Hibiscus sabdariffa, a Treatment for Uncontrolled Hypertension. Pilot Comparative Intervention
Abstract
1. Introduction
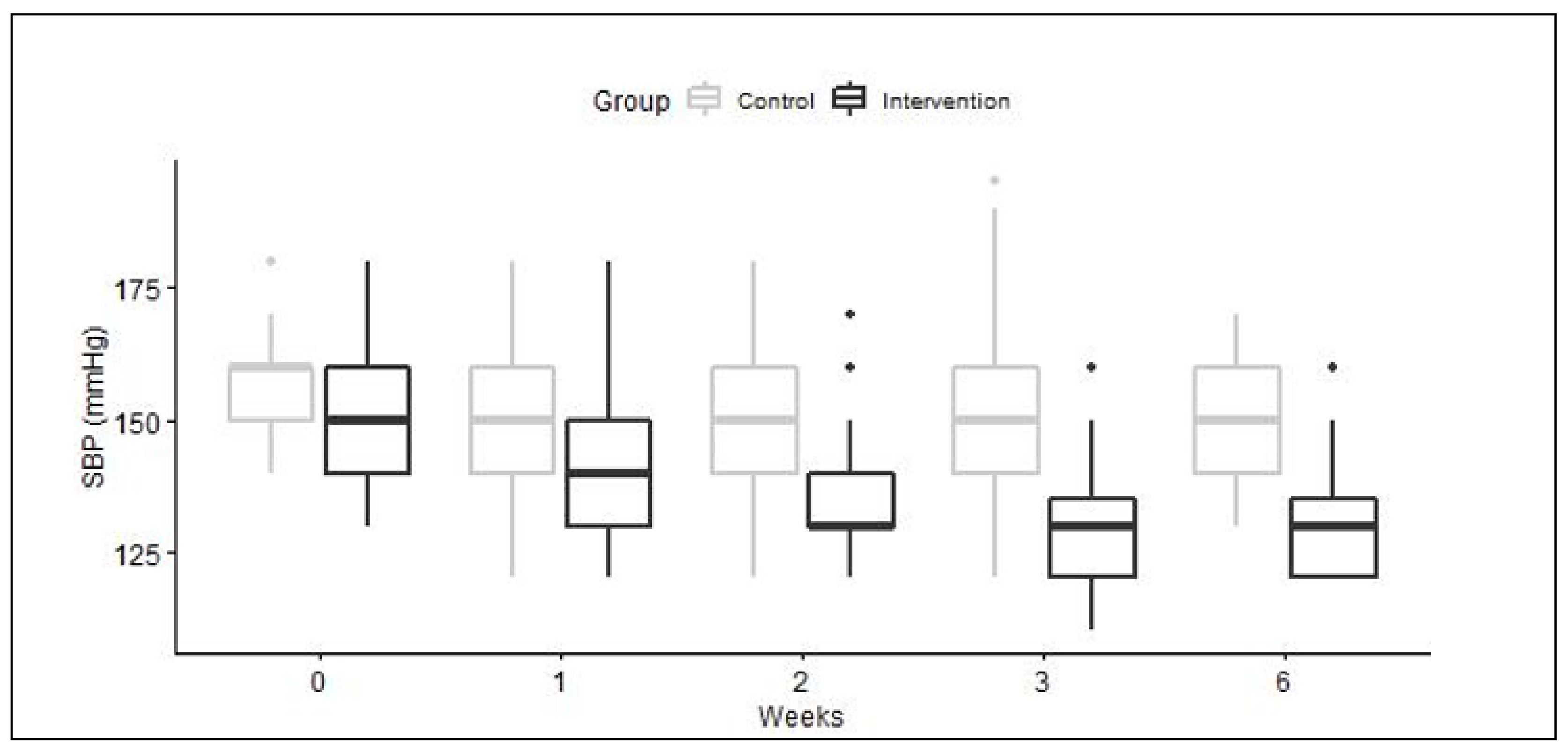
2. Results
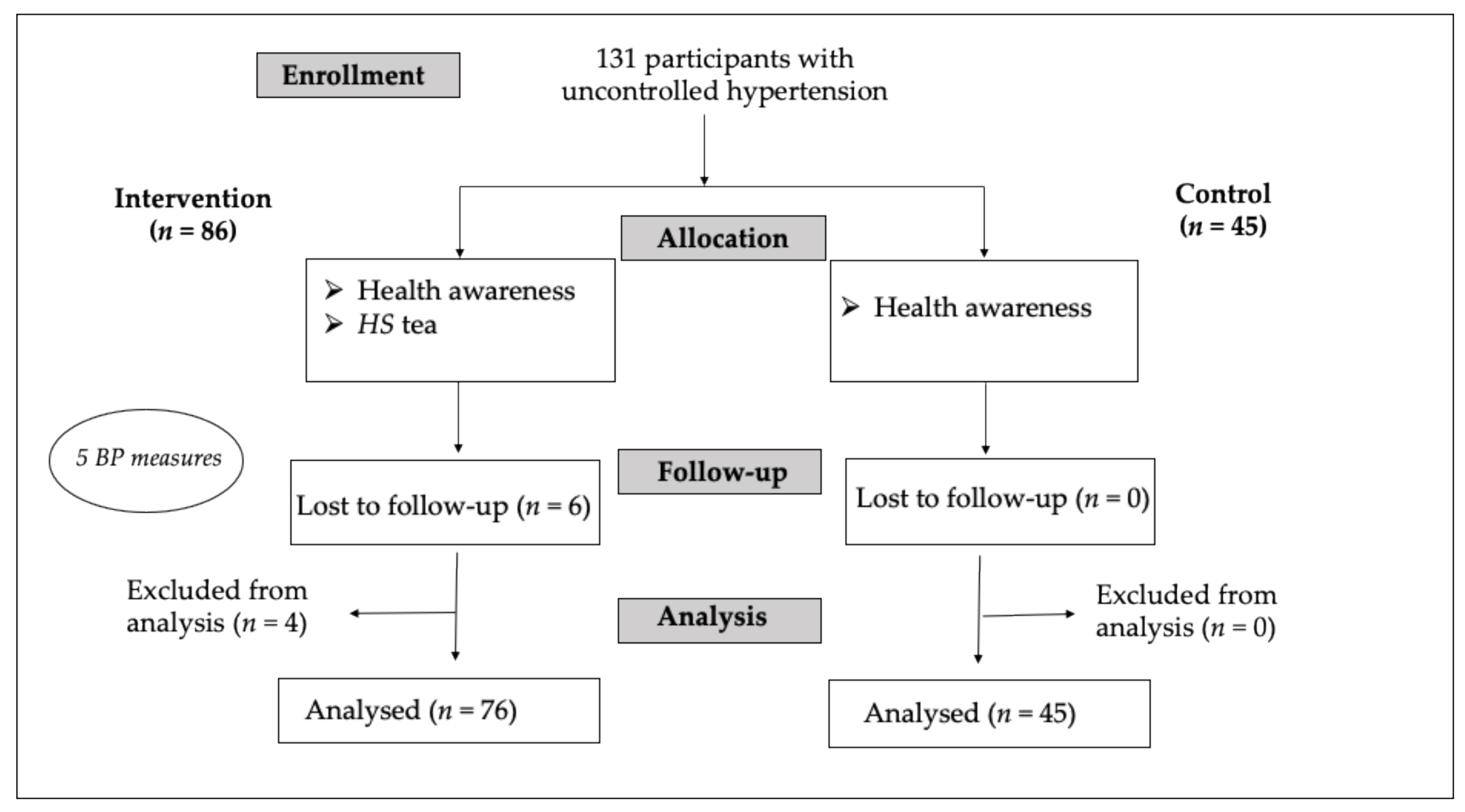
2.1. Recruitment and Baseline Characteristics
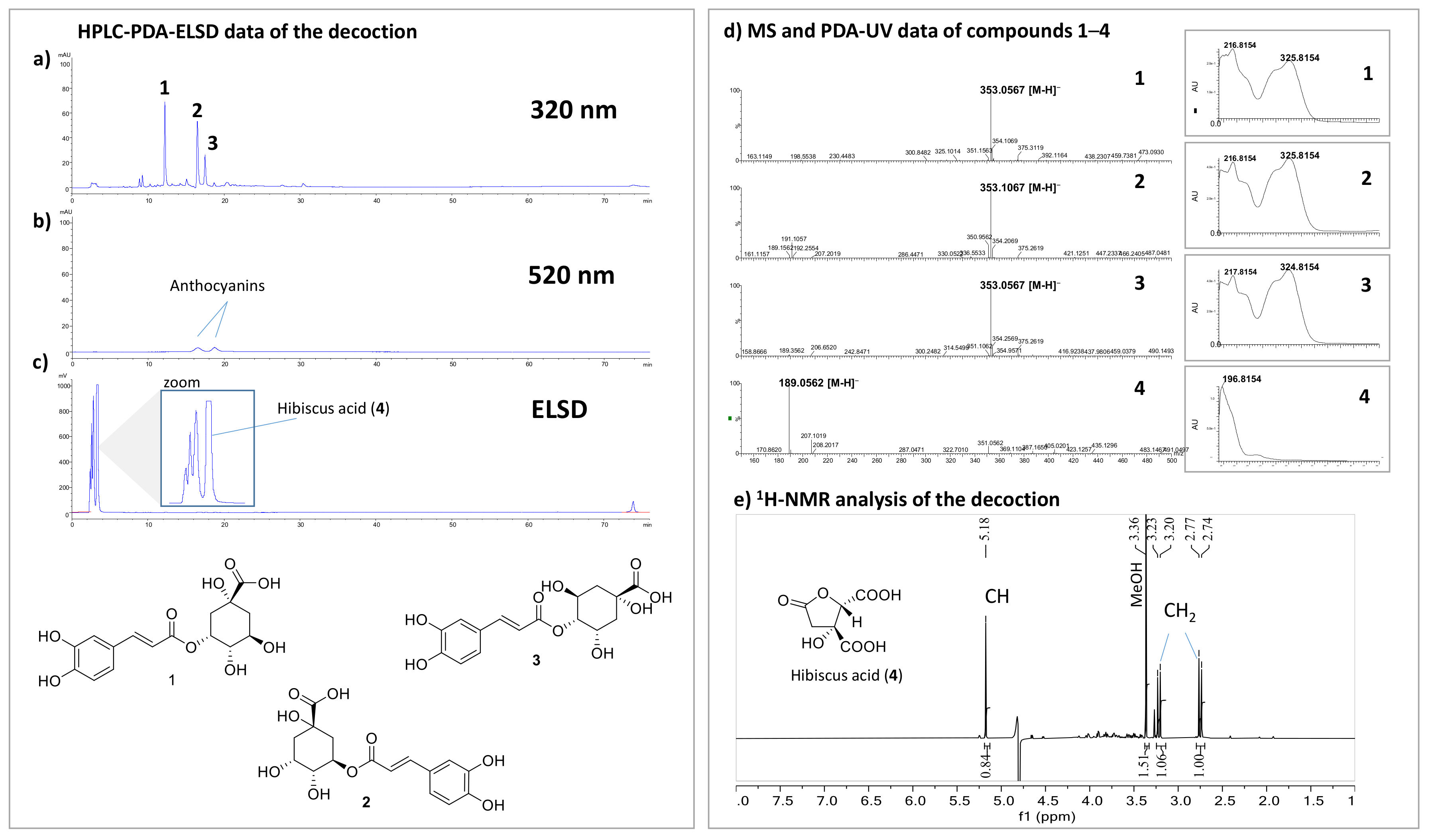
2.2. Chemical Composition of HS Decoction
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Design
4.2. Participants
- Inclusion criteria:
- Age > 18 years.
- Systolic BP (SBP) ≥ 140 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg, with or without ongoing antihypertensive medication.
- No evidence of cardiovascular, renal, or retinal complication.
- 2.
- Exclusion criteria:
- Hypertensive crisis requiring urgent medication.
- Overt kidney failure (serum creatinine > 1.4 mg/dL).
- Pregnant or lactating women (excluded on principle, although there is no evidence of any problems encountered with the tested food product).
- Previous adverse reaction to HS.
4.3. Intervention
4.4. Measurement Procedures
4.5. Ethical Issues
4.6. Outcome Measurements
- 1.
- SBP and DBP change after 6 weeks.
- 2.
- Proportion of participants reaching target BP (< 140/90 mmHg) after 6 weeks.
- 3.
- Percentage of participants for whom the SBP change was clinically significant (defined as a decrease of at least 10 mmHg).
- 4.
- Adverse events (any new symptoms, plausibility of a causal link).
- 5.
- Interaction with other medication, plausibility of a causal link.
- 6.
- Need to increase HS dosage during follow-up.
