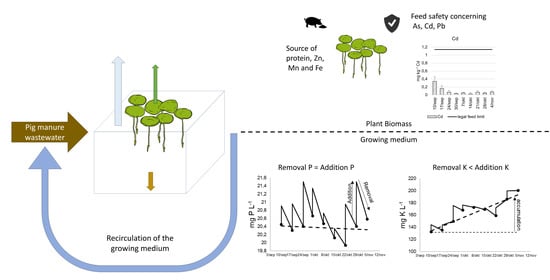
Lemna minor Cultivation for Treating Swine Manure and Providing Micronutrients for Animal Feed
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.1.1. Pilot
2.1.2. Growing Medium
- the total mass of LF, BE, and RW equals 1000 kg, as this is the approximate limit of the cubicontainers,
- all fractions of LF, BE, and RW are greater than zero,
- the total N and total P content of the final mixture are below the limits proposed for Lemna minor [13],
- the N/P ratio of the medium equals 3.0, as this is the ratio between the N removal and P removal determined in a duckweed system grown on diluted BE in outdoor systems [7].
2.1.3. Sampling
2.2. Plant Analysis
2.3. Medium Analysis
2.4. Calculations
3. Results and Discussion
3.1. Medium Composition and Accumulation of Elements
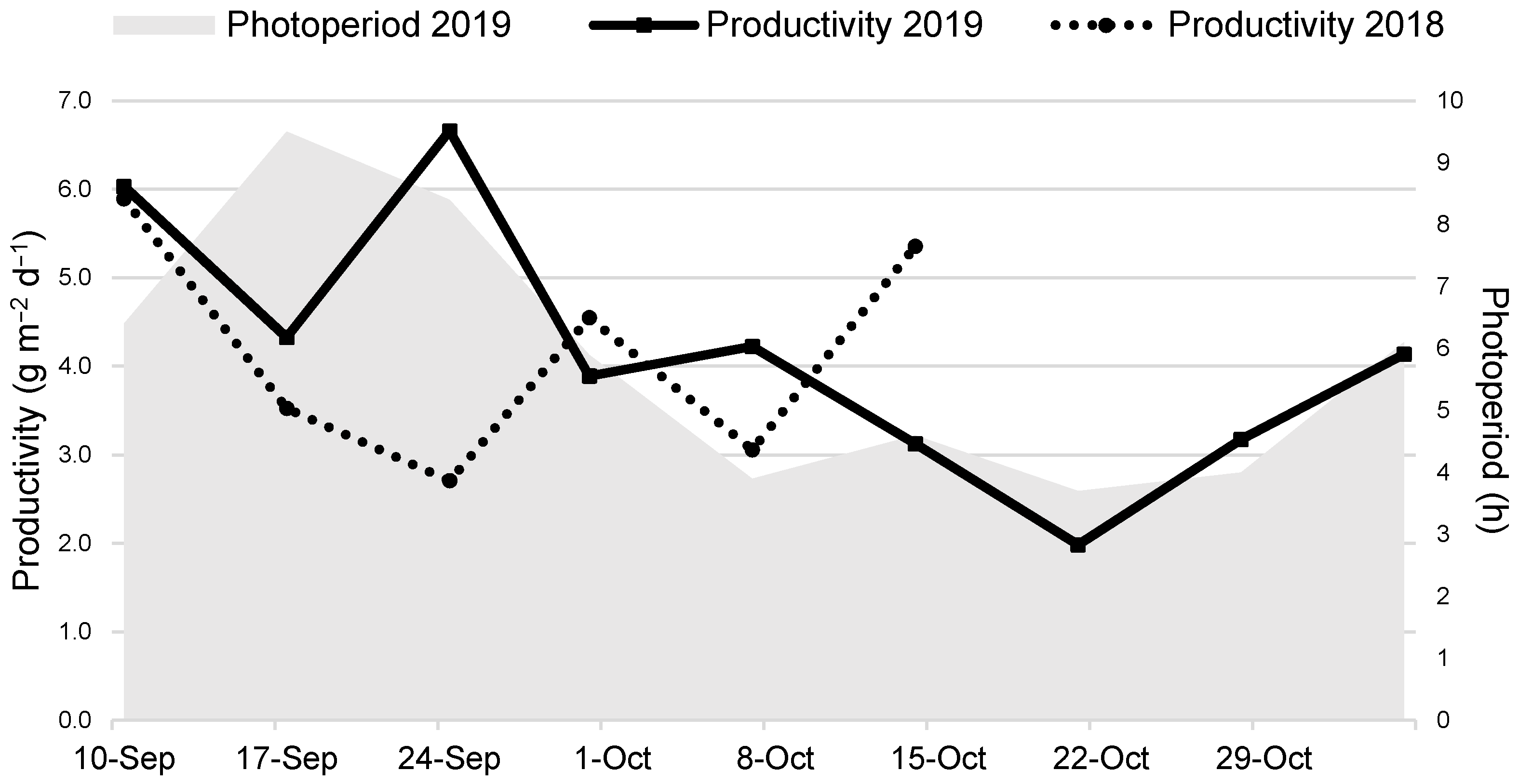
3.2. Mineral Removal and Recovery Potential
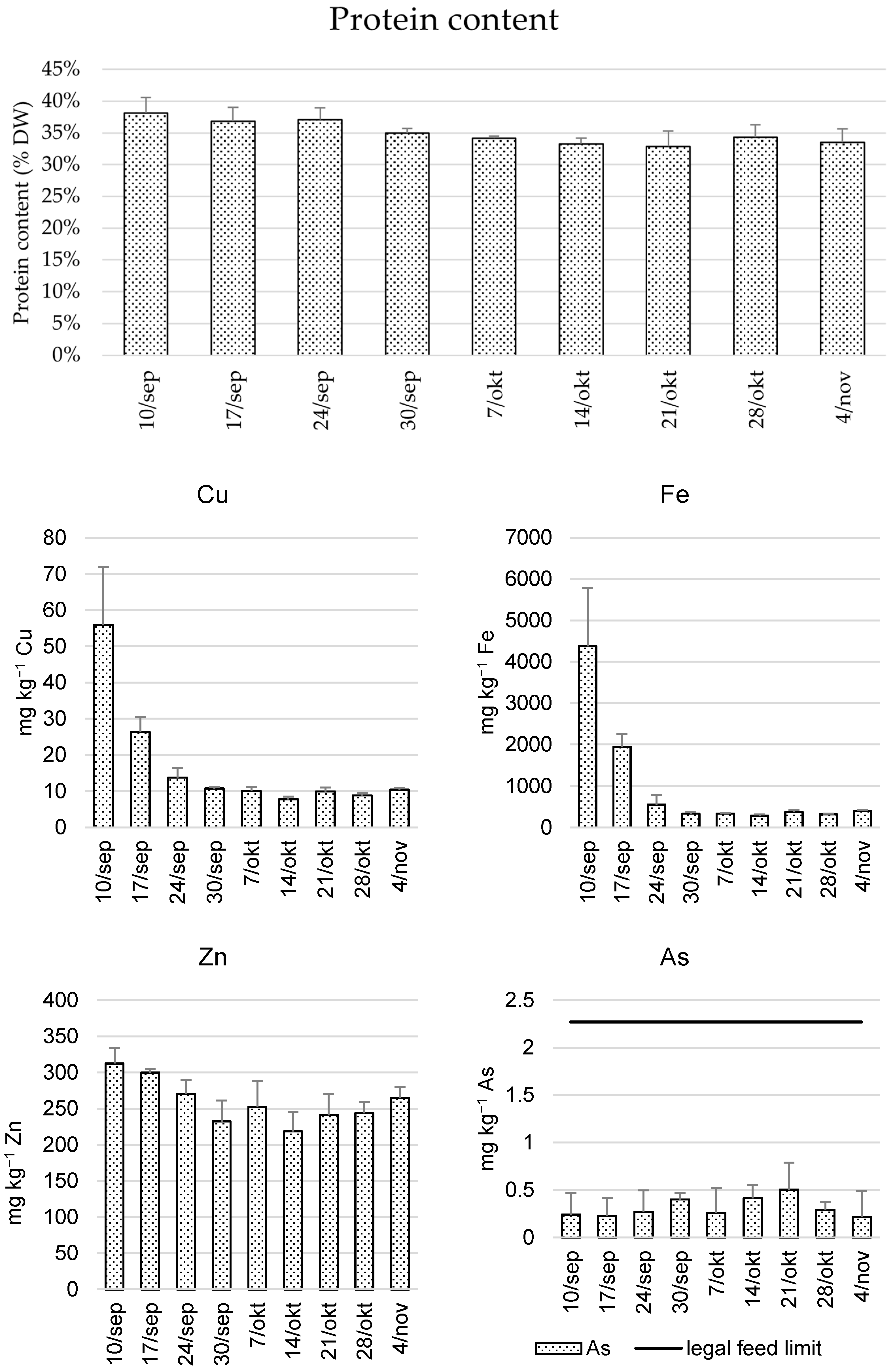
