Investigating the Invasion Pattern of the Alien Plant Solanum elaeagnifolium Cav. (Silverleaf Nightshade): Environmental and Human-Induced Drivers
Abstract
1. Introduction
2. Results
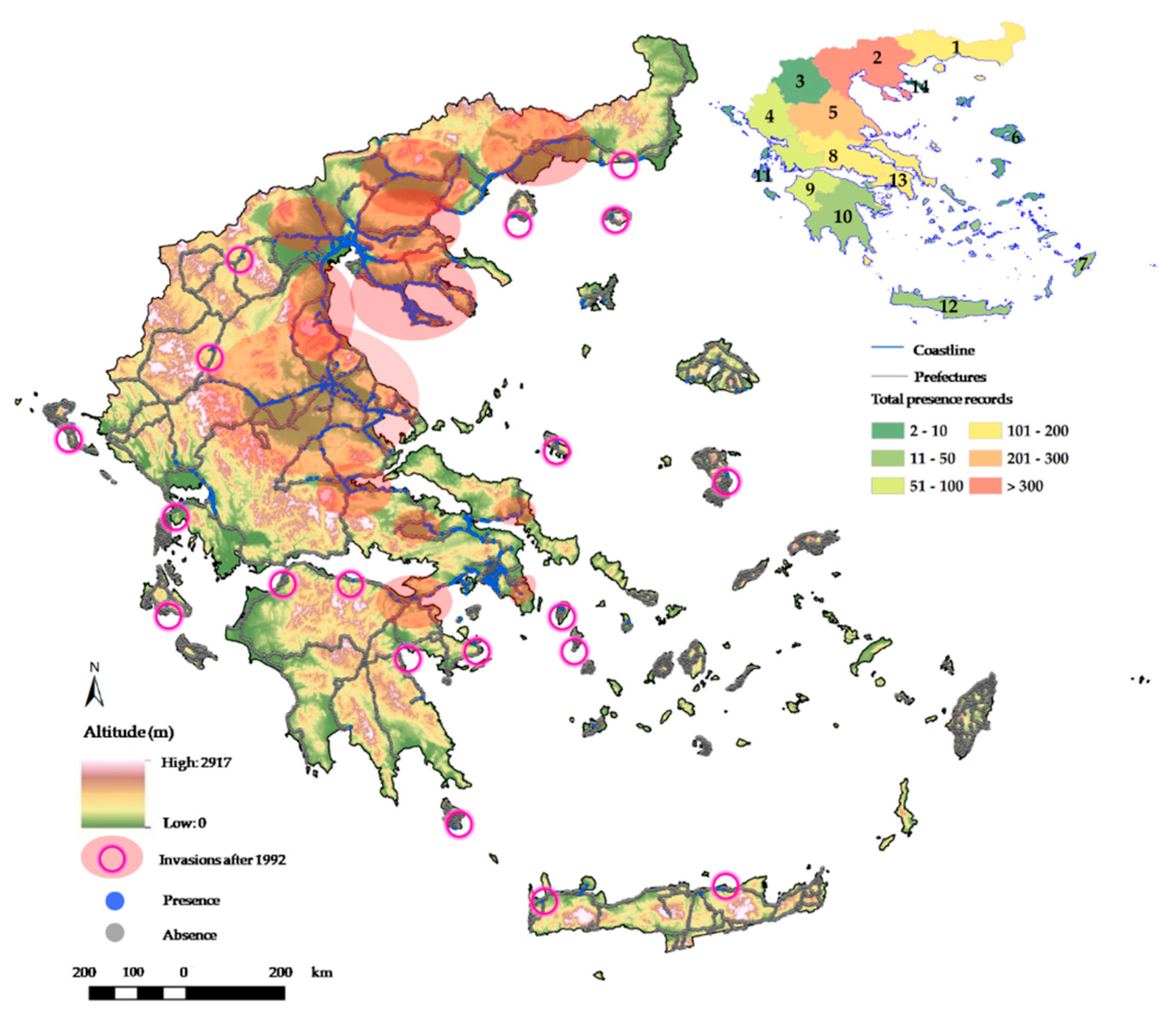
2.1. Distribution of Solanum Elaeagnifolium in Greece
2.2. Elevation and Soil Types as Possible Underlying Factors
2.3. Climate as Possible Underlying Factor
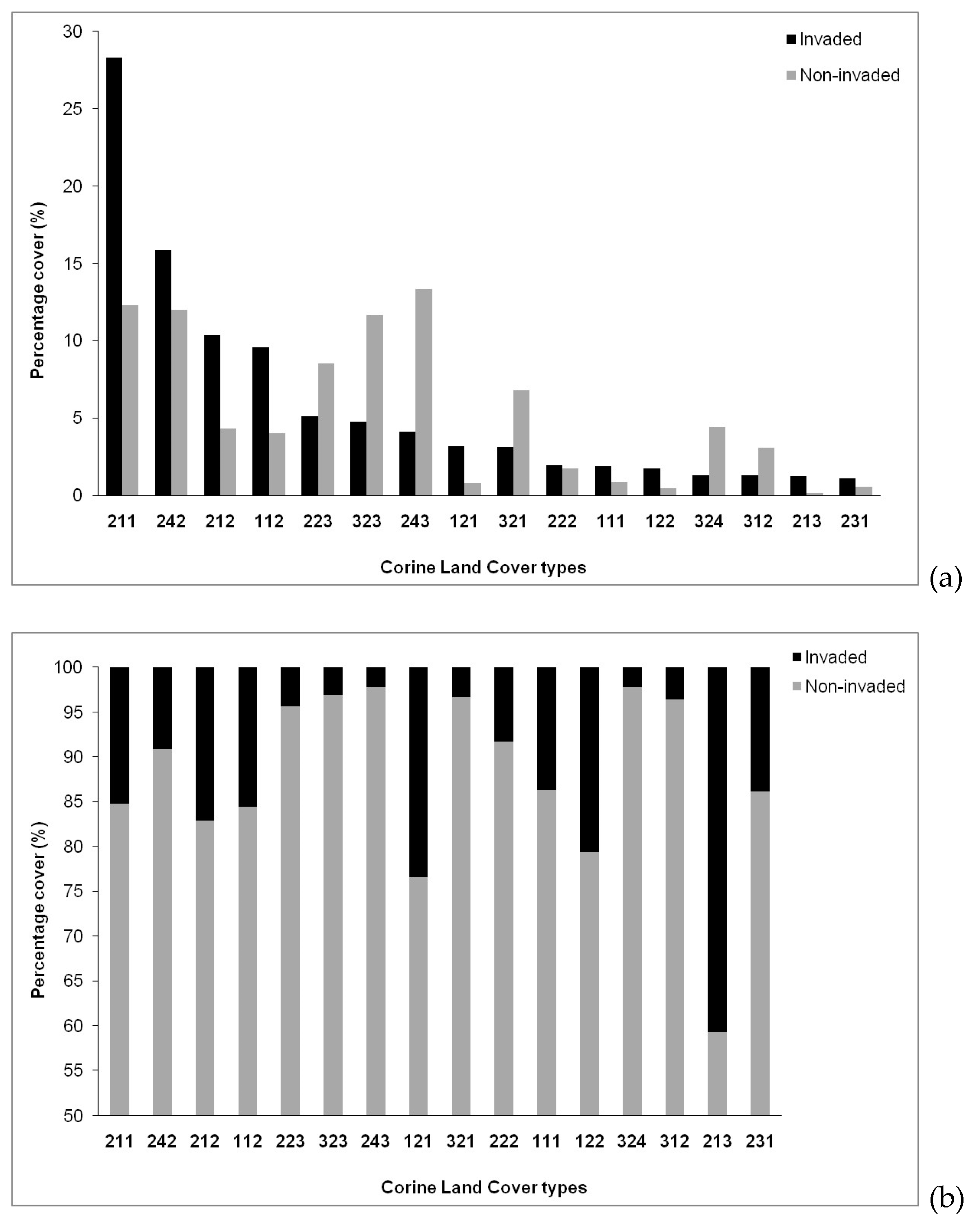
2.4. Land Uses as Underlying Factor
2.5. Road Type as Underlying Factor
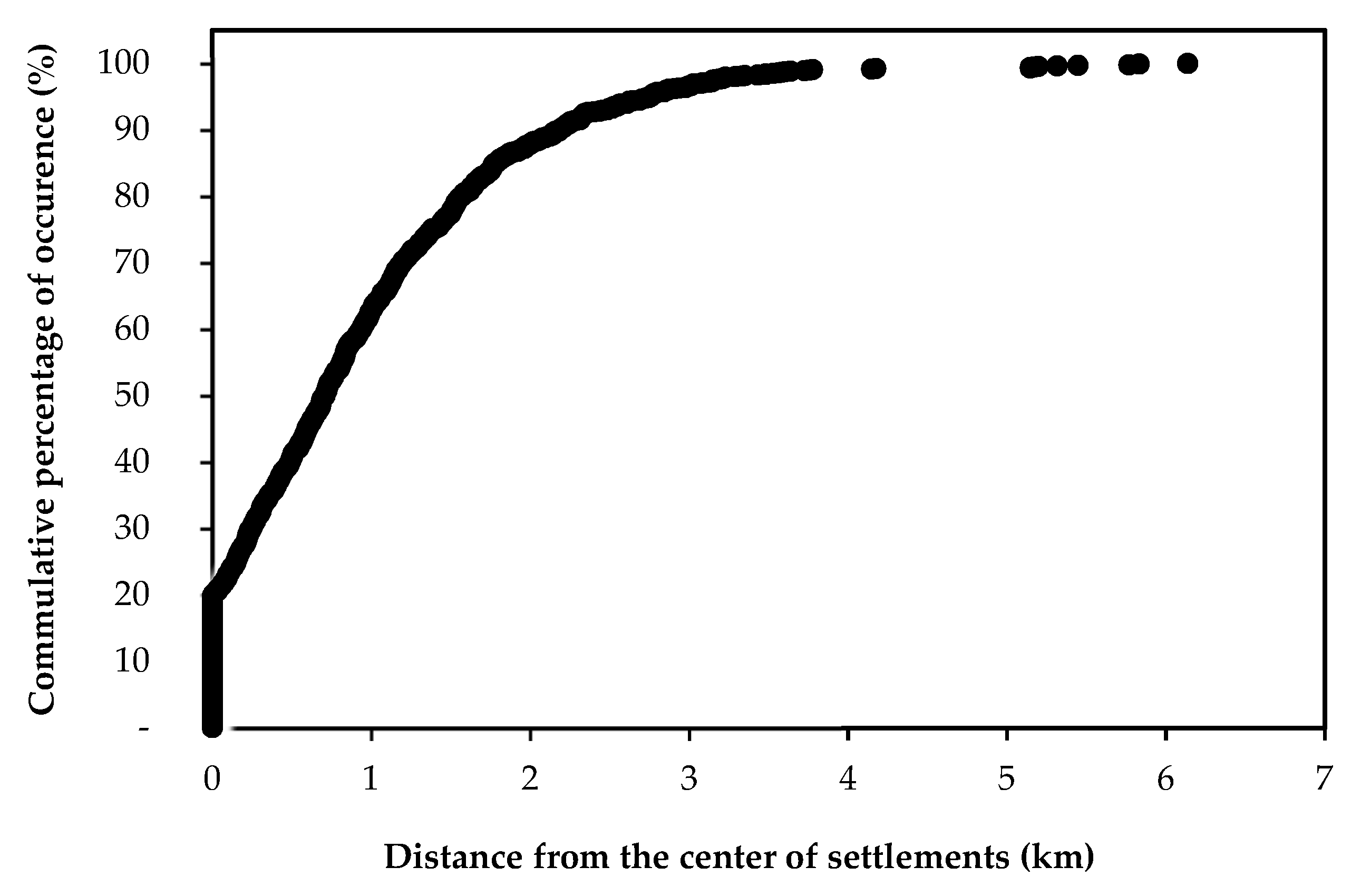
2.6. Distance from Settlements as Underlying Factor
3. Discussion
3.1. Current Invasion of Solanum Elaeagnifolium in Greece
3.2. Elevation and Climate Limitations
3.3. Influence of Soil
3.4. Type and Intensity of Land Use as Drivers
4. Materials and Methods
4.1. Study Area, Sampling Strategy and Data Collection
4.2. Data-Set Compilation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millenium Ecosystem Assessment. Ecosystems and Human Well-Being: Scenarios; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef]
- Pauchard, A.; Alaback, P.B. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of south-central Chile. Conserv. Biol. 2004, 18, 238–248. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, X.; Wei, H.; Wanga, D.; Chen, R.; Wanga, L.; Gua, W. Predicting the invasive trend of exotic plants in China based on the ensemble model under climate change: A case for three invasive plants of Asteraceae. Sci. Total Environ. 2021, 756, 14384. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.; Xianfeng Jiang, T.; Hu, X.; Feng, J. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef]
- Pyšek, P.; Perg, J.; Ess, F.; Lenzner, B.; Dawson, W.; Kreft, H.; Weige, P.; Winter, M.; Kartesz, J.; Nishino, M.; et al. Naturalized alien flora of the world: Species diversity, taxonomic and phylogenetic patterns, geographic distribution and global hotspots of plant invasion. Preslia 2017, 89, 203–274. [Google Scholar] [CrossRef]
