Downregulation of Polyamine and Diamine Oxidases in Silicon-Treated Cucumber
Abstract
1. Introduction
2. Results
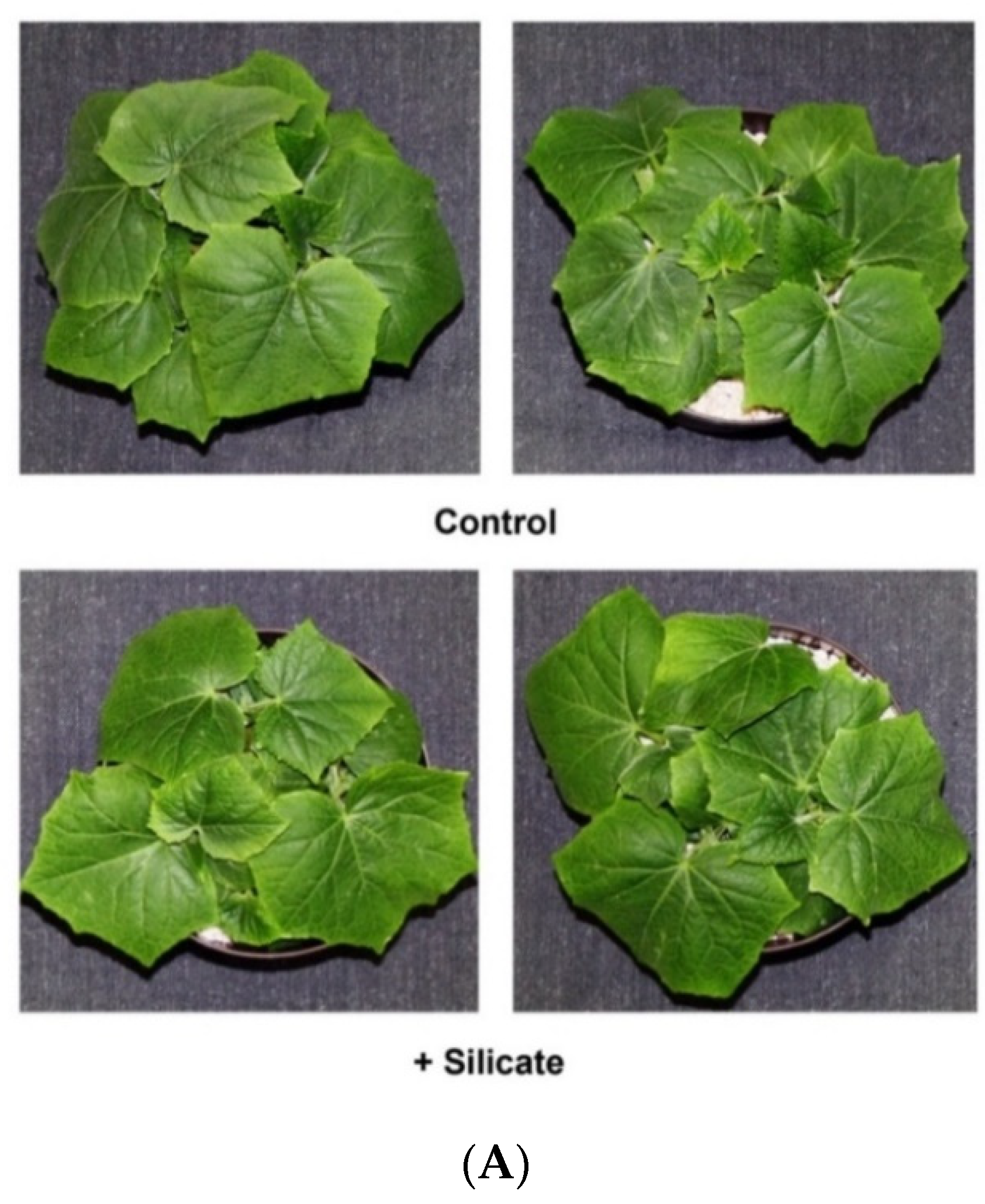
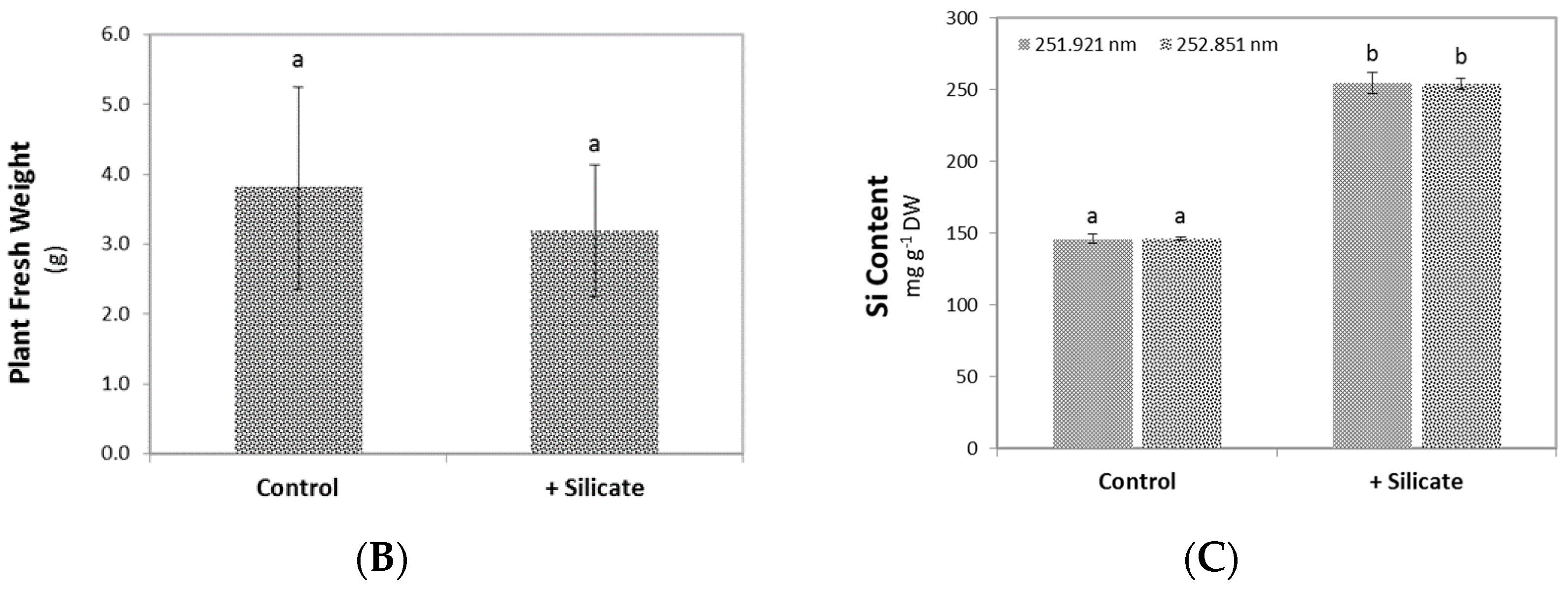
2.1. Plant Growth and Silicate Content
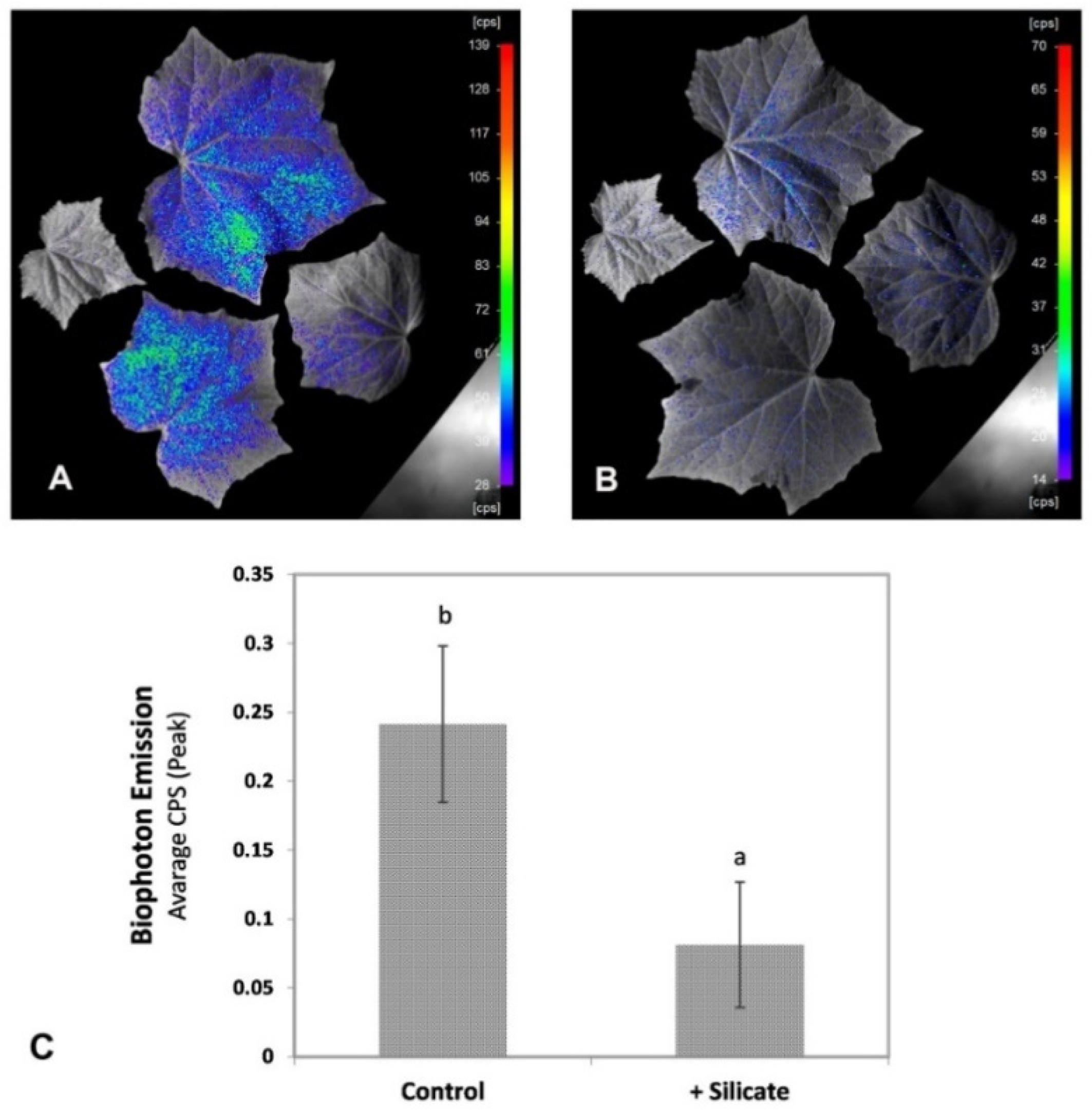
2.2. Bio-Photon Emission Imaging
2.3. H2O2 Content and Lipid Peroxidation
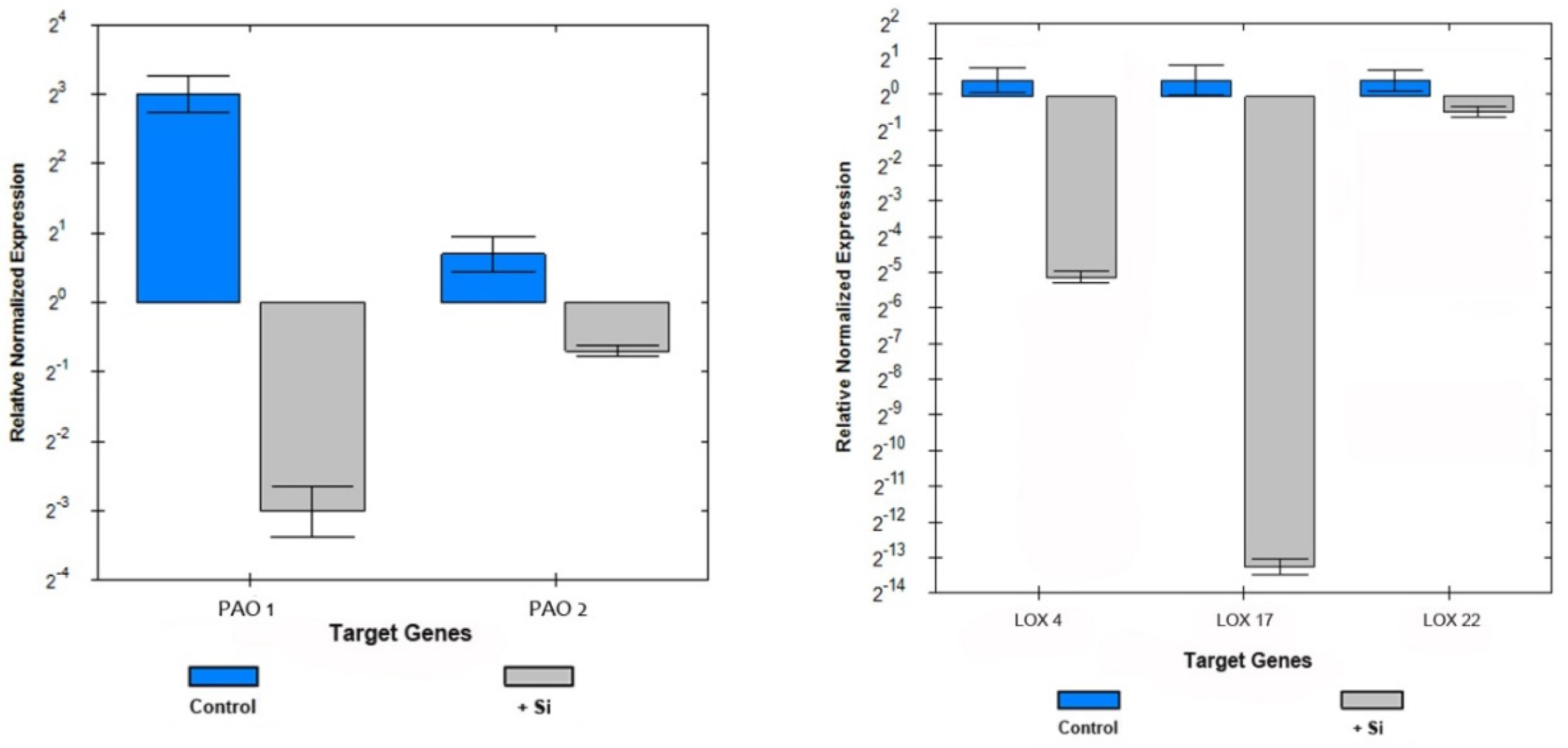
2.4. Polyamine Oxidase, Diamine Oxidase, and Ascorbate Peroxidase Enzyme Activities
2.5. Transcriptional Analysis of Selected Redox-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Silicate Treatment
4.2. Ultra-Weak Bio-Photon Emission
4.3. Measurement of Si, H2O2, and Lipid Hydroperoxide Content, DAB Staining
4.4. Amine Oxidases and Ascorbate Peroxidase Enzyme Activities
4.5. RNA Isolation, cDNA Preparation, RT-PCR, and RT-qPCR
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Yan, G.; Nikolic, M.; Ye, M.; Xiao, Z.; Liang, Y. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Lee, I.J. Silicon: A duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit. Rev. Biotechnol. 2015, 36, 1099–1109. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adrees, M.; Bharwana, S.A.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Fretté, X.; Jensen, B.; Shetty, N.P.; Jensen, J.D.; Jørgensen, H.J.L.; Newman, M.A.; Christensen, L.P. Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid pathway genes during the interaction between miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol. 2011, 157, 2194–2205. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Yin, J.; Jia, J.; Lian, Z.; Hu, Y.; Guo, J.; Huo, H.; Zhu, Y.; Gong, H. Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotoxicol. Environ. Saf. 2019, 169, 8–17. [Google Scholar] [CrossRef]
- Podgórska, A.; Burian, M.; Szal, B. Extra-cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Yu, Z.; Jia, D.; Liu, T. Polyamine oxidases play various roles in plant development and abiotic stress tolerance. Plants 2019, 6, 184. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant lipoxygenases and their role in plant physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Ye, S.; Jiang, L.; Liu, S. Genome-wide identification of glutathione peroxidase (GPX) gene family and their response to abiotic stress in cucumber. 3 Biotech 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Yang, X.Y.; Jiang, W.J.; Yu, H.J. The expression profiling of the lipoxygenase (LOX) family genes during fruit development, abiotic stress and hormonal treatments in cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2012, 13, 2481–2500. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yin, J.; Liang, Y.; Liu, J.; Jia, J.; Huo, H.; Wu, Z.; Yang, R.; Gong, H. Transcriptomic dynamics provide an insight into the mechanism for silicon-mediated alleviation of salt stress in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 174, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Birtic, S.; Ksas, B.; Genty, B.; Mueller, M.J.; Triantaphylidès, C.; Havaux, M. Using spontaneous photon emission to image lipid oxidation patterns in plant tissues. Plant J. 2011, 67, 1103–1115. [Google Scholar] [CrossRef]
