability of SCoT markers for detection of variations in Fusarium yellows resistant lines of ginger (Zingiber officinale Rosc.) induced through gamma irradiations. S. Afr. J. Bot. 2021. [Google Scholar] [CrossRef]
- Ghobadi, G.; Etminan, A.; Mehrabi, A.M.; Shooshtari, L. Molecular diversity analysis in hexaploid wheat (Triticum aestivum L.) and two Aegilops species (Aegilops crassa and Aegilops cylindrica) using CBDP and SCoT markers. J. Genet. Eng. Biotechnol. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai., P. iMEC: Online Marker Efficiency Calculator. Appl. Plant Sci. 2018, 6, e1159. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, T.L.; Afolayan, A.J. Evaluation of genetic relationship among varieties of Capsicum annuum L. and Capsicum frutescens L. in West Africa using ISSR markers. Heliyon 2019, 5, e01700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayan, W.A.; Osman, S.A.H. Phylogenetic relationships of some Egyptian soybean cultivars (Glycine max L.) using SCoT marker and protein pattern. Bull. Natl. Res. Cent. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Teixeira, G.C.; Konzen, E.R.; Faria, J.C.T.; Gonçalves, D.S.; De Carvalho, D.; Brondani, G.E. Genetic diversity analysis of two Eucalyptus species using ISSR markers. Ciência Florestal 2020, 30, 270–278. [Google Scholar] [CrossRef]
- Alzahib, R.; Migdadi, H.; Ghamdi, A.; Alwahibi, M.; Afzal, M.; Elharty, E.; Alghamdi, S. Exploring genetic variability among and within hail tomato landraces based on sequence-related amplified polymorphism markers. Diversity 2021, 13, 135. [Google Scholar] [CrossRef]
- Guzmán, F.A.; Moore, S.; De Vicente, M.C.; Jahn, M.M. Microsatellites to enhance characterization, conservation and breeding value of Capsicum germplasm. Genet. Resour. Crop. Evol. 2019, 67, 569–585. [Google Scholar] [CrossRef] [Green Version]
- Greenbaum, G.; Templeton, A.; Zarmi, Y.; Bar-David, S. Allelic richness following population founding events—A stochastic modeling framework incorporating gene flow and genetic drift. PLoS ONE 2014, 9, e115203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-Q. Genetic diversity and population structure of the endangered species Paeonia decomposita endemic to China and implications for its conservation. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stojnić, S.; Avramidou, E.V.; Fussi, B.; Westergren, M.; Orlović, S.; Matović, B.; Trudić, B.; Kraigher, H.; Aravanopoulos, F.A.; Konnert, M. Assessment of genetic diversity and population genetic structure of norway spruce (Picea abies (l.) Karsten) at its southern Lineage in Europe. Implications for conservation of forest genetic resources. Forests 2019, 10, 258. [Google Scholar] [CrossRef] [Green Version]
- Konopiński, M.K. Shannon diversity index: A call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies. Peer. J. 2020, 8, e9391. [Google Scholar] [CrossRef]
- Gorji, A.M.; Poczai, P.; Polgar, Z.; Taller, J. Efficiency of arbitrarily amplified dominant markers (SCOT, ISSR and RAPD) for diagnostic fingerprinting in tetraploid potato. Am. J. Potato Res. 2011, 88, 226–237. [Google Scholar] [CrossRef]