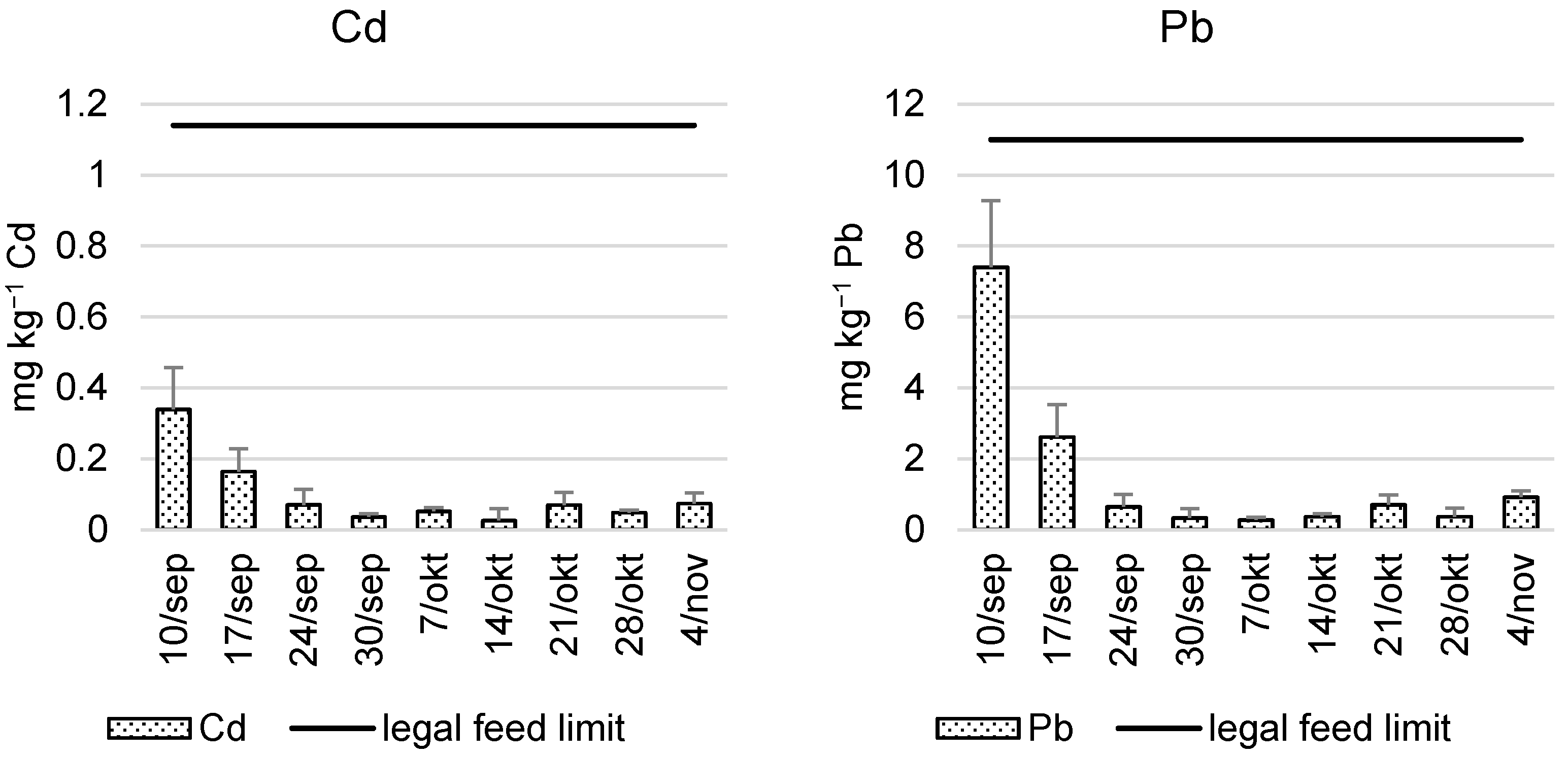
3.3. Feeding Value
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Date | Average Temperature (°C) | Average Relative humidity (%) | Average Light Intensity (µmol m−2 s−1) | Average Photoperiod (h) | Total Precipitation (mm) | |
|---|---|---|---|---|---|---|
| 10/Sep | 13 ± 2 | 82 ± 5 | 112 ± 14 | 6.4 ± 1.9 | 18.9 | |
| 17/Sep | 16 ± 2 | 83 ± 8 | 101 ± 16 | 9.5 ± 2.7 | 0.7 | |
| 24/Sep | 15 ± 0 | 72 ± 3 | 99 ± 15 | 8.4 ± 2.5 | 5.7 | |
| 30/Sep | 15 ± 2 | 91 ± 6 | 106 ± 20 | 5.9 ± 1.4 | 44 | |
| 7/Oct | 13 ± 2 | 86 ± 4 | 155 ± 64 | 3.9 ± 1.9 | 49.7 | |
| 14/Oct | 14 ± 2 | 89 ± 6 | 114 ± 36 | 4.6 ± 3.0 | 17.5 | |
| 21/Oct | 13 ± 2 | 90 ± 5 | 135 ± 69 | 3.7 ± 2.4 | 21.1 | |
| 28/Oct | 13 ± 3 | 90 ± 6 | 92 ± 30 | 4.0 ± 2.6 | 9.4 | |
| 4/Nov | 9 ± 3 | 90 ± 4 | 74 ± 9 | 6.1 ± 1.4 | 23.3 | |
| Average | 14 ± 4 | 83 ± 9 | 109 ± 36 | 7.0 ± 3.5 | Total | 190.3 |
References
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, T.; Kiron, V.; Kaushik, S.J.; Lei, X.G. Meeting global feed protein demand: Challenge, Opportunity, and Strategy. Annu. Rev. Anim. Biosci. 2019, 7, 221–243. [Google Scholar] [CrossRef]
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Sec. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single cell protein-state-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Meul, M.; Ginneberge, C.; Van Middelaar, C.E.; de Boer, I.J.M.; Fremaut, D.; Haesaert, G. Carbon footprint of five pig diets using three land use change accounting methods. Livest. Sci. 2012, 149, 215–223. [Google Scholar] [CrossRef]
- Snauwaert, E.; Vannecke, T. VCM-Enquëte Operationele Stand van Zaken Mestverwerking in Vlaanderen; VCM: Brugge, Belgium, 2017. [Google Scholar]
- OECD. Nutrient Balance (Indicator). Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-compendium-of-agri-environmental-indicators_9789264186217-en (accessed on 14 May 2019).
