Foliar Application of Salicylic Acid Improves Water Stress Tolerance in Conocarpus erectus L. and Populus deltoides L. Saplings: Evidence from Morphological, Physiological, and Biochemical Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Water Stress and Salicylic Acid Application
2.3. Growth and Biomass Production
2.4. Leaf Gas Exchange Measurements
2.5. Determination of Chlorophyll a, b, Carotenoid, and Various Osmolytes
2.6. Determination of Malondialdehyde Contents and Electrolyte Leakage
2.7. Production of Reactive Oxygen Species (ROS)
2.8. Antioxidant Enzyme Activity
2.9. Statistical Analysis
3. Results
3.1. Effect of Soil Water Deficit and SA Application on Growth and Dry Weight Production
3.2. Effect of Soil Water Deficit and SA Application on Chlorophyll a, b, and Carotenoids Contents
3.3. Effect of Soil Water Deficit and SA Application on the Production of Osmolytes
3.4. Effect of Soil Water Deficit and SA Application on the Variations in Leaf Gas Exchange Parameters
3.5. Effect of Soil Water Deficit and SA Application on the Concentration of MDA, EL % and Oxidant and Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Executive Summary of the Intergovernmental Panel on Climate Change. February 2007. Available online: https://www.ipcc.ch/sr15/ (accessed on 18 May 2021).
- Stuart, M.E.; Gooddy, D.C.; Bloomfield, J.P.; Williams, A.T. A review of the impact of climate change on future nitrate concentrations in groundwater of the UK. Sci. Total. Environ. 2011, 409, 2859–2873. [Google Scholar] [CrossRef]
- Aryal, J.P.; Sapkota, T.B.; Khurana, R.; Khatri-Chhetri, A.; Rahut, D.B.; Jat, M.L. Climate change and agriculture in South Asia: Adaptation options in smallholder production systems. Dev. Sustain. Environ. 2020, 22, 5045–5075. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; Van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Zafar, Z.; Rasheed, F.; Atif, R.M.; Maqsood, M.; Gailing, O. Salicylic acid-induced morpho-physiological and biochemical changes triggered water deficit tolerance in Syzygium cumini L. saplings. Forests 2021, 12, 491. [Google Scholar] [CrossRef]
- Beniwal, R.S.; Heyser, R.L.; Polle, A. Ectomycorrhiza and hydrogel protect hybrid poplar from water deficit and unravel plastic responses of xylem anatomy. Environ. Exp. Bot. 2010, 69, 189–197. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Field, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, G.; Innes, I.; Nitschke, C.; Kang, H. Climatic niche models and their consensus projections for future climates for four major forest tree species in the Asia–Pacific region. For. Ecol. Mang. 2016, 360, 357–366. [Google Scholar] [CrossRef]