- Al Salameen, F.; Habibi, N.; Kumar, V.; Al Amad, S.; Dashti, J.; Talebi, L.; Al Doaij, B. Genetic diversity and population structure of Haloxylon salicornicum moq. in Kuwait by ISSR markers. PLoS ONE 2018, 13, e0207369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etminan, A.; Pour-Aboughadareh, A.; Noori, A.; Ahmadi-Rad, A.; Shooshtari, L.; Mahdavian, Z.; Yousefiazar-Khanian, M. Genetic relationships and diversity among wild Salvia accessions revealed by ISSR and SCoT markers. Biotechnol. Biotechnol. Equip. 2018, 32, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Xanthopoulou, A.; Ganopoulos, I.; Kalivas, A.; Nianiou-Obeidat, I.; Ralli, P.; Moysiadis, T.; Tsaftaris, A.; Madesis, P. Comparative analysis of genetic diversity in Greek Genebank collection of summer squash (‘Cucurbita pepo’) landraces using start codon targeted (SCoT) polymorphism and ISSR markers. Aust. J. Crop Sci. 2015, 9, 14–21. [Google Scholar] [CrossRef]
- Klie, M.; Menz, I.; Linde, M.; Debener, T. Lack of structure in the gene pool of the highly polyploid ornamental chrysanthemum. Mol. Breed. 2013, 32, 339–348. [Google Scholar] [CrossRef]
- Pakseresht, F.; Talebi, R.; Karami, E. Comparative assessment of ISSR, DAMD and SCoT markers for evaluation of genetic diversity and conservation of landrace chickpea (Cicer arietinum L.) genotypes collected from north-west of Iran. Physiol. Mol. Biol. Plants 2013, 19, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Shim, M.S.; Sim, S.-C.; Oh, K.H.; Seo, J.Y. Genetic variation of flower characteristics in a population derived from a cross between the chrysanthemum cultivars ‘Falcao’ and ‘Frill Green’. Hortic. Environ. Biotechnol. 2014, 55, 322–328. [Google Scholar] [CrossRef]
- Song, X.; Xu, Y.; Gao, K.; Fan, G.; Zhang, F.; Deng, C.; Dai, S.; Huang, H.; Xin, H.; Li, Y. High-density genetic map construction and identification of loci controlling flower-type traits in Chrysanthemum (Chrysanthemum × morifolium Ramat.). Hortic. Res. 2020, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 39–40. [Google Scholar]
- Mondal, T.K. Assessment of genetic diversity of tea (Camellia sinensis (L.) O. Kuntze) by inter-simple sequence repeat polymerase chain reaction. Euphytica 2002, 128, 307–315. [Google Scholar] [CrossRef]
- Fang, W.; Cheng, H.; Duan, Y.; Jiang, X.; Li, X. Genetic diversity and relationship of clonal tea (Camellia sinensis) cultivars in China as revealed by SSR markers. Plant Syst. Evol. 2012, 298, 469–483. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2011, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://darwin.cirad.fr/overview (accessed on 24 May 2021).
| Locus | Approx. Band Size, bp | Total Number of Bands | Number of Monomorphic Bands | P | PIC | D | I | h |
|---|---|---|---|---|---|---|---|---|
| SSR markers | ||||||||
| gi298296818 | 1000–1070 | 4 | 2 | 15.6 | 0.50 | 0 | 0.31 | 0.00 |
| 357 | 250–280 | 2 | 1 | 12.8 | 0.48 | 0.27 | 0.38 | 0.25 |
| gi298297301 | 150–160 | 2 | 0 | 14.6 | 0.47 | 0.27 | 0.41 | 0.25 |
| gi298295865 | 120–140 | 4 | 0 | 46.5 | 0.38 | 0.72 | 0.63 | 0.50 |
| 320 | 200–300 | 4 | 1 | 50.3 | 0.40 | 0.79 | 0.33 | 0.56 |
| MEAN ± SD | 3.2 ± 0.1 | 0.8 ± 0.0 | 28.2 ± 1.9 | 0.45 ± 0.05 | 0.41 ± 0.33 | 0.41 ± 0.07 | 0.31 ± 0.22 | |
| ISSR markers | ||||||||
| ISSR810 | 250–2000 | 19 | 1 | 48.7 | 0.33 | 0.69 | 0.48 | 0.49 |
| ISSR813 | 750–1800 | 7 | 1 | 66.1 | 0.36 | 0.90 | 0.42 | 0.44 |
| ISSR815 | 450–1950 | 15 | 0 | 64.8 | 0.36 | 0.89 | 0.44 | 0.44 |
| ISSR873 | 300–2800 | 24 | 0 | 92.2 | 0.44 | 0.99 | 0.17 | 0.15 |
| ISSR880 | 450–2200 | 19 | 0 | 63.0 | 0.34 | 0.85 | 0.40 | 0.47 |
| ISSR13 | 980–1500 | 5 | 0 | 56.5 | 0.36 | 0.87 | 0.52 | 0.50 |
| ISSR15 | 500–1600 | 11 | 0 | 77.5 | 0.40 | 0.95 | 0.38 | 0.51 |
| ISSR814.1 | 450–1550 | 18 | 0 | 82.3 | 0.42 | 0.97 | 0.31 | 0.40 |