- Devlamynck, R.; de Souza, M.F.; Michels, E.; Sigurnjak, I.; Donoso, N.; Coudron, C.; Leenknegt, J.; Vermeir, P.; Eeckhout, M.; Meers, E. Agronomic and environmental performance of Lemna minor cultivated on agricultural wastewater Streams—A Practical Approach. Sustainability 2021, 13, 1570. [Google Scholar] [CrossRef]
- Gwaze, F.; Mwale, M. The prospect of duckweed in pig nutrition: A Review. J. Agric. Sci. 2015, 7, 189. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, P.; Liu, F.; Li, H.; Zhang, S.; Xiao, R.; Yin, L.; Zhou, J.; Wu, J. Nitrogen removal and distribution of ammonia-oxidizing and denitrifying genes in an integrated constructed wetland for swine wastewater treatment. Ecol. Eng. 2017, 104, 30–38. [Google Scholar] [CrossRef]
- Li, X.; Wu, S.; Yang, C.; Zeng, G. Microalgal and duckweed based constructed wetlands for swine wastewater treatment: A review. Bioresour. Technol. 2020, 318, 123858. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U.; Niklas, K.J. Darwin-Wallace Demons: Survival of the fastest in populations of duckweeds and the evolutionary history of an enigmatic group of angiosperms. Plant Biol. 2015, 17, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.-J. Relative in vitro growth rates of duckweeds (Lemnaceae)—The most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef]
- Landolt, E.; Kandeler, R. Biosystematic Investigations in the Family of Duckweeds (Lemnaceae), Vol. 4: The Family of LEMNACEAE-a Monographic Study, Vol. 2 Phytochemistry, Physiology; Geobotanische Institut ETH: Zürich, Switzerland, 1987. [Google Scholar]
- Zhao, Y.; Fang, Y.; Jin, Y.; Huang, J.; Bao, S.; Fu, T.; He, Z.; Wang, F.; Wang, M.; Zhao, H. Pilot-scale comparison of four duckweed strains from different genera for potential application in nutrient recovery from wastewater and valuable biomass production. Plant Biol. 2015, 17, 82–90. [Google Scholar] [CrossRef]
- Skillicorn, P.; Spira, W.; Journey, W. Duckweed Aquaculture—A New Aquatic Farming Fystem for Developing Countries; The World Bank: Washington, DC, USA, 1993; ISBN 0-8213-2067-x. [Google Scholar]
- Sikorski, Ł.; Piotrowicz-Cieślak, A.I.; Adomas, B. Phytotoxicity of sodium chloride towards common duckweed (Lemna minor L.) and yellow lupin (Lupinus Luteus L.). Arch. Environ. Prot. 2013, 39, 117–128. [Google Scholar] [CrossRef][Green Version]
- Suzuki, K.; Waki, M.; Yasuda, T.; Fukumoto, Y.; Kuroda, K.; Sakai, T.; Suzuki, N.; Suzuki, R.; Matsuba, K. Distribution of phosphorus, copper and zinc in activated sludge treatment process of swine wastewater. Bioresour. Technol. 2010, 101, 9399–9404. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Chambers, B.J.; Williams, J.R.; Unwin, R.J. Heavy metal contents of livestock feeds and animal manures in England and Wales. Bioresour. Technol. 1999, 70, 23–31. [Google Scholar] [CrossRef]
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef]
- European Commission. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Commun. 2002, 140, 10–23. [Google Scholar]
- Devlamynck, R.; Fernandes de Souza, M.; Bog, M.; Leenknegt, J.; Eeckhout, M.; Meers, E. Effect of the growth medium composition on nitrate accumulation in the novel protein crop Lemna minor. Ecotoxicol. Environ. Saf. 2020, 206, 111380. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.C.; Mc Farlane, J.C. 1. Radiation. In Plant Growth Chamber Handbook; Langhans, R.W., Tibbitts, T.W., Eds.; Iowa State University of Science and Technology: Ames, IA, USA, 1997; pp. 1–30. [Google Scholar]
- Corbala-Robles, L.; Sastafiana, W.N.D.; van Linden, V.; Volcke, E.I.P.; Schaubroeck, T. Life cycle assessment of biological pig manure treatment versus direct land application—A trade-off story. Resour. Conserv. Recycl. 2018, 131, 86–98. [Google Scholar] [CrossRef]