- Klein, T.; Yakir, D.; Buchmann, N.; Grünzweig, J.M. Towards an advanced assessment of the hydrological vulnerability of forests to climate change induced drought. New Phytol. 2014, 201, 712–716. [Google Scholar] [CrossRef]
- Choat, B.; Timothy, J.B.; Craig, R.B.; Remko, A.D.; Rosana, L.; Belinda, E.M. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Blum, A. Plant water relations, plant stress and plant production. In Plant Breeding for Water-Limited Environments; Springer: New York, NY, USA, 2011; pp. 11–52. [Google Scholar]
- Muller, B.; Pantin, F.; Genard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef]
- Sabir, M.A.; Rasheed, F.; Zafar, Z.; Khan, I.; Nawaz, M.F.; Haq, I.U.; Bilal, M. A consistent CO2 assimilation rate and an enhanced root development drives the tolerance mechanism in Ziziphus jujuba under soil water deficit. Arid. Land. Res. Manag. 2020, 34, 392–404. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.C.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, T. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.E.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.M.; Barbaroux, C.; Thiec, D.L.; Bréchet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef]
- Rasheed, F.; Delagrange, S. Acclimation of Betula alleghaniensis Britton to moderate soil water deficit: Small morphological changes make for important consequences in crown display. Tree Physiol. 2016, 36, 1320–1329. [Google Scholar] [PubMed]
- Rasheed, F.; Gondal, A.; Kudus, K.A.; Zafar, Z.; Nawaz, M.F.; Khan, W.R.; Abdullah, M.; Ibrahim, F.H.; Depardieu, C.; Pazi, A.M.M.; et al. Effects of soil water deficit on three tree species of the arid environment: Variations in growth, physiology, and antioxidant enzyme activities. Sustainability 2021, 13, 3336. [Google Scholar] [CrossRef]
- Zarafshar, M.; Akbarinia, M.; Askari, H.; Hosseini, S.M.; Rahaie, M.; Struve, D.; Striker, G.G. Morphological, physiological and biochemical responses to soil water deficit in seedlings of three populations of wild pear (Pyrus boisseriana). Biotechnol. Agron. Soc. Environ. 2014, 18, 353–366. [Google Scholar]