4.7. Statistical Analysis
4.8. General Experimental Procedures for the Chemical Analysis
4.9. Plant Material
4.10. HS Decoction Chemical Content Analysis
4.10.1. HPLC-PDA-ELSD Analysis of the HS Decoction
4.10.2. Quantitation of Anthocyanins
4.10.3. Identification and Quantitation of Hibiscus Acid by 1H-NMR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Haji Faraji, M.; Haji Tarkhani, A. The effect of sour tea (Hibiscus sabdariffa) on essential hypertension. J. Ethnopharmacol. 1999, 65, 231–236. [Google Scholar] [CrossRef]
- Herrera-Arellano, A.; Flores-Romero, S.; Chavez-Soto, M.A.; Tortoriello, J. Effectiveness and tolerabil-ity of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: A controlled and randomized clinical trial. Phytomedicine 2004, 11, 375–382. [Google Scholar] [CrossRef]
- Herrera-Arellano, A.; Miranda-Sánchez, J.; Ávila-Castro, P.; Herrera-Alvarez, S.; Jiménez-Ferrer, J.; Zamilpa, A.; Román-Ramos, R.; Ponce-Monter, H.; Tortoriello, J. Clinical Effects Produced by a Standardized Herbal Medicinal Product of Hibiscus sabdariffa on Patients with Hypertension. A Randomized, Double-blind, Lisinopril-Controlled Clinical Trial. Planta Med. 2006, 73, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Zarrabal, O.; Barradas-Dermitz, D.M.; Orta-Flores, Z.; Hayward-Jones, P.M.; Nolasco-Hipólito, C.; Aguilar-Uscanga, M.G.; Miranda-Medina, A.; Bin Bujang, K. Hibiscus sabdariffa L., roselle calyx, from ethnobotany to pharmacology. J. Exp. Pharmacol. 2012, 4, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Boushehri, S.N.; Karimbeiki, R.; Ghasempour, S.; Ghalishourani, S.; Pourmasoumi, M.; Hadi, A.; Mbabazi, M.; Pour, Z.K.; Assarroudi, M.; Mahmoodi, M.; et al. The efficacy of sour tea (Hibiscus sabdariffa L.) on selected cardiovascular disease risk factors: A systematic review and meta-analysis of randomized clinical trials. Phytotherapy Res. 2020, 34, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Zheoat, A.M.; Gray, A.I.; Igoli, J.O.; Ferro, V.A.; Drummond, R.M. Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia 2019, 134, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bourqui, A.; Niang, E.A.B.; Graz, B.; Diop, E.A.; Dahaba, M.; Thiaw, I.; Soumare, K.; Valmaggia, P.; Nogueira, R.C.; Cavin, A.L.; et al. Hypertension treatment with Combretum micranthum or Hibiscus sabdariffa, as decoction or tablet: A randomized clinical trial. J. Hum. Hypertens 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- AL-Anbaki, M.; Nogueira, R.C.; Cavin, A.L.; AL-Hadid, M.; AL-Ajlouni, I.; Shuhaiber, L.; Graz, B. Treat-ing Uncontrolled Hypertension with Hibiscus sabdariffa When Standard Treatment Is Insufficient: Pilot Intervention. J. Altern. Complementary Med. 2019, 25, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- UNHCR. Global Focus Iraq [Online]. UNHCR. 2020. Available online: https://reporting.unhcr.org/iraq (accessed on 28 July 2020).