| MEAN ± SD | 14.8 ± 6.6 | 0.3 ± 0.0 | 69.0 ± 1.4 | 0.38 ± 0.04 | 0.89 ± 0.09 | 0.39 ± 0.02 | 0.43 ± 0.12 | |
| SCoT markers | ||||||||
| SCoT12 | 300–1750 | 15 | 0 | 73.8 | 0.42 | 0.93 | 0.47 | 0.39 |
| SCoT20 | 350–1500 | 8 | 2 | 49.6 | 0.37 | 0.75 | 0.30 | 0.50 |
| SCoT23 | 750–1500 | 4 | 1 | 51.6 | 0.37 | 0.77 | 0.43 | 0.50 |
| SCoT29 | 500–1850 | 8 | 0 | 45.7 | 0.38 | 0.71 | 0.36 | 0.51 |
| SCoT31 | 560–1000 | 9 | 2 | 56.3 | 0.38 | 0.81 | 0.53 | 0.49 |
| SCoT33 | 400–1240 | 14 | 0 | 53.4 | 0.38 | 0.79 | 0.47 | 0.52 |
| SCoT34 | 880–2100 | 13 | 2 | 51.3 | 0.38 | 0.77 | 0.53 | 0.51 |
| MEAN ± SD | 10.1 ± 4.0 | 1.0 ± 0.0 | 54.5 ± 0.9 | 0.39 ± 0.02 | 0.79 ± 0.07 | 0.45 ± 0.03 | 0.49 ± 0.05 | |
| Branch | No of Accessions | Phenotypical Traits Common to More Than 85% of Accessions of the Branch | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant Height | Stem Thickness | Peduncle Thickness | Inflorescence | Composite Flower | Floret Type | Floret Color | Disc Color | ||
| I | 6 | tall | thick, medium | thick, medium | disbud, spray | big, daisy-eyed doubled | strait flat | different | green |
| II | 13 | medium tall | thick, medium | medium | disbud, pompon | doubled, medium size | tubular twisted, bounded | pink, red, red-purple | yellow, light yellow |
| IIIa | 5 | medium tall | medium | thick | disbud, pompon | big, doubled | bent, hanging tubular | red, red-purple, purple | yellow-green |
| IIIb | 12 | different | thin, medium | thin, medium | spray | small, daisy-eyed doubled | Flat | light yellow, yellow, orange | yellow, yellow-orange |
| IIIc1 | 6 | medium tall | medium | medium | spray, flat corymb | doubled | strait simmetrical flat | different | different |
| IIIc2 | 14 | medium tall | medium | medium thick | disbud, pompon spray | big, doubled | quilled, broken, incurved | different | different |
| IIIc3.1 | 15 | short, medium | medium | medium thick | spray, compact corymb | small, doubled, anemone-shaped | funnel-shaped | different | yellow, light yellow, orange |
| IIIc3.2 | 16 | tall, very tall | medium, thick | medium thick | disbud, spray | daisy-eyed semi-doubled, doubled | Different | pink, red-purple | yellow, yellow-orange |
| IIIc4 | 1 | very tall | medium | thick | disbud | doubled | Flat | pink | yellow-green |
| Name | Primer Sequence 5′3′ | Origin Species | Reference |
|---|---|---|---|
| SCoT Marker | |||
| SCoT1 | CAACAATGGCTACCACCA | Oryza sativa | [19] |
| SCoT2 | CAACAATGGCTACCACCC | Oryza sativa | [19] |
| SCoT3 | CAACAATGGCTACCACCG | Oryza sativa | [19] |
| SCoT4 | CAACAATGGCTACCACCT | Oryza sativa | [19] |
| SCoT5 | CAACAATGGCTACCACGA | Oryza sativa | [19] |
| SCoT6 | CAACAATGGCTACCACGC | Oryza sativa | [19] |
| SCoT7 | CAACAATGGCTACCACGG | Oryza sativa | [19] |
| SCoT8 | CAACAATGGCTACCACGT | Oryza sativa | [19] |
| SCoT9 | CAACAATGGCTACCAGCA | Oryza sativa | [19] |
| SCoT10 | CAACAATGGCTACCAGCC | Oryza sativa | [19] |
| SCoT11 | AAGCAATGGCTACCACCA | Oryza sativa | [19] |
| SCoT12 | ACGACATGGCGACCAACG | Oryza sativa | [19] |
| SCoT13 | ACGACATGGCGACCATCG | Oryza sativa | [19] |
| SCoT14 | ACGACATGGCGACCACGC | Oryza sativa | [19] |
| SCoT15 | ACGACATGGCGACCGCGA | Oryza sativa | [19] |
| SCoT16 | ACCATGGCTACCACCGAC | Oryza sativa | [19] |
| SCoT17 | ACCATGGCTACCACCGAG | Oryza sativa | [19] |
| SCoT18 | ACCATGGCTACCACCGCC | Oryza sativa | [19] |
| SCoT19 | ACCATGGCTACCACCGGC | Oryza sativa | [19] |
| SCoT20 | ACCATGGCTACCACCGCG | Oryza sativa | [19] |
| SCoT21 | ACGACATGGCGACCCACA | Oryza sativa | [19] |
| SCoT22 | AACCATGGCTACCACCAC | Oryza sativa | [19] |