- Talbi, S.; Romero-Puertas, M.C.; Hernandez, A.; Terron, L.; Ferchichi, A.; Sandalio, L.M. Drought tolerance in a Saharian plant Oudneya africana: Role of antioxidant defences. Environ. Exp. Bot. 2015, 111, 114–126. [Google Scholar] [CrossRef]
- Jesus, C.; Meijón, M.; Monteiro, P.; Correia, B.; Amaral, J.; Escandón, M.; Cañal, J.M.; Pinto, G. Salicylic acid application modulates physiological and hormonal changes in Eucalyptus globulus under water deficit. Environ. Exp. Bot. 2015, 118, 56–66. [Google Scholar] [CrossRef]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Laxa, M.; Michael, L.; Wilena, T.; Kamel, C.; Karl, J.D. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mishra, A.; Jha, B. Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Mar. Biotechnol. 2014, 16, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Saheri, F.; Barzin, G.; Pishkar, L.; Boojar, M.M.A.; Babaeekhou, L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia 2020, 75, 2189–2200. [Google Scholar] [CrossRef]
- Shen, C.; Hu, Y.; Du, X.; Li, T.; Tang, H.; Wu, J. Salicylic acid induces physiological and biochemical changes in Torreya grandis cv. Merrillii seedlings under drought stress. Trees 2014, 28, 961–970. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.; Ferreira, H.; Moutinho-Pereira, J.; Correia, C. The role of nighttime water balance on Olea europaea plants subjected to contrasting water regimes. J. Plant Physiol. 2018, 226, 56–63. [Google Scholar] [CrossRef]
- Singh, B.; Usha, K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul. 2003, 39, 137–141. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, S.S.; Aref, I.M.; Al-Mefarrej, H.; El-Juhany, L.I. Effect of spacing on the biomass production and allocation in Conocarpus erectus L. trees grown in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2008, 15, 315–322. [Google Scholar]
- Wood, J.R. A Handbook of Yemen Flora; Royal Botanical Gardens: Kew, UK, 1997. [Google Scholar]
- Redha, A.; Al-Hasan, R.; Afzal, M. Synergistic and concentration-dependent toxicity of multiple heavy metals compared with single heavy metals in Conocarpus lancifolius. Environ. Sci. Pollut. Res. 2021, 28, 23258–23272. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, F.; Dreyer, E.; Richard, B.; Brignolas, F.; Brendel, O.; Le Thiec, D. Vapour pressure deficit during growth has little impact on genotypic differences of transpiration efficiency at leaf and whole plant level: An example from Populus nigra L. Plant Cell Environ. 