- Pharmacopee Francaise. Dosage des Anthocyanides Dans la Vigne Rouge (Vitis vinifera), 11th ed.; ANSN: Saint-Denis, France, 1996. [Google Scholar]
- Wang, J.; Cao, X.; Ferchaud, V.; Qi, Y.; Jiang, H.; Tang, F.; Yue, Y.; Chin, K.L. Variations in chemical finger-prints and major flavonoid contents from the leaves of thirty-one accessions of Hibiscus sabdariffa L. Biomed. Chromatogr. 2016, 30, 8807. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green Coffee Bean Extract and Its Metabolites Have a Hypotensive Effect in Spontaneously Hypertensive Rats. Hypertens. Res. 2002, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Keasley, J.; Oyebode, O.; Shantikumar, S.; Proto, W.; McGranahan, M.; Sabouni, A.; Kidy, F. A systematic review of the burden of hypertension, access to services and patient views of hypertension in humanitarian crisis settings. BMJ Glob. Health 2020, 5, e002440. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009, 338, b1665. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.; Mashoto, K.O.; Rumisha, S.F.; Malebo, H.M.; Shija, A.; Oriyo, N. Iron and Ascorbic Acid Content in Hibiscus sabdariffa Calyces in Tanzania: Modeling and Optimization of Extraction Conditions. Int. J. Food Sci. Nutr. Eng. 2014, 4, 27–35. [Google Scholar]
- Ghiasi, S.S.; Jalalyazdi, M.; Ramezani, J.; Izadi-Moud, A.; Madani-Sani, F.; Shahlaei, S. Effect of Hibiscus sabdariffa on blood pressure in patients with stage 1 hypertension. J. Adv. Pharm. Technol. Res. 2019, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Zheoat, A.M.; Gray, A.I.; Igoli, J.O.; Kennedy, A.R.; Ferro, V.A. Crystal structures of hibiscus acid and hi-biscus acid dimethyl ester isolated from Hibiscus sabdariffa (Malvaceae). Acta Crystallogr. Sect. E: Crystallogr. Commun. 2017, 73, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Holzgrabe, U. Quantitative NMR spectroscopy in pharmaceutical applications. Prog. Nucl. Magn. Reson. Spectrosc. 2010, 57, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.F.; Chen, S.-N.; Simmler, C.; Lankin, D.C.; Gödecke, T.; Jaki, B.U.; Friesen, J.B.; McAlpine, J.B.; Napolitano, J.G. Importance of Purity Evaluation and the Potential of Quantitative1H NMR as a Purity Assay. J. Med. Chem. 2014, 57, 9220–9231. [Google Scholar] [CrossRef] [PubMed]
| Intervention n = 76 | Control n = 45 | |
|---|---|---|
| Age (mean ± SD) | 51.0 ± 10.3 | 53.5 ± 12.8 |
| Gender (%Female) | 60.5 | 48.9 |
| % on anti-hypertensive medication | 61.8 | 88.9 |
| Baseline SBP | 151.6 ± 11.7 | 155.9 ± 10.6 |
| Baseline DBP | 93.9 ± 8.8 | 88.7 ± 12.2 |
| SBP after 6 weeks | 128.6 ± 9.2 | 151.4 ± 10.7 |
| DBP after 6 weeks | 81.9 ± 7.7 | 85.1 ± 7.9 |
| Mean reduction SBP * | 23.1 ± 11.8 | 4.4 ± 10.2 |
| Mean reduction DBP ** | 12.0 ± 11.2 | 3.6 ± 8.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Anbaki, M.; Cavin, A.-L.; Nogueira, R.C.; Taslimi, J.; Ali, H.; Najem, M.; Shukur Mahmood, M.; Abdullah Khaleel, I.; Saad Mohammed, A.; Ramadhan Hasan, H.; et al. Hibiscus sabdariffa, a Treatment for Uncontrolled Hypertension. Pilot Comparative Intervention. Plants 2021, 10, 1018. https://doi.org/10.3390/plants10051018
Al-Anbaki M, Cavin A-L, Nogueira RC, Taslimi J, Ali H, Najem M, Shukur Mahmood M, Abdullah Khaleel I, Saad Mohammed A, Ramadhan Hasan H, et al. Hibiscus sabdariffa, a Treatment for Uncontrolled Hypertension. Pilot Comparative Intervention. Plants. 2021; 10(5):1018. https://doi.org/10.3390/plants10051018
Chicago/Turabian StyleAl-Anbaki, Marwah, Anne-Laure Cavin, Renata Campos Nogueira, Jaafar Taslimi, Hayder Ali, Mohammed Najem, Mustafa Shukur Mahmood, Ibrahim Abdullah Khaleel, Abdulqader Saad Mohammed, Hasan Ramadhan Hasan, and et al. 2021. "Hibiscus sabdariffa, a Treatment for Uncontrolled Hypertension. Pilot Comparative Intervention" Plants 10, no. 5: 1018. https://doi.org/10.3390/plants10051018
APA StyleAl-Anbaki, M., Cavin, A.-L., Nogueira, R. C., Taslimi, J., Ali, H., Najem, M., Shukur Mahmood, M., Abdullah Khaleel, I., Saad Mohammed, A., Ramadhan Hasan, H., Marcourt, L., Félix, F., Vinh Tri Low-Der’s, N., Ferreira Queiroz, E., Wolfender, J.-L., Watissée, M., & Graz, B. (2021). Hibiscus sabdariffa, a Treatment for Uncontrolled Hypertension. Pilot Comparative Intervention. Plants, 10(5), 1018. https://doi.org/10.3390/plants10051018







