Adonis amurensis Is a Promising Alternative to Haematococcus as a Resource for Natural Esterified (3S,3′S)-Astaxanthin Production
Abstract
1. Introduction
2. Results
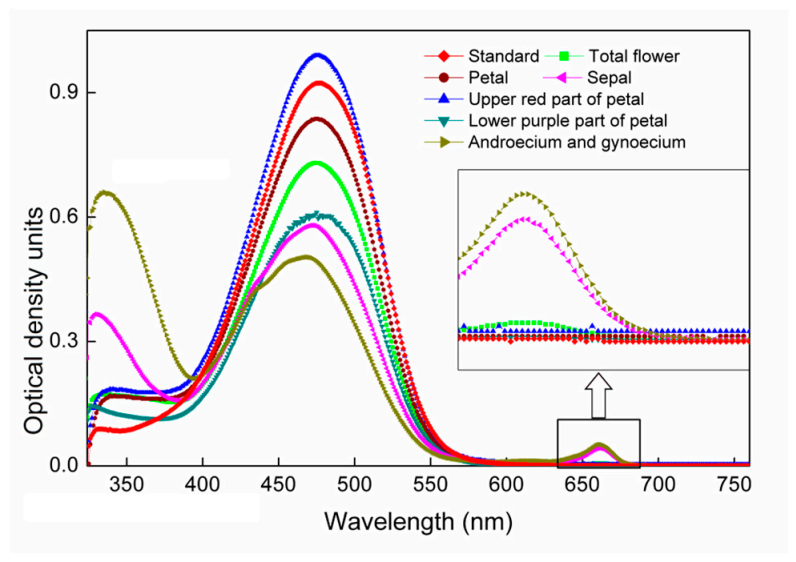
2.1. UV-Visible Absorption Spectra of Acetone Extracts
2.2. Pigment Contents of Flower Parts
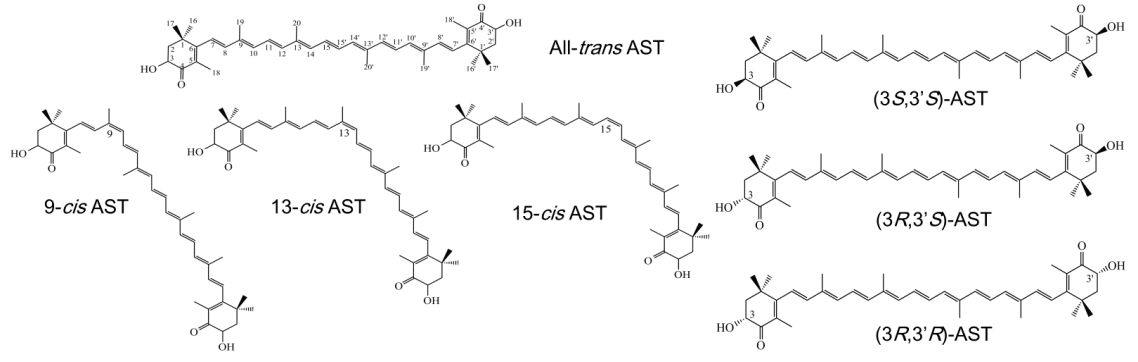
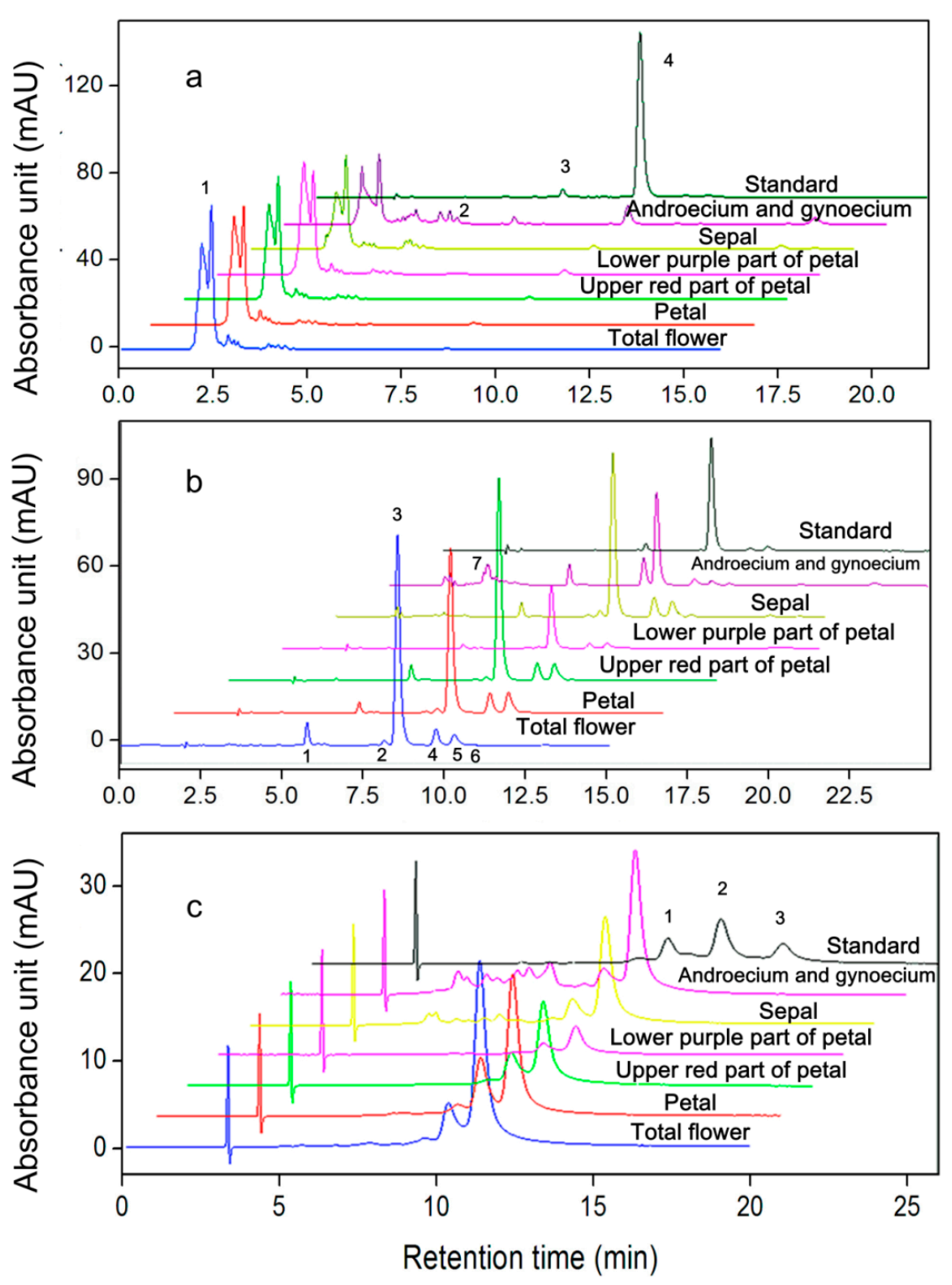
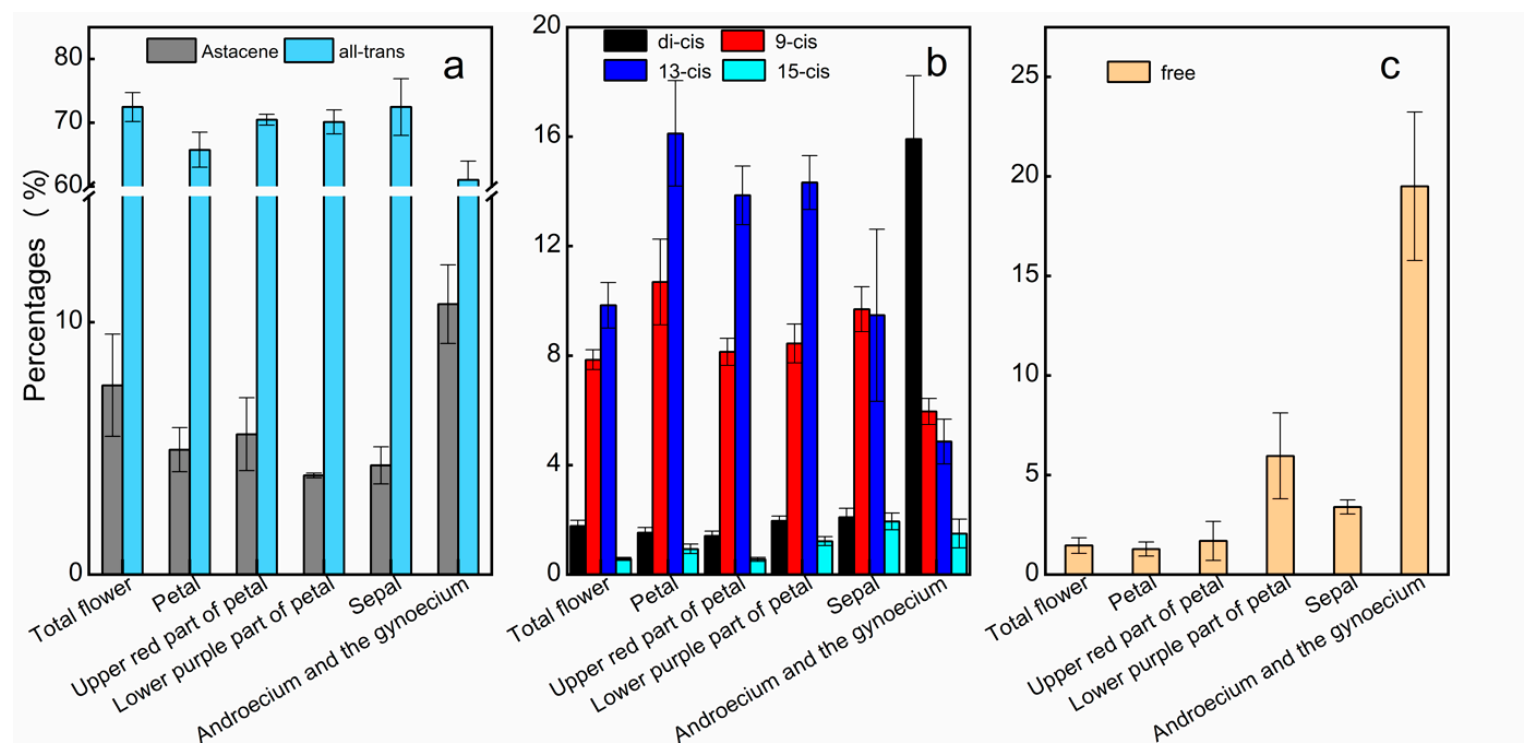
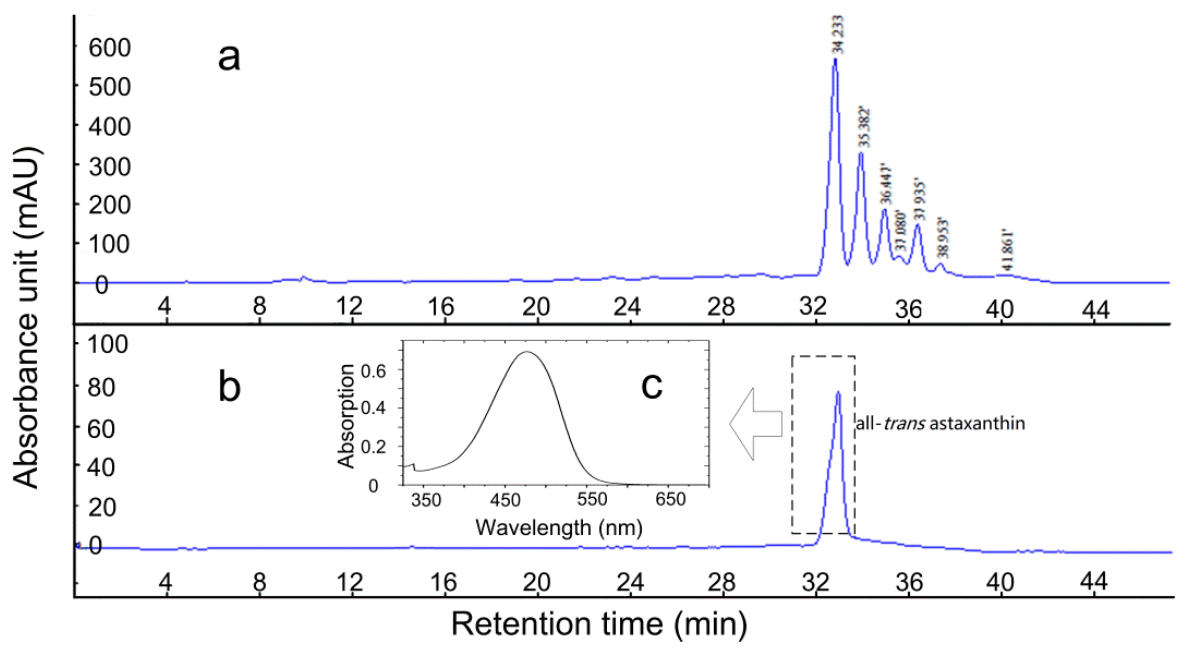
2.3. AST Isomers of Flower Parts
2.4. Isolation of the All-Trans Isomer of (3S,3′S)-AST
3. Discussion
3.1. Distribution of AST and Its Isomers in Adonis Flowers
3.2. Potential Uses of Adonis Flower AST
3.3. Adonis Flower as a Promising Source of AST
4. Materials and Methods
4.1. Plant Materials and Reagents
4.2. Preparation of Pigment Extracts from Adonis
4.3. Quantification of Pigments
4.4. Identification of Carotenoids by HPLC
4.5. Preparation of the All-Trans Isomer of (3S,3′S)-AST
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Liu, X.H.; Wang, B.J.; Li, Y.F.; Wang, L.; Liu, J.G. Effects of dietary botanical and synthetic astaxanthin on E/Z and R/S isomer composition, growth performance, and antioxidant capacity of white shrimp, Litopenaeus vannamei, in the nursery phase. ISJ Invert. Surviv. J. 2018, 15, 131–140. [Google Scholar]
- Liu, J.G.; Meer, J.V.D.; Zhang, L.; Zhang, Y. Cultivation of Haematococcus pluvialis for Astaxanthin Production. In Microalgal Production for Biomass and High-Value Products; Slocombe, S.P., Benemann, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 267–294. [Google Scholar]
- Zhang, C.S.; Su, F.; Li, S.H.; Yu, Y.; Xiang, J.H.; Liu, J.G.; Li, F.H. Isolation and identification of the main carotenoid pigment from a new variety of the ridgetail white prawn Exopalaemon carinicauda. Food Chem. 2018, 269, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs. 2011, 9, 447–465. [Google Scholar] [CrossRef]
- Schultz, H. Despite Some Exaggerations, Astaxanthin demand Growing Strongly, Expert Says. NutraIngredients-USA. 2018. Available online: https://www.nutraingredients-usa.com/Article/2018/08/30/Despite-some-exaggerations-astaxanthin-demand-growing-strongly-expert-says (accessed on 10 March 2019).