2015, 38, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, F.; Dreyer, E.; Richard, B.; Brignolas, F.; Montpied, P.; Le Thiec, D. Genotype differences in13 C discrimination between atmosphere and leaf matter match differences in transpiration efficiency at leaf and whole-plant levels in hybrid Populus deltoides × nigra. Plant Cell Environ. 2012, 36, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Z.; Rasheed, F.; Abdullah, M.; Salam, M.M.A.; Mohsin, M. Effects of water deficit on growth and physiology of Young Conocarpus erectus L. and Ficus benjamina L. saplings. Bangladesh J. Bot. 2019, 48, 1215–1221. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ainsworth, E.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Nayyar, H. Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ. Exp. Bot. 2003, 50, 253–264. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Ma, F.; Feng, F.; Shu, H. Responses of growth and antioxidant system root-zone hypoxia stress in two Malus species. Plant Soil. 2009, 327, 95–105. [Google Scholar] [CrossRef]
- Bayer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Knörzer, O.C.; Burner, J.; Boger, P. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol. Plantarum. 1996, 97, 388–396. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. CR Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Correia, B.; Pintó-Marijuan, M.; Neves, L.; Brossa, R.; Dias, M.C.; Costa, A.; Castro, B.B.; Araujo, C.; Santos, C.; Chaves, M.M.; et al. Water stress and recovery in the performance of two Eucalyptus globulus clones: Physiological and biochemical profiles. Physiol Plant 2014, 150, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Possen, B.J.H.M.; Oksanen, E.; Rousi, M.; Ruhanen, H.; Ahonen, V.; Tervahauta, A.; Heinonen, J.; Heiskanen, J.; Karenlampi, S.; Vapaavuori, E. Adaptability of birch (Betula pendula Roth) and aspen (Populus tremula L.) genotypes to different soil moisture conditions. For. Ecol. Manag. 2011, 262, 1387–1399. [Google Scholar] [CrossRef]
- Bogeat-Triboulot, M.B.; Mikael, B.; Jenny, R.; Laurent, J.; Didier, L.T.; Payam, F.; Basia, V.; Erwin, W.; Kris, L.; Thomas, T.; et al. Gradual soil water depletion results in reversible changes of gene expression, protein profiles ecophysiology and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol. 2007, 143, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, B.; Layeghhaghighi, M.; Azimi, R.; Hadi, N. Improving water use efficiency through drought stress and using salicylic acid for proper production of Rosmarinus officinalis L. Ind. Crop. Prod. 2020, 144, 111893. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestic, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A.; Hossain, M.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil. Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef]
- Sakhabutdinova, A.R.; Fatkhutdinova, D.R.; Bezrukova, M.V.; Shakiova, F.M. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg. J. Plant Physiol. 2003, 29, 314–319. [Google Scholar]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life. Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends. Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Jutur, P.P.; Sumithra, K. Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ. Exp. Bot. 2004, 52, 33–42. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhris, M.; Ben Abdallah, F. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Regier, N.; Streb, S.; Cocozza, C.; Schaub, M.; Cherubini, P.; Zeeman, S.C.; Rrey, B. Drought tolerance of two black poplar (Populus nigra L.) clones: Contribution of carbohydrates and oxidative stress defence. Plant Cell Environ. 2009, 32, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Dianat, M.; Saharkhiz, M.J.; Tavassolian, I. Salicylic acid mitigates drought stress in Lippia citriodora L. effects on biochemical traits and essential oil yield. Biocatal. Agric. Biotechnol. 2016, 8, 286–293. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; Cd, O.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Idrees, M.; Khan, M.M.A.; Aftab, T.; Naeem, M.; Hashmi, N. Salicylic acid-induced physiological and biochemical changes in lemongrass varieties under water stress. J. Plant Interact. 2010, 5, 293–303. [Google Scholar] [CrossRef]
- Preciado-Rangel, P.; Reyes-Pérez, J.J.; Ramírez-Rodríguez, S.C.; Salas-Pérez, L.; Fortis-Hernández, M.; Murillo-Amador, B.; Troyo-Diéguez, E. Foliar aspersion of salicylic acid improves phenolic and flavonoid compounds and also the fruit yield in cucumber (Cucumis sativus L.). Plants 2019, 8, 44. [Google Scholar] [CrossRef]
- Gondor, O.K.; Janda, T.; Soós, V.; Pál, M.; Majláth, I.; Adak, M.K.; Balázs, E.; Szalai, G. Salicylic acid induction of flavonoid biosynthesis pathways in wheat varies by treatment. Front. Plant Sci. 2016, 7, 1447. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crop. Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Lima, A.L.S.; DaMatta, F.M.; Pinheiro, H.A.; Totola, M.R.; Loureiro, M.E. Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environ. Exp. Bot. 2002, 47, 239–247. [Google Scholar] [CrossRef]