- Bodemkundige Dienst van België. Eindrapport: Valorisatie van Resteffluenten Afkomstig van de Mestverwerking (Samenvatting); BDB: Leuven, Belgium, 2004. [Google Scholar]
- Van Ranst, E.; Verloo, M.; Demeyer, A.; Pauwesl, J.M. Manual for the Soil Chemistry and Fertility Laboratory: Analytical Methods for Soils and Plants Equipment and Management of Consumables; NUGI 835; University of Ghent: Ghent, Belgium, 1999; ISBN 90-76603-01-4. [Google Scholar]
- Casal, J.A.; Vermaat, J.E.; Wiegman, F. A test of two methods for plant protein determination using duckweed. Aquat. Bot. 2000, 67, 61–67. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Tan, L.; Guo, L.; Yang, G.-L.; Li, Q.; Lai, F.; He, K.-Z.; Jin, Y.; Du, A.; Fang, Y.; et al. Increasing starch productivity of Spirodela polyrhiza by precisely control the spectral composition and nutrients status. Ind. Crops Prod. 2019, 134, 284–291. [Google Scholar] [CrossRef]
- Mohedano, R.A.; Costa, R.H.R.; Tavares, F.A.; Belli Filho, P. High nutrient removal rate from swine wastes and protein biomass production by full-scale duckweed ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef]
- Frick, H. Micronutrient tolerance and accumulation in the duckweed, Lemna. J. Plant Nutr. 1985, 8, 1131–1145. [Google Scholar] [CrossRef]
- VLAREM II EMIS Navigator. Available online: https://navigator.emis.vito.be/mijn-navigator?woId=10112 (accessed on 19 February 2018).
- Xu, J.; Cheng, J.J.; Stomp, A.-M. Growing Spirodela polyrrhiza in Swine Wastewater for the Production of Animal Feed and Fuel Ethanol: A Pilot Study. CLEAN Soil Air Water 2012, 40, 760–765. [Google Scholar] [CrossRef]
- Lasfar, S.; Monette, F.; Millette, L.; Azzouz, A. Intrinsic growth rate: A new approach to evaluate the effects of temperature, photoperiod and phosphorus–nitrogen concentrations on duckweed growth under. Water Res. 2007, 41, 2333–2340. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice. In Sustainable Agriculture; Springer Netherlands: Dordrecht, The Netherlands, 2009; Volume 2, pp. 953–986. ISBN 9789048126651. [Google Scholar]
- Liu, C.; Dai, Z.; Sun, H. Potential of duckweed (Lemna minor) for removal of nitrogen and phosphorus from water under salt stress. J. Environ. Manage. 2017, 187, 497–503. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.Lewis Publishers: New York, NY, USA, 1996; ISBN 0-87371-930-1. [Google Scholar]
- Zimmo, O.R.O.; Steen, N.; van der Gijzen, H.J.H.; van der Steen, N.P.; Gijzen, H.J.H. Nitrogen mass balance across pilot-scale algae and duckweed-based wastewater stabilisation ponds. Water Res. 2004, 38, 913–920. [Google Scholar] [CrossRef]
- Meers, E.; Tack, F.M.G.; Tolpe, I.; Michels, E. Application of a Full-scale Constructed Wetland for Tertiary Treatment of Piggery Manure: Monitoring Results. Water. Air. Soil Pollut. 2008, 193, 15–24. [Google Scholar] [CrossRef]
- CBS. Standardised Calculation Methods for Animal Manure and Nutrients; Statistics: Den Haag, The Netherlands, 2012. [Google Scholar]
- European Commission. EU Feed Protein Balance Sheet; EC: Brussels, Belgium, 2020. [Google Scholar]
- Papadopoulos, G.A.; Maes, D.G.D.; Janssens, G.P.J. Mineral accretion in nursing piglets in relation to sow performance and mineral source. Vet. Med. 2009, 54, 41–46. [Google Scholar] [CrossRef]
- Chojnacka, K. Using biosorption to enrich the biomass of seaweeds from the Baltic Sea with microelements to produce mineral feed supplement for livestock. Biochem. Eng. J. 2008, 39, 246–257. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J. 2014, 12. [CrossRef]
- Heuzé, V.; Tran, G.; Kaushik, S. Soybean Meal; Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO. Available online: https://www.feedipedia.org/node/674 (accessed on 14 May 2021).