- Wan, X.; Zhou, X.R.; Moncalian, G.; Su, L.; Chen, W.C.; Zhu, H.Z.; Chen, D.; Gong, Y.M.; Huang, F.H.; Deng, Q.C. Reprogramming microorganisms for the biosynthesis of astaxanthin via metabolic engineering. Prog. Lipid Res. 2020, 81, 101083. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Nakada, T.; Ota, S. What is the correct name for the type of Haematococcus Flot. (Volvocales, Chlorophyceae)? Taxon 2016, 65, 343–348. [Google Scholar] [CrossRef]
- Zhang, C.H.; Zhang, L.T.; Liu, J.G. The role of photorespiration during astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae). Plant Physiol. Biochenm. 2016, 107, 75–81. [Google Scholar] [CrossRef]
- Tsubokura, A.; Yoneda, H.; Mizuta, H. Paracoccus carotinifaciens sp. nov., a new aerobic gram-negative astaxanthin-producing bacterium. Int. J. Syst. Bacteriol. 1999, 49, 277–282. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S.; Choi, T.J.; Lee, W.J.; Kim, Y.T. Paracoccus haeundaensis sp. nov., a Gram-negative, halophilic, astaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1699–1702. [Google Scholar] [CrossRef]
- Maj, A.; Dziewit, L.; Drewniak, L.; Garstka, M.; Krucon, T.; Piatkowska, K.; Gieczewska, K.; Czarnecki, J.; Furmanczyk, E.; Lasek, R.; et al. In vivo creation of plasmid pCRT01 and its use for the construction of carotenoid producing Paracoccus spp. strains that grow efficiently on industrial wastes. Microb. Cell Fact. 2020, 19, 141. [Google Scholar] [CrossRef]
- Ye, L.; Xie, W.; Zhou, P.; Yu, H. Biotechnological production of astaxanthin through metabolic engineering of yeasts. Chembioeng. Rev. 2015, 2, 107–117. [Google Scholar] [CrossRef]
- Mann, V.; Harker, M.; Pecker, I.; Hirschberg, J. Metabolic engineering of astaxanthin production in tobacco flowers. Nat. Biotech. 2000, 18, 888. [Google Scholar] [CrossRef]
- Farré, G.; Perez-Fons, L.; Decourcelle, M.; Breitenbach, J.; Hem, S.; Zhu, C.; Capell, T.; Christou, P.; Fraser, P.; Sandmann, G. Metabolic engineering of astaxanthin biosynthesis in maize endosperm and characterization of a prototype high oil hybrid. Transgenic Res. 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Chelebieva, E.S.; Chubchikova, I.N.; Dantsyuk, N.V.; Drobetskaya, I.V.; Sakhon, E.G.; Chivkunova, O.B.; Chekanov, K.A.; Lobakova, E.S.; Sidorov, R.A.; et al. pH and CO2 effects on Coelastrella (Scotiellopsis) rubescens growth and metabolism. Russ. J. Plant. Physiol. 2016, 63, 566–574. [Google Scholar] [CrossRef]