- Jin, R.; Shi, H.; Han, C.; Zhong, B.; Wang, Q.; Chan, Z. Physiological changes of purslane (Portulaca oleracea L.) after progressive drought stress and rehydration. Sci. Hortic. 2015, 194, 215–221. [Google Scholar] [CrossRef]
- Pourghasemian, N.; Moradi, R.; Naghizadeh, M.; Landberg, T. Mitigating drought stress in sesame by foliar application of salicylic acid, beeswax waste and licorice extract. Agric. Water. Manag. 2020, 231, 105997. [Google Scholar] [CrossRef]


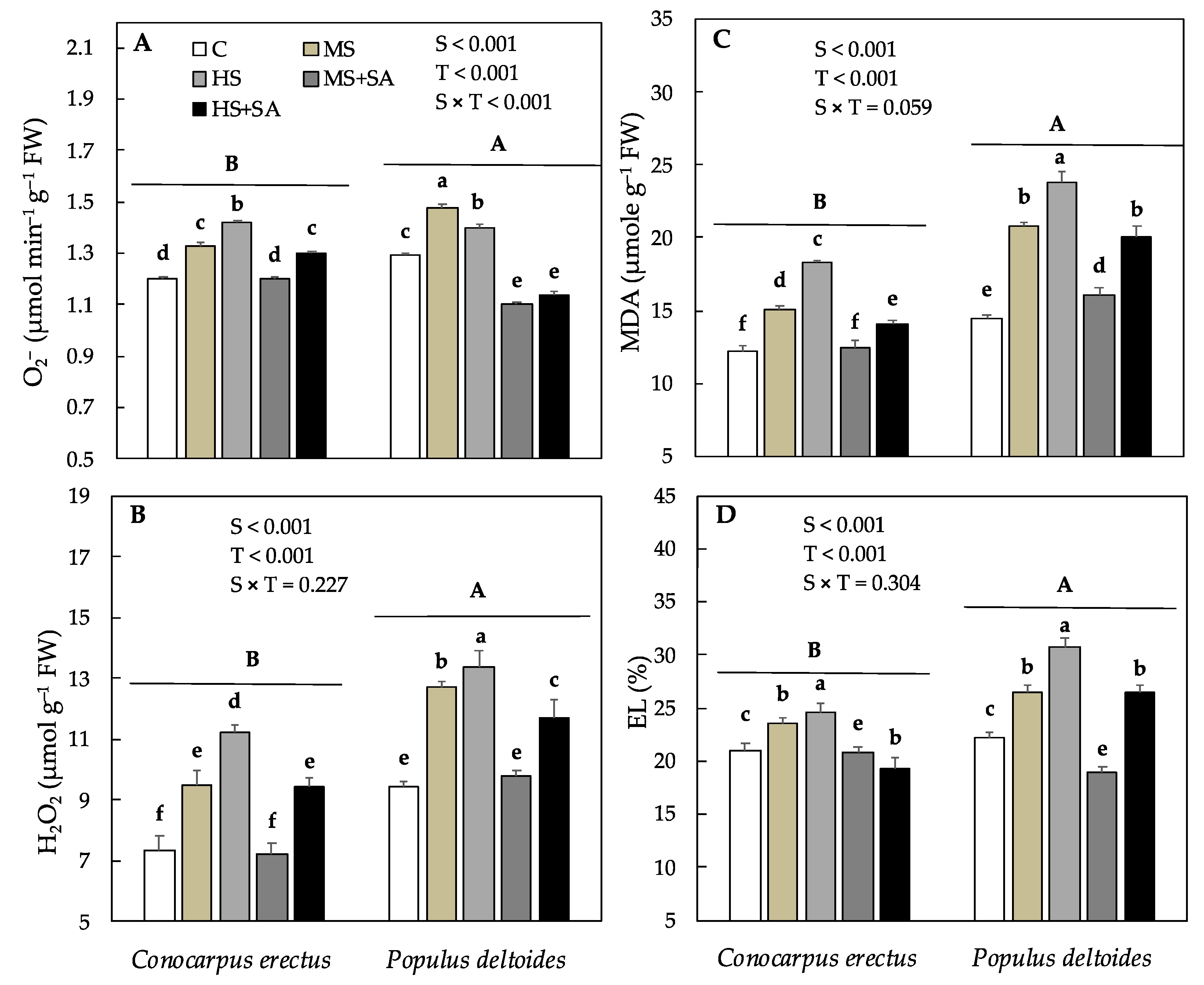
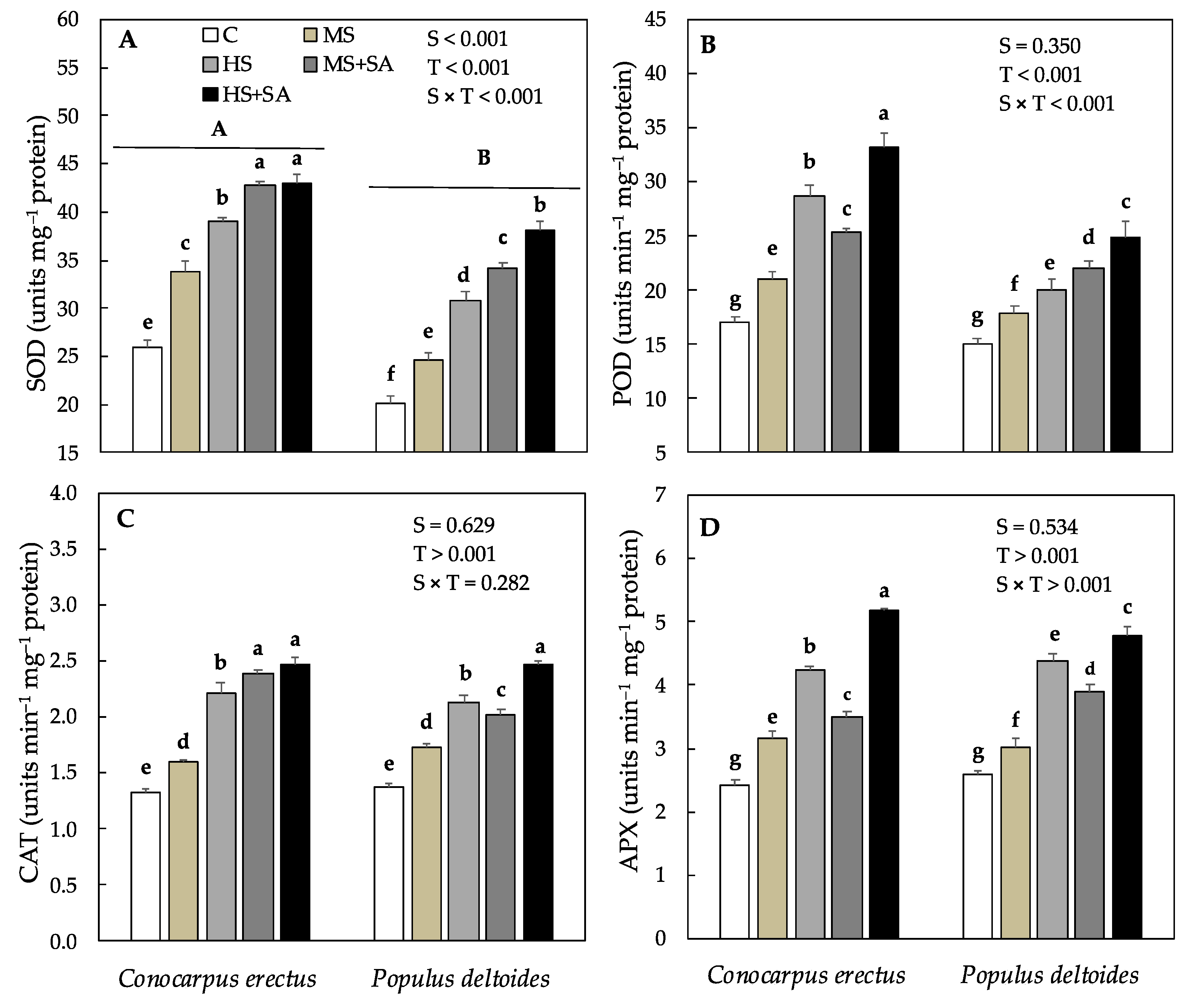
| Species | Treatments | H (cm) | D (mm) | R:S Ratio | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Cc (mg g−1 FW) |
|---|---|---|---|---|---|---|---|
| C | 74.1 ± 2.33 a | 6.18 ± 0.21 a | 0.64 ± 0.07 d | 2.18 ± 0.07 a | 2.21 ± 0.02 a | 0.82 ± 0.02 a | |
| MS | 69.8 ± 2.05 ab | 5.86 ± 0.40 ab | 0.68 ± 0.05 c | 1.66 ± 0.05 c | 1.89 ± 0.04 b | 0.72 ± 0.02 b | |
| Conocarpus | HS | 56.3 ± 3.35 d | 4.03 ± 0.09 d | 1.09 ± 0.07 a | 1.34 ± 0.05 d | 1.44 ± 0.05 d | 0.65 ± 0.03 c |
| erectus | MS + SA | 71.3 ± 2.66 ab | 5.52 ± 0.24 b | 0.72 ± 0.01 c | 1.87 ± 0.06 b | 1.99 ± 0.05 b | 0.83 ± 0.03 a |
| HS + SA | 68.0 ± 2.61 bc | 4.35 ± 0.35 c | 0.95 ± 0.08 a | 1.65 ± 0.06 c | 1.71 ± 0.06 c | 0.85 ± 0.02 a | |
| C | 64.0 ± 1.70 c | 4.52 ± 0.17 c | 0.61 ± 0.01 d | 1.55 ± 0.20 c | 1.41 ± 0.15 d | 0.89 ± 0.04 a | |
| MS | 48.7 ± 1.52 de | 3.64 ± 0.21 de | 0.75 ± 0.09 c | 1.14 ± 0.05 e | 1.09 ± 0.07 e | 0.76 ± 0.01 b | |
| Populus | HS | 32.0 ± 1.47 f | 3.02 ± 0.14 e | 0.81 ± 0.01 b | 0.86 ± 0.07 f | 0.84 ± 0.01 f | 0.66 ± 0.02 c |
| deltoides | MS + SA | 53.3 ± 1.52 d | 3.91 ± 0.17 d | 0.82 ± 0.08 b | 1.34 ± 0.06 d | 1.29 ± 0.06 d | 0.99 ± 0.06 a |
| HS + SA | 43.1 ± 1.87 e | 3.51 ± 0.12 d | 0.58 ± 0.10 c | 1.21 ± 0.14 e | 1.21 ± 0.18 d | 0.84 ± 0.04 a | |
| S-effect | p < 0.001 | p = 0.021 | p = 0.161 | p < 0.001 | p < 0.001 | p < 0.001 | |
| T-effect | p < 0.001 | p = 0.051 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| S × T effect | p < 0.001 | p = 0.405 | p = 0.005 | p = 0.046 | p = 0.046 | p < 0.001 |
| Species | Treatments | Pc (µmol g−1 FW) | TSS (mg g−1 FW) | TPC (mg g−1 FW) | SP (mg g−1 FW) |