- Pagad, S.; Genovesi, P.; Carnevali, L.; Schigel, D.; McGeoch, M.A. Data Descriptor: Introducing the global register of introduced and invasive Species. In Sci. Data; 2018; Volume 5, p. 170102. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Naturalization of introduced plants: Ecological drivers of biogeographical patterns. New Phytol. 2012, 196, 383–396. [Google Scholar] [CrossRef]
- van Kleunen, M.; Pyšek, P.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Weigelt, P.; Stein, A.; Dullinger, S.; König, C.; et al. The Global Naturalized Alien Flora (GloNAF) database. Ecology 2019, 100, e02542. [Google Scholar] [CrossRef]
- Brunel, S. Pest risk analysis for Solanum elaeagnifolium and international management measures proposed. EPPO Bull. 2011, 41, 232–242. [Google Scholar] [CrossRef]
- Uludag, A.; Gbehounou, G.; Kashefi, J.; Boulache, M.; Bon, M.-C.; Bell, C.; Lagopodi, A.L. Review of the current situation for Solanum elaeagnifolium in the Mediterranean Basin. EPPO Bull. 2016, 46, 139–147. [Google Scholar] [CrossRef]
- Boyd, J.W.; Murray, D.S.; Tyrl, R.J. Silverleaf nightshade, Solanum elaeagnifolium, origin, distribution and relation to man. Econ. Bot. 1984, 38, 210–217. [Google Scholar] [CrossRef]
- Economidou, E.; Yannitsaros, A. Recherches sur la flore adventice de Grèce: V. Distribution et écologie de Solanum elaeagnifolium Cav. Rev. Biol. Écol. Medit. 1975, 2, 29–44. [Google Scholar] [CrossRef]
- Brunel, S.; Schrader, G.; Brundu, G.; Fried, G. Emerging invasive alien plants for the Mediterranean Basin. EPPO Bull. 2010, 40, 219–238. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Delipetrou, P.; Celesti-Grapow, L.; Basnou, C.; Bazos, I.; Kokkoris, Y.; Blasi, C.; Vilá, M. Comparing naturalized alien plants and recipient habitats across an east-west gradient in the Mediterranean Basin. J. Biogeogr. 2010, 37, 1811–1823. [Google Scholar] [CrossRef]