| SCoT23 | CACCATGGCTACCACCAG | Oryza sativa | [19] |
| SCoT24 | CACCATGGCTACCACCAT | Oryza sativa | [19] |
| SCoT25 | ACCATGGCTACCACCGGG | Oryza sativa | [19] |
| SCoT26 | ACCATGGCTACCACCGTC | Oryza sativa | [19] |
| SCoT27 | ACCATGGCTACCACCGTG | Oryza sativa | [19] |
| SCoT28 | CCATGGCTACCACCGCCA | Oryza sativa | [19] |
| SCoT29 | CCATGGCTACCACCGGCC | Oryza sativa | [19] |
| SCoT30 | CCATGGCTACCACCGGCG | Oryza sativa | [19] |
| SCoT31 | CCATGGCTACCACCGCCT | Oryza sativa | [19] |
| SCoT32 | CCATGGCTACCACCGCAC | Oryza sativa | [19] |
| SCoT33 | CCATGGCTACCACCGCAG | Oryza sativa | [19] |
| SCoT34 | ACCATGGCTACCACCGCA | Oryza sativa | [19] |
| SCoT35 | CATGGCTACCACCGGCCC | Oryza sativa | [19] |
| SCoT36 | GCAACAATGGCTACCACC | Oryza sativa | [19] |
| ISSR Marker | |||
| ISSR810 | GAGAGAGAGAGAGAGAT | Camellia sinensis | [51] |
| ISSR813 | CTCTCTCTCTCTCTCTT | Camellia sinensis | [54] |
| ISSR815 | CTCTCTCTCTCTCTCTG | Camellia sinensis | [54] |
| ISSR851 | TATTATTATTATTAT | Camellia sinensis | [54] |
| ISSR873 | CTTCACTTCACTTCA | Camellia sinensis | [54] |
| ISSR880 | GGAGAGGAGAGGAGA | Camellia sinensis | [54] |
| ISSR13 | ACACACACACACACACC | Camellia sinensis | [55] |
| ISSR14 | TGTGTGTGTGTGTGTGG | Camellia sinensis | [55] |
| ISSR15 | TCTCTCTCTCTCTCTCC | Camellia sinensis | [55] |
| ISSR814.1 | CTCTCTCTCTCTCTCTTG | Camellia sinensis | [55] |
| SSR Marker | |||
| gi298295865 | F: ACTCACTTGCCCCATTTGTC R: AGAGAAGCTCTCCAGGGACC | Ch. morifolium | [4] |
| gi298296818 | F: ATGTCCAGCTTGATGGGAAG R: GGCCCCTTGCAAATCCTC | Ch. morifolium | [4] |
| gi298297301 | F: TCAAACACCACCACCAACAC R: ATGTCACCAAGTCCTGGTCC | Ch. morifolium | [4] |
| 357 | F: ACCCAACCTGAACAAGATGC R: ATACTGCTGCCACTGACCCT | Ch. morifolium | [4] |
| 320 | F: GGTCCTTCGTTTCATTTGGA R: CGGGGGTAGGAATAGAAAGC | Ch. morifolium | [4] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samarina, L.S.; Malyarovskaya, V.I.; Reim, S.; Yakushina, L.G.; Koninskaya, N.G.; Klemeshova, K.V.; Shkhalakhova, R.M.; Matskiv, A.O.; Shurkina, E.S.; Gabueva, T.Y.; et al. Transferability of ISSR, SCoT and SSR Markers for Chrysanthemum × Morifolium Ramat and Genetic Relationships Among Commercial Russian Cultivars. Plants 2021, 10, 1302. https://doi.org/10.3390/plants10071302
Samarina LS, Malyarovskaya VI, Reim S, Yakushina LG, Koninskaya NG, Klemeshova KV, Shkhalakhova RM, Matskiv AO, Shurkina ES, Gabueva TY, et al. Transferability of ISSR, SCoT and SSR Markers for Chrysanthemum × Morifolium Ramat and Genetic Relationships Among Commercial Russian Cultivars. Plants. 2021; 10(7):1302. https://doi.org/10.3390/plants10071302
Chicago/Turabian StyleSamarina, Lidia S., Valentina I. Malyarovskaya, Stefanie Reim, Lyudmila G. Yakushina, Natalia G. Koninskaya, Kristina V. Klemeshova, Ruset M. Shkhalakhova, Alexandra O. Matskiv, Ekaterina S. Shurkina, Tatiana Y. Gabueva, and et al. 2021. "Transferability of ISSR, SCoT and SSR Markers for Chrysanthemum × Morifolium Ramat and Genetic Relationships Among Commercial Russian Cultivars" Plants 10, no. 7: 1302. https://doi.org/10.3390/plants10071302
APA StyleSamarina, L. S., Malyarovskaya, V. I., Reim, S., Yakushina, L. G., Koninskaya, N. G., Klemeshova, K. V., Shkhalakhova, R. M., Matskiv, A. O., Shurkina, E. S., Gabueva, T. Y., Slepchenko, N. A., & Ryndin, A. V. (2021). Transferability of ISSR, SCoT and SSR Markers for Chrysanthemum × Morifolium Ramat and Genetic Relationships Among Commercial Russian Cultivars. Plants, 10(7), 1302. https://doi.org/10.3390/plants10071302