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of carotenogenic microalgae in the White Sea polar region. FEMS Microbial. Ecol. 2020, 96, fiz183. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Wijffels, R.H.; Martens, D.E. Dynamics of biomass composition and growth during recovery of nitrogen-starved Chromochloris zofingiensis. Appl. Microbiol. Biotechnol. 2015, 99, 1873–1884. [Google Scholar] [CrossRef]
- Su, F.; Huang, B.; Liu, J.G. The carotenoid characteristics of cultured shrimps in China. J. Crustacean Biol. 2018, 38, 523–530. [Google Scholar] [CrossRef]
- Su, F.; Liu, J.G. The carotenoid characteristics of the important wild shrimp Trachysalambria curvirostris (Stimpson, 1860) in China. J. Ocean. Limnol. 2019, 37, 706–712. [Google Scholar] [CrossRef]
- Yu, W.J.; Liu, J.G. Astaxanthin isomers: Selective distribution and isomerization in aquatic animals. Aquaculture 2020, 520, 734915. [Google Scholar] [CrossRef]
- Yang, C.; Hassan, Y.I.; Liu, R.H.; Zhang, H.; Chen, Y.H.; Zhang, L.F.; Tsao, R. Antiinflammatory effects of different astaxanthin isomers and the roles of lipid transporters in the cellular transport of astaxanthin isomers in Caco-2 cell monolayers. J. Agric. Food Chem. 2019, 67, 6222–6231. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.F.; Zhang, H.; Sun, Q.R.; Liu, R.H.; Li, J.; Wu, L.Y.; Tsao, R. Rapid and Efficient Conversion of All-E-astaxanthin to 9Z- and 13Z-Isomers and Assessment of Their Stability and Antioxidant Activities. J. Agric. Food Chem. 2017, 65, 818–826. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2014, 12, 145–152. [Google Scholar] [CrossRef]
- Wang, C.L.; Armstrong, D.W.; Chang, C.D. Rapid baseline separation of enantiomers and a mesoform of all-trans-astaxanthin, 13-cis-astaxanthin, adonirubin, and adonixanthin in standards and commercial supplements. J. Chromatogr. A 2008, 1194, 172–177. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Zhang, L.H.; Peng, Y.J.; Xu, X.D.; Wang, S.N.; Yu, L.M.; Hong, Y.M.; Ma, J.P. Determination of other related carotenoids substances in astaxanthin crystals extracted from Adonis amurensis. J. Oleo Sci. 2015, 64, 751–759. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Hassanien, R.H.E.; Li, M.; Lin, W.D. Advanced applications of solar energy in agricultural greenhouses. Renew. Sust. Energ. Rev. 2016, 54, 989–1001. [Google Scholar] [CrossRef]
- Yang, W.H.; Zhang, X.W.; Xu, W.J.; Huang, H.Y.; Ma, Y.R.; Bai, H.; Gong, J.; Ni, S.F. Overview of pharmacological research on Adonis L. Agric. Sci. Technol. 2015, 16, 626–628. [Google Scholar]
- Cunningham, F.X.; Gantt, E. A study in scarlet: Enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis. Plant. J. 2005, 41, 478–492. [Google Scholar] [CrossRef]
- Cunningham, F.X.; Gantt, E. Elucidation of the pathway to astaxanthin in the flowers of Adonis aestivalis. Plant. Cell 2011, 23, 3055–3069. [Google Scholar] [CrossRef]
- Maoka, T.; Etoh, T.; Kishimoto, S.; Sakata, S. Carotenoids and their fatty acid esters in the petals of Adonis aestivalis. J. Oleo Sci. 2011, 60, 47–52. [Google Scholar] [CrossRef]
- Renstrøm, B.; Berger, H.; Liaaen-Jensen, S. Esterified, optical pure (3S,3′S)-astaxanthin from flowers of Adonis annua. Biochem. Syst. Ecol. 1981, 9, 249–250. [Google Scholar] [CrossRef]
- Rodney, M. Astaxanthin from Flowers of the Genus Adonis. U.S. Patent 5453565, 26 September 1995. [Google Scholar]
- Rayton, S.; Rayton, J.; Foley, L. Ketocarotenoids from Adonis palaestina. International Patent Application WO/2006/11934, 2 February 2006. [Google Scholar]
- Tan, A.M.; Tao, Z.G.; Wu, X.L.; Zhao, D.H. Method for Extracting Astaxanthin from Adonis vernalis Flowers. Chinese Patent 20101191682, 28 May 2010. [Google Scholar]
- Kamata, T.; Simpson, K.L. Study of astaxanthin diester extracted from Adonis aestivalis. Comp. Biochem. Phys. B 1987, 86, 587–591. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Arai, S.; Mori, T.; Miki, W.; Yamaguchi, K.; Konosu, S.; Satake, M.; Fujita, T. Pigmentation of juvenile coho salmon with carotenoid oil extracted from Antarctic Krill. Aquaculture 1987, 66, 255–264. [Google Scholar] [CrossRef]
- Lambertsen, G.; Braekkan, O.R. Method of analysis of astaxanthin and its occurrence in some marine products. J. Sci. Food Agric. 2010, 22, 99–101. [Google Scholar] [CrossRef]