- Votsi, N.E.P.; Drakou, E.G.; Mazaris, A.D.; Kallimanis, A.S.; Pantis, J.D. Distance-based assessment of open country Quiet Areas in Greece. Landscape Urban. Plan. 2012, 104, 279–288. [Google Scholar] [CrossRef]
- Pollnac, F.; Seipel, T.; Repath, C.; Rew, L.J. Plant invasion at landscape and local scales along roadways in the mountainous region of the Greater Yellowstone Ecosystem. Biol. Invasions 2012, 14, 1753–1763. [Google Scholar] [CrossRef]
- Zahariadi, C. Quelques taxons rares ou nouvellement découverts e la flore de la Grèce. Ann. Mus. Goulandris 1973, 1, 165–183. [Google Scholar]
- Boratynski, A.; Browicz, K.; Zieliński, J. Chorology of Trees and Shrubs in Greece; Polish Academy of Sciences: Sorus, Poznan/Kornik, Poland, 1992. [Google Scholar]
- Browicz, K. Nicotiana glauca and Solanum elaeagnifolium (Solanaceae): Two xenophytes from South America and the history of their spreading in the eastern Mediterranean. Fragm. Flor. Geobot. 1993, 2 (Suppl. 2), 299–305. [Google Scholar]
- Mekki, M. Biology, distribution and impacts of silverleaf nightshade (Solanum elaeagnifolium Cav.). EPPO Bull. 2007, 37, 114–118. [Google Scholar] [CrossRef]
- Krigas, N.; Kokkini, S. A survey of the alien vascular flora of the urban and suburban area of Thessaloniki (N Greece). Willdenowia 2004, 34, 77–98. [Google Scholar] [CrossRef]
- Krigas, N.; Kokkini, S. Indigenous vascular flora of the urban and suburban area of Thessaloniki (N Greece). Bot. Chron. 2005, 18, 29–85. [Google Scholar]
- Celesti-Grapow, L.; di Marzio, P.; Blasi, C. Temporal niche separation of the alien flora of Rome (Italy). In Plant Invasions: Ecological Threats and Management Solutions; Child, L.E., Brock, J.H., Brundu, G., Prach, K., Pyšek, P., Wade, P.M., Williamson, M., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2003; pp. 101–111. [Google Scholar]