|---|---|---|---|---|---|
| C | 20.1 ± 0.53 d | 74.3 ± 0.63 c | 1.38 ± 0.02 ef | 23.6 ± 0.49 d | |
| MS | 24.7 ± 0.94 c | 83.0 ± 1.12 b | 2.20 ± 0.05 c | 28.6 ± 0.30 b | |
| Conocarpus | HS | 29.0 ± 0.39 b | 84.3 ± 1.00 b | 2.55 ± 0.02 b | 33.2 ± 0.63 a |
| erectus | MS + SA | 30.2 ± 0.48 b | 86.9 ± 1.17 a | 2.54 ± 0.02 b | 31.6 ± 0.45 a |
| HS + SA | 34.9 ± 0.58 a | 88.6 ± 1.25 a | 2.82 ± 0.02 a | 33.6 ± 0.90 a | |
| C | 19.0 ± 0.38 e | 69.3 ± 1.39 e | 1.25 ± 0.01 f | 24.8 ± 0.32 d | |
| MS | 22.4 ± 1.12 cd | 74.6 ± 0.46 d | 1.59 ± 0.04 e | 26.9 ± 1.12 c | |
| Populus | HS | 26.5 ± 0.42 c | 83.8 ± 0.93 b | 2.1 ± 0.03 cd | 30.0 ± 0.71 ab |
| deltoides | MS + SA | 29.6 ± 0.63 b | 78.5 ± 0.87 c | 1.83 ± 0.01 d | 28.7 ± 0.86 b |
| HS + SA | 30.1 ± 0.32 b | 89.3 ± 1.63 a | 2.35 ± 0.04 c | 32.3 ± 0.89 a | |
| S-effect | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| T-effect | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| S × T effect | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, Z.; Rasheed, F.; Atif, R.M.; Javed, M.A.; Maqsood, M.; Gailing, O. Foliar Application of Salicylic Acid Improves Water Stress Tolerance in Conocarpus erectus L. and Populus deltoides L. Saplings: Evidence from Morphological, Physiological, and Biochemical Changes. Plants 2021, 10, 1242. https://doi.org/10.3390/plants10061242
Zafar Z, Rasheed F, Atif RM, Javed MA, Maqsood M, Gailing O. Foliar Application of Salicylic Acid Improves Water Stress Tolerance in Conocarpus erectus L. and Populus deltoides L. Saplings: Evidence from Morphological, Physiological, and Biochemical Changes. Plants. 2021; 10(6):1242. https://doi.org/10.3390/plants10061242
Chicago/Turabian StyleZafar, Zikria, Fahad Rasheed, Rana Muhammad Atif, Muhammad Asif Javed, Muhammad Maqsood, and Oliver Gailing. 2021. "Foliar Application of Salicylic Acid Improves Water Stress Tolerance in Conocarpus erectus L. and Populus deltoides L. Saplings: Evidence from Morphological, Physiological, and Biochemical Changes" Plants 10, no. 6: 1242. https://doi.org/10.3390/plants10061242
APA StyleZafar, Z., Rasheed, F., Atif, R. M., Javed, M. A., Maqsood, M., & Gailing, O. (2021). Foliar Application of Salicylic Acid Improves Water Stress Tolerance in Conocarpus erectus L. and Populus deltoides L. Saplings: Evidence from Morphological, Physiological, and Biochemical Changes. Plants, 10(6), 1242. https://doi.org/10.3390/plants10061242