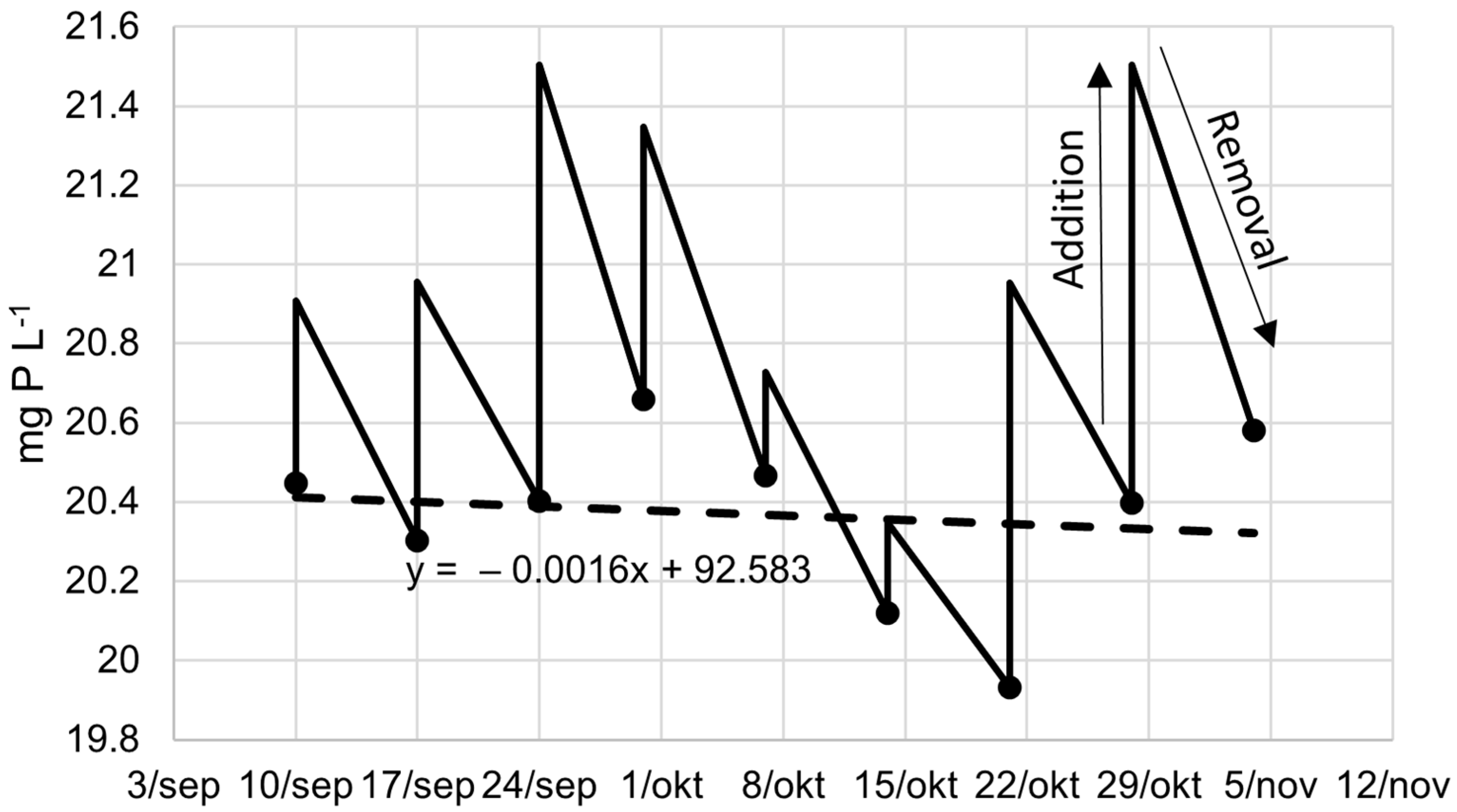
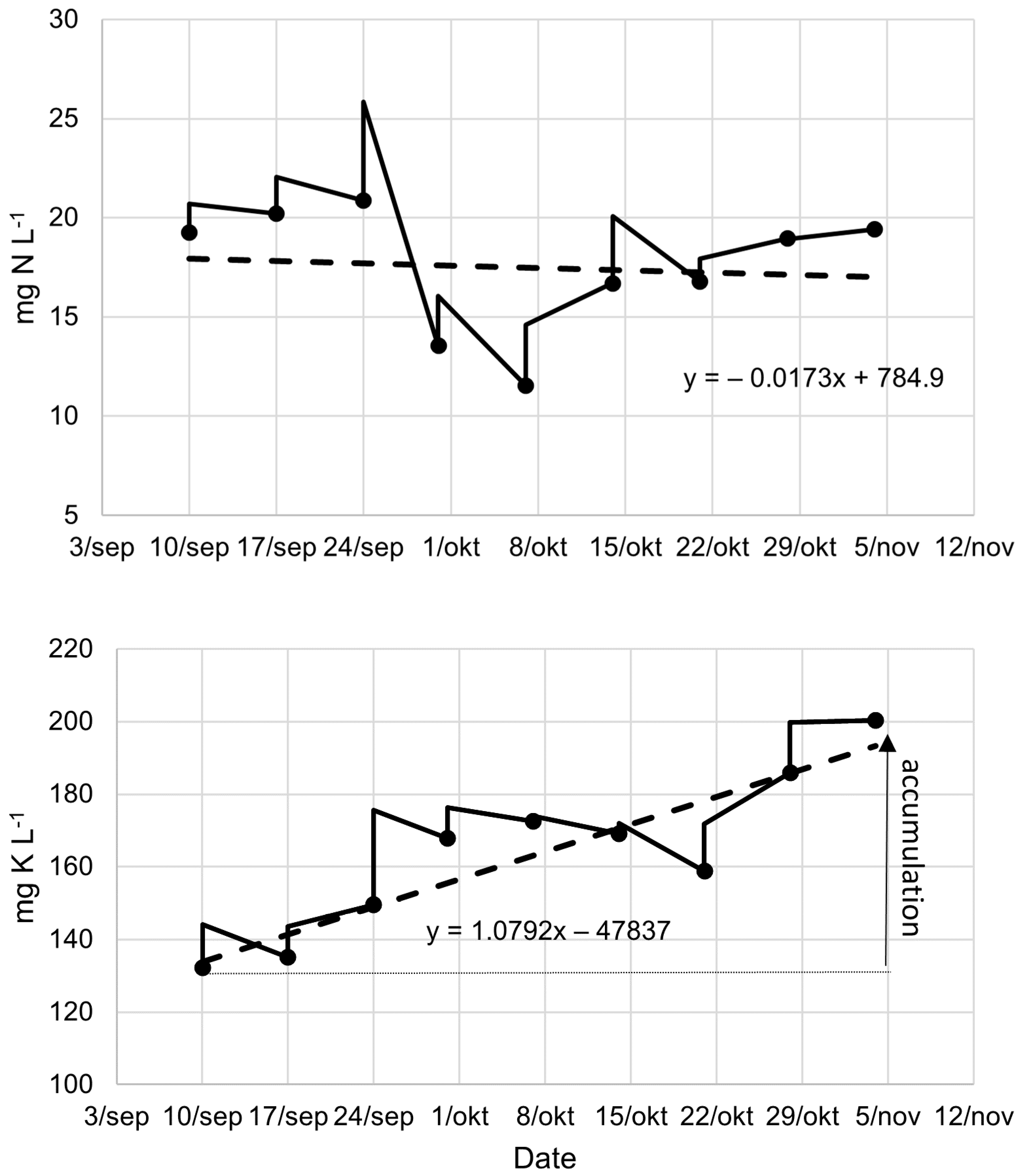
| Total N | Total P | Ratio | Mass | |
|---|---|---|---|---|
| [mg kg−1] | [mg/mg] | [kg] | ||
| LF | 4800 | 225 | 4 | |
| BE | 331 | 247 | 41 | |
| RW | 0 | 0 | 955 | |
| Mixture | 33 | 11 | 3.0 | 1000 |
| Restrictions | 350 | 11 | 3.0 |
| Start (10 September 2019) | End (28 October 2019) | Optimal Growing Ranges | Maximal Growing Ranges | Unit | |
|---|---|---|---|---|---|
| pH | 7.0 ± 0.1 | 6.8 ± 0.1 | 6.5–7.5 α | 5.0–9.0 α | |
| EC | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.6–1.4 α | <10.9 α | mS/cm |
| T-N | 19 ± 2 | 19 ± 1 | 2.8–350 α | <2100 α | mg L−1 |
| NO3- | 0.2 ± 0.2 | 5.6 ± 0.3 | 2.8–350 α | <2100 α | mg L−1 |
| PO43- | 0.8 ± 0.0 | 0.5 ± 0 | 0.4–11 α | <55 α | mg L−1 |
| T-P | 20 ± 0 | 21 ± 0 | 0.4–11 α | <55 α | mg L−1 |
| K | 132 ± 9 | 200 ± 13 | 39–780 α | <2000 α | mg L−1 |
| Cl | 16 ± 3 | 29 ± 3 | 0.4–36 α | <3500 α | mg L−1 |
| T-S | 17 ± 1 | 23 ± 2 | 48–1900 α | <4800 α | mg L−1 |
| Ca | 15 ± 0 | 17 ± 0 | 20–400 α | <2000 α | mg L−1 |
| Mg | 5.9 ± 0.3 | 8.8 ± 0.7 | 5.0–97 α | <1200 α | mg L−1 |
| Fe | 0.53 ± 0.07 | 0.51 ± 0.06 | <27.9 β | <100 β | mg L−1 |
| Mn | 16 ± 14 | 9 ± 7 | <54,900 β | <274,500 β | µg L−1 |
| Cu | 8.3 ± 6.3 | 3.4 ± 2.6 | <3200 β | <6300 β | µg L−1 |
| Zn | 28 ± 23 | 11 ± 8 | <6500 β | <65,300 β | µg L−1 |
| As | <LOD | <LOD | |||
| Cd | <LOD | <LOD | |||
| Pd | <LOD | <LOD |
| Parameter | Accumulation Rate | Unit | p-Value | Interpretation |
|---|---|---|---|---|
| pH | 0.432 | Constant | ||