- Greaves, R.F.; Woollard, G.A.; Hoad, K.E.; Walmsley, T.A.; Johnson, L.A.; Briscoe, S.; Koetsier, S.; Harrower, T.; Gill, J.P. Laboratory medicine best practice guideline: Vitamins a, e and the carotenoids in blood. Clin. Biochem. Rev. 2014, 35, 81–113. [Google Scholar] [PubMed]
- Fan, Y.; Cai, H.X.; Li, S.; Wang, X.P. The Ultraviolet Absorption Spectra of the Chlorophyll. J. Light Scatt. 2011, 23, 80–82. [Google Scholar]
- Coral-Hinostroza, G.N.; Ytrestoyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’ R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Phys. C 2004, 139, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Østerlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Følling, M.; Lagocki, S.; Storebakken, T.; Olli, J.J.; Alsted, N. Bioavailability of all-E-astaxanthin and Z-isomers of astaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture 1997, 157, 63–82. [Google Scholar] [CrossRef]
- Schiedt, K.; Bischof, S.; Glinz, E. Recent progress on carotenoid metabolism in animals. Pure Appl. Chem. 1991, 63, 89–100. [Google Scholar] [CrossRef]
- Schiedt, K.; Bischof, S.; Glinz, E. Metabolism of carotenoids and in vivo racemization of (3S,3′S)-astaxanthin in the crustacean Penaeus. Methods Enzymol. 1993, 214, 148–168. [Google Scholar]
- Maoka, T.; Kawashima, Y.; Takaki, M. Structures of yellow xantophylls and metabolism of astaxanthin in the prawn Penaeus japonicus. J. Oleo Sci. 2018, 67, 1425–1433. [Google Scholar] [CrossRef]
- Morison, W.L. Effects of ultraviolet radiation on the immune system in humans. Photochem. Photobiol. 1989, 50, 515. [Google Scholar] [CrossRef]
- Alarcón-Alarcón, C.; Inostroza-Riquelme, M.; Torres-Gallegos, C.; Araya, C.; Miranda, M.; Sánchez-Caamaño, J.C.; Moreno-Villoslada, I.; Oyarzun-Ampuero, F.A. Protection of astaxanthin from photodegradation by its inclusion in hierarchically assembled nano and microstructures with potential as food. Food Hydrocoll. 2018, 83, 36–44. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 551–1558. [Google Scholar] [CrossRef]
- Kamata, T.; Neamtu, G.; Tanaka, Y.; Sameshima, M.; Simpson, K.L. Utilization of Adonis aestivalis as a dietary pigment source for rainbow trout Salmo gairdneri. Nippon Suisan Gakk. 1990, 56, 783–788. [Google Scholar] [CrossRef]