- Petanidou, T.; Price, V.M.; Waser, N.M.; Kantsa, A.; Tscheulin, T.; Kariyat, R.; Krigas, N.; Mescher, M.S.; De Moraes, C.M.; Bronstein, J.L. Pollination and reproduction of an invasive plant inside and outside its ancestral range. Acta Oecol. 2018, 89, 11–20. [Google Scholar] [CrossRef]
- Mkula, N.P. Allelopathic Interference of Silverleaf Nightshade (Solanum elaeagnifolium Cav.) with the Early Growth of Cotton (Gossypium hirsutum L.). Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2006. [Google Scholar]
- Pauchard, A.; Kueffer, C.; Dietz, H.; Daehler, C.C.; Alexander, J.; Edwards, P.J.; Arévalo, J.R.; Cavieres, L.A.; Guisan, A.; Haider, S.; et al. Ain’t no mountain high enough: Plant invasions reaching new elevations. Front. Ecol. Environ. 2009, 7, 479–486. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošik, V.; Pergl, J.; Wild, J. Colonization of high altitudes by alien plants over the last two centuries. Proc. Natl. Acad. Sci. USA 2011, 108, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Sforza, R.; Jones, W.A. Potential for classical biocontrol of silverleaf nightshade in the Mediterranean Basin. EPPO Bull. 2007, 37, 156–162. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef]
- Scaldaferro, M.; Chiarini, F.; Santiñaque, F.F.; Bernardello, G.; Moscone, E.A. Geographical pattern and ploidy levels of the weed Solanum elaeagnifolium (Solanaceae) from Argentina. Genet. Resour. Crop. Evol. 2012, 59, 1833–1847. [Google Scholar] [CrossRef]
- Heap, J.W.; Carter, R.J. The biology of Australian weeds, 35. Solanum elaeagnifolium Cav. Plant. Prot. Q. 1999, 14, 2–12. [Google Scholar]
- Stanton, R.; Wu, H.; Lemerle, D. Factors affecting silverleaf nightshade (Solanum elaeagnifolium) germination. Weed Sci. 2012, 60, 42–47. [Google Scholar] [CrossRef]
- Wassermann, V.D.; Zimmerman, H.G.; Neser, S. The Weed Silverleaf Bitter Apple (“Santasbos”) (Solanum elaeagnifolium) with Special Reference to Its Status in South Africa; Technical Communication, Department of Agriculture and Water Supply: Pretoria, South Africa, 1988. [Google Scholar]