| EC | 3.88 | mS cm−1 d−1 | 0.042 | Accumulation |
| T-N | 0.541 | Constant | ||
| NO3− | 0.12 | mg L−1 d−1 | 0.000 | Accumulation |
| PO43− | −0.004 | mg L−1 d−1 | 0.000 | Depletion |
| T-P | 0.685 | Constant | ||
| K | 1.09 | mg L−1 d−1 | 0.000 | Accumulation |
| Cl | 0.18 | mg L−1 d−1 | 0.000 | Accumulation |
| T-S | 0.11 | mg L−1 d−1 | 0.000 | Accumulation |
| Ca | 0.037 | mg L−1 d−1 | 0.0004 | Accumulation |
| Mg | 0.045 | mg L−1 d−1 | 0.000 | Accumulation |
| Fe | 0.363 | Constant | ||
| Mn | −0.15 | µg L−1 d−1 | 0.022 | Depletion |
| Cu | 0.402 | Constant | ||
| Zn | 0.662 | Constant | ||
| As | <LOD | <LOD | ||
| Cd | <LOD | <LOD | ||
| Pd | <LOD | <LOD |
| Content | Uptake | Removal | Relative Uptake | |
|---|---|---|---|---|
| [mg g−1 DW] | [mg m−2 d−1] | [mg m−2 d−1] | ||
| N | 60 ± 4 | 264 ± 123 | 1107 ± 715 | 24% |
| P | 13 ± 1 | 58 ± 31 | 149 ± 150 | 39% |
| K | 52 ± 10 | 233 ± 85 | 72 ± 3002 | 102% |
| S | 6.1 ± 0.8 | 27 ± 15 | 47 ± 318 | 59% |
| Mg | 2.8 ± 0.2 | 12 ± 5 | 32 ± 103 | 38% |
| Ca | 11 ± 1 | 44 ± 18 | 242 ± 383 | 18% |
| Fe | 1 ± 1 | 4.7 ± 14.5 | 44 ± 63 | 11% |
| Zn | 0.25 ± 0.04 | 1.2 ± 0.7 | 2.4 ± 2.8 | 49% |
| [µg g−1 DW] | [µg m−2 d−1] | [µg m−2 d−1] | ||
| Cu | 16 ± 15 | 82 ± 179 | 910 ± 1100 | 9% |
| As | 0.32 ± 0.19 | 1.2 ± 2.0 | <LOD | |
| Cd | 0.1 ± 0.1 | 0.43 ± 1.05 | <LOD | |
| Pb | 3.0 ± 5.2 | 0.41 ± 11.36 | <LOD |
| Feeding Standard/Material | Mn | Zn | Cu | Fe |
|---|---|---|---|---|
| [mg kg−1 DM] | [mg kg−1 DM] | [mg kg−1 DM] | [mg kg−1 DM] | |
| Feeding standard for laying hens | 60–70 | 50–60 | 5–6 | 60–70 |