- Su, F.; Yu, W.; Liu, J. Comparison of effect of dietary supplementation with Haematococcus pluvialis powder and synthetic astaxanthin on carotenoid composition, concentration, esterification degree and astaxanthin isomers in ovaries, hepatopancreas, carapace, epithelium of adult female Chinese mitten crab (Eriocheir sinensis). Aquaculture 2020, 523, 735146. [Google Scholar]
- Shi, K.; Su, F.; Guo, W.; Hu, F.W.; Pang, T.; Gao, Z.; Liu, J.G. Application of Haematococcus pluvialis residues in the adult rainbow trout culture. Jiangsu Agric. Sci. 2017, 45, 153–157. (In Chinese) [Google Scholar]
- Li, H.; Liu, J.G. Effects of defatted Haematococcus pluvialis meal (DHPM) supplementation on the growth performance, and the carotenoid content and composition in the rotifer (Brachionus plicatilis). Aquaculture 2019, 505, 34–40. [Google Scholar] [CrossRef]
- Storebakken, T.; Sørensen, M.; Bjerkeng, B.; Harris, J.; Monahan, P.; Hiu, S. Stability of astaxanthin from red yeast, Xanthophyllomyces dendrorhous, during feed processing: Effects of enzymatic cell wall disruption and extrusion temperature. Aquaculture 2004, 231, 489–500. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Safety and efficacy of Panaferd-AX (red carotenoidrich bacterium Paracoccus carotinifaciens) as feed additive for salmon and trout—Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed. EFSA J. 2007, 546, 1–30. [Google Scholar] [CrossRef]
- Huang, Q.J. Pigment Crops-Field Cultural Techniques of Adonis amurensis. Chin. Hortic. Abstr. 2011, 11, 176–177. (In Chinese) [Google Scholar]
- Li, J.; Xiu, J.Q. Difference analysis of wheat yield and planting cost in main producing areas of China. Agric. Dev. Financ. 2016, 10, 44–47. (In Chinese) [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts Phenoloxidases in Beta Vulgaris. Plant. Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A.; Alderson, R.; Sandham, C.; Dingwall, A.; Mitchell, D.; Selkirk, C.; Nickell, D.; Baker, R.; Robertson, B.; Whyte, D. Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L.). Aquaculture 2000, 189, 335–349. [Google Scholar] [CrossRef]