- Raviv, M.; Lieth, J.H.; Bar-Tal, A. (Eds.) Growing plants in soilless culture: Operational conclusions. In Soilless Culture, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 637–669. [Google Scholar]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef]
- Morard, P.; Silvestre, J. Plant injury due to oxygen deficiency in the root environment of soilless culture: A review. Plant Soil. 1996, 184, 243–254. [Google Scholar] [CrossRef]
- Bat-Erdene, O.; Szegő, A.; Gyöngyik, M.; Mirmazloum, I.; Papp, I. Long term silicon exposure coordinately downregulates lipoxygenase genes, decreases reactive oxygen species level and promotes growth of cucumber plants in a semi-hydroponic cultivation system. Russ. J. Plant Physiol. 2021, accepted, in press. [Google Scholar] [CrossRef]
- Law, C.; Exley, C. New insight into silica deposition in horsetail (Equisetum arvense). BMC Plant Biol. 2011, 11, 112. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Qi, L.; Yin, L.; Wang, S.; Deng, X. Silicon-moderated K-deficiency-induced leaf chlorosis by decreasing putrescine accumulation in sorghum. Ann. Bot. 2016, 118, 305–315. [Google Scholar] [CrossRef]
- Kim, D.W.; Watanabe, K.; Murayama, C.; Izawa, S.; Niitsu, M.; Michael, A.J.; Berberich, T.; Kusano, T. Polyamine oxidase 5 regulates arabidopsis growth through thermospermine oxidase activity. Plant Physiol. 2014, 165, 1575–1590. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Kurabachew, H.; Wydra, K. Induction of systemic resistance and defense-related enzymes after elicitation of resistance by rhizobacteria and silicon application against Ralstonia solanacearum in tomato (Solanum lycopersicum). Crop Prot. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Kellős, T.; Tímár, I.; Szilágyi, V.; Szalai, G.; Galiba, G.; Kocsy, G. Stress hormones and abiotic stresses have different effects on antioxidants in maize lines with different sensitivity. Plant Biol. 2008, 10, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Friesen, T. DAB staining and visualization of hydrogen peroxide in wheat leaves. Bio. Protoc. 2012, 2, e309. [Google Scholar] [CrossRef]
- Takács, Z.; Poór, P.; Tari, I. Comparison of polyamine metabolism in tomato plants exposed to different concentrations of salicylic acid under light or dark conditions. Plant Physiol. Biochem. 2016, 108, 266–278. [Google Scholar] [CrossRef]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J. Soil Sci. Plant Nutr. 2016, 16, 662–676. [Google Scholar] [CrossRef]
- Jaakola, L.; Pirttilä, A.M.; Halonen, M.; Hohtola, A. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Appl. Biochem. Biotechnol.-Part B Mol. Biotechnol. 2001, 19, 201–203. [Google Scholar] [CrossRef]