| Feeding standard for swine | 30–40 | 20–165 | 70–150 | 90–100 |
| Maximum limit piglets & sows | <150 | |||
| Corn (grain) | 5 | 15 | 3.3 | 26 |
| Oat (grain) | 38 | 18 | 3.3 | 52 |
| Wheat (grain) | 26 | 23 | 2.7 | 43 |
| Rye (grain) | 58 | 26 | 3.3 | 60 |
| Potatoes (steamed) | 3 | 5 | 1.1 | 16 |
| Fodder yeasts | 14 | 9 | 12.6 | 90 |
| Soybean meal | 44 | 57 | 17 | 201 |
| Duckweed | 410 ± 60 | 250 ± 40 | 10 ± 2 | 372 ± 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devlamynck, R.; de Souza, M.F.; Leenknegt, J.; Jacxsens, L.; Eeckhout, M.; Meers, E. Lemna minor Cultivation for Treating Swine Manure and Providing Micronutrients for Animal Feed. Plants 2021, 10, 1124. https://doi.org/10.3390/plants10061124
Devlamynck R, de Souza MF, Leenknegt J, Jacxsens L, Eeckhout M, Meers E. Lemna minor Cultivation for Treating Swine Manure and Providing Micronutrients for Animal Feed. Plants. 2021; 10(6):1124. https://doi.org/10.3390/plants10061124
Chicago/Turabian StyleDevlamynck, Reindert, Marcella Fernandes de Souza, Jan Leenknegt, Liesbeth Jacxsens, Mia Eeckhout, and Erik Meers. 2021. "Lemna minor Cultivation for Treating Swine Manure and Providing Micronutrients for Animal Feed" Plants 10, no. 6: 1124. https://doi.org/10.3390/plants10061124
APA StyleDevlamynck, R., de Souza, M. F., Leenknegt, J., Jacxsens, L., Eeckhout, M., & Meers, E. (2021). Lemna minor Cultivation for Treating Swine Manure and Providing Micronutrients for Animal Feed. Plants, 10(6), 1124. https://doi.org/10.3390/plants10061124