- Su, F.; Xu, H.R.; Yang, N.; Liu, W.; Liu, J.G. Hydrolytic efficiency and isomerization during de-esterification of natural astaxanthin esters by saponification and enzymolysis. Electron. J. Biotechn. 2018, 34, 37–42. [Google Scholar] [CrossRef]
- Capelli, B.; Cysewski, G. Natural Astaxanthin: King of the Carotenoids; Cyanotech Corporation: Kona-Kailua, HI, USA, 2007; ISBN 13: 9780979235306. [Google Scholar]
- Gong, F.Y.; Zhang, C.H.; Zhang, L.T.; Liu, J.G. Changes of carotenoids contents and analysis of astaxanthin geometrical isomerization in Haematococcus pluvialis under outdoor high light conditions. Aquac. Res. 2020, 51, 770–778. [Google Scholar] [CrossRef]
- Qiu, D.; Wu, Y.C.; Zhu, W.L.; Yin, H.; Yi, L.T. Identification of geometrical isomers and comparison of different isomeric samples of astaxanthin. J. Food Sci. 2012, 77, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613–1622. [Google Scholar] [CrossRef] [PubMed]


| Sample | Moisture (%) | Carotenoids (dw, %) | Chlorophyll a (dw, %) | Chlorophyll b (dw, %) |
|---|---|---|---|---|
| Total flower | 69.13 | 1.31 ± 0.21 | 0.029 ± 0.003 | 0.021 ± 0.005 |
| Petal | 71.97 | 2.58 ± 0.10 | N. D. | 0.019 ± 0.005 |
| Upper Red part of petal | 67.72 | 3.31 ± 0.04 | N. D. | 0.027 ± 0.007 |
| Lower Purple part of petal | 73.85 | 1.21 ± 0.06 | N. D. | 0.010 ± 0.001 |
| Sepal | 67.70 | 0.32 ± 0.07 | 0.074 ± 0.013 | 0.029 ± 0.005 |
| Androecium and gynoecium | 69.42 | 0.14 ± 0.00 | 0.035 ± 0.002 | 0.018 ± 0.001 |
| Species | Astaxanthin Content | Proportion of Geometric Isomers (% of AST) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All-trans | 9-cis | 13-cis | 15-cis | di-cis | |||||
| Plant | Microalga | Haematococcus pluvialis | 0.2–3.93 (% dw) | 80–90 | 7–10 | 2–8 | <1 | N.D. | 42 |
| Higher plant | Adonis amurensis | 1.31 (% dw) | 72 | 7.8 | 9.8 | 0.57 | 1.8 | This study | |
| Aquatic animals | Shrimp | Litopenaeus vannamei | 1.9–271.4 (mg kg−1ww) | 72–85 | 5–10 | 7–14 | <2.5 | <2 | [2,23] |
| Penaeus monodon | 26.2–105.4 (mg kg−1ww) | 70–80 | 6–15 | 2–17 | 2–4 | <9 | [23] | ||
| Fenneropenaeus chinensis | 1.9–137.7 (mg kg−1ww) | 63–76 | 4–10 | 11–16 | <9 | <9 | [23] | ||
| Exopalaemon carinicauda | 3.1–25.6 (mg kg−1ww) | 72–92 | 3–9 | 5–17 | <2 | <3 | [4,23] | ||