- Oszlányi, R.; Mirmazloum, I.; Pónya, Z.; Szegő, A.; Jamal, S.; Bat-Erdene, O.; Papp, I. Oxidative stress level and dehydrin gene expression pattern differentiate two contrasting cucumber F1 hybrids under high fertigation treatment. Int. J. Biol. Macromol. 2020, 161, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; Mangelsdorf, D.J. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. 2003, 1, e012. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szegő, A.; Mirmazloum, I.; Pónya, Z.; Bat-Erdene, O.; Omran, M.; Kiss-Bába, E.; Gyöngyik, M.; Papp, I. Downregulation of Polyamine and Diamine Oxidases in Silicon-Treated Cucumber. Plants 2021, 10, 1248. https://doi.org/10.3390/plants10061248
Szegő A, Mirmazloum I, Pónya Z, Bat-Erdene O, Omran M, Kiss-Bába E, Gyöngyik M, Papp I. Downregulation of Polyamine and Diamine Oxidases in Silicon-Treated Cucumber. Plants. 2021; 10(6):1248. https://doi.org/10.3390/plants10061248
Chicago/Turabian StyleSzegő, Anita, Iman Mirmazloum, Zsolt Pónya, Oyuntogtokh Bat-Erdene, Mohammad Omran, Erzsébet Kiss-Bába, Márta Gyöngyik, and István Papp. 2021. "Downregulation of Polyamine and Diamine Oxidases in Silicon-Treated Cucumber" Plants 10, no. 6: 1248. https://doi.org/10.3390/plants10061248
APA StyleSzegő, A., Mirmazloum, I., Pónya, Z., Bat-Erdene, O., Omran, M., Kiss-Bába, E., Gyöngyik, M., & Papp, I. (2021). Downregulation of Polyamine and Diamine Oxidases in Silicon-Treated Cucumber. Plants, 10(6), 1248. https://doi.org/10.3390/plants10061248