| Trachysalambria curvirostris | 11.0–106.0 (mg kg−1ww) | 65–75 | 4–12 | 11–16 | <4 | 4–7 | [24] | ||
| Crab | Eriocheir sinensis | 12.9–343.4 (mg kg−1ww) | 80–97 | <6 | 1–8 | 1–13 | N.D. | [60] | |
| Fish | Oncorhychus mykiss | 2.1–4.3 (mg kg−1ww) | 87–90 | 1–2 | 8–12 | N.D. | N.D. | [61] | |
| Zooplankton | Rotifer | Brachionus plicatilis | 0.06–0.6 (mg g−1ww) | 82–92 | 7–10 | 2–3 | 0.5–5 | N.D. | [62] |
| Cladoceran | Moina macrocopa | 0.043–0.059 (mg g−1ww) | >90 | N.D. | <10 | N.D. | N.D. | Unpublished data | |
| Yeast/Bacteria | Phaffia rhodozyma | 11.4–13.4 (mg g−1ww) | 70–78 | 2–3 | 15–21 | 4 | 2–3 | [63] | |
| Paracoccus carotinifaciens | 21.8 (mg g−1ww) | 95.5 | 1.7 | 2.8 | N.D. | N.D. | [64] | ||
| Resource | Adonis Flower | Haematococcus pluvialis | ||
|---|---|---|---|---|
| Location | Inner Mongolia | Livadeia [32] | Amsterdam [32] | Shenzhen [45] |
| Production of biomass (kg/ha/year, dw) | 1125 a | 18,280 | 6150 | 450 |
| Astaxanthin content in the biomass | 1.31% | --- | --- | 2.50% |
| Production of astaxanthin (kg/ha/year) | 14.74 | 426 | 143 | 11.25 |
| Production costs of astaxanthin (€/kg) | 388–393 b | 1536–1857 | 6403–6723 | 632 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Gong, F.; Guo, S.; Yu, W.; Liu, J. Adonis amurensis Is a Promising Alternative to Haematococcus as a Resource for Natural Esterified (3S,3′S)-Astaxanthin Production. Plants 2021, 10, 1059. https://doi.org/10.3390/plants10061059
Li Y, Gong F, Guo S, Yu W, Liu J. Adonis amurensis Is a Promising Alternative to Haematococcus as a Resource for Natural Esterified (3S,3′S)-Astaxanthin Production. Plants. 2021; 10(6):1059. https://doi.org/10.3390/plants10061059
Chicago/Turabian StyleLi, Yongfu, Fengying Gong, Shuju Guo, Wenjie Yu, and Jianguo Liu. 2021. "Adonis amurensis Is a Promising Alternative to Haematococcus as a Resource for Natural Esterified (3S,3′S)-Astaxanthin Production" Plants 10, no. 6: 1059. https://doi.org/10.3390/plants10061059
APA StyleLi, Y., Gong, F., Guo, S., Yu, W., & Liu, J. (2021). Adonis amurensis Is a Promising Alternative to Haematococcus as a Resource for Natural Esterified (3S,3′S)-Astaxanthin Production. Plants, 10(6), 1059. https://doi.org/10.3390/plants10061059