- Brett, M.W.; Orrock, J.L. Historic land use influences contemporary establishment of invasive plant species. Oecologia 2013, 172, 1147–1157. [Google Scholar] [CrossRef]
- Orgiazzi, A.; Panagos, P.; Yigini, Y.; Dunbar, M.B.; Gardi, C.; Montanarella, L.; Ballabio, C. A knowledge-based approach to estimating the magnitude and spatial patterns of potential threats to soil biodiversity. Sci. Total Environ. 2016, 545–546, 11–20. [Google Scholar] [CrossRef]
- de Vries, F.T.; Thébault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Jørgensen, H.B.; Brady, M.V.; Christensen, S.; De Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Setälä, H.; Bardgett, R.D.; Birkhofer, K.; Brady, M.; Byrne, L.; de Ruiter, P.C.; de Vries, F.T.; Gardi, C.; Hedlund, K.; Hemerik, L.; et al. Urban and agricultural soils: Conflicts and trade-offs in the optimization of ecosystem services. Urban. Ecosyst. 2014, 17, 239–253. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Chalghaf, E.; Aissa, M.; Mellassi, H.; Mekki, M. Maîtrise de la propagation de la morelle jaune (Solanum elaeagnifolium Cav.) dans le gouvernorat de Kairouan (Tunisie). EPPO Bull. 2007, 37, 132–136. [Google Scholar] [CrossRef]
- Chytrý, M.; Jarošik, V.; Pyšek, P.; Hajek, O.; Knollová, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.J.; Tung, P.J.; Leishman, M.R. Relationships between anthropogenic disturbance, soil properties and plant invasion in endangered Cumberland Plain Woodland, Australia. Austral. Ecol. 2005, 30, 775–788. [Google Scholar] [CrossRef]
- Trnka, M.; Rötter, R.P.; Ruiz-Ramos, M.; Kersebaum, K.C.; Olesen, J.E.; Žalud, Z.; Semenov, M.A. Adverse weather conditions for European wheat production will become more frequent with climate change. Nat. Clim. Chang. 2014, 4, 637–643. [Google Scholar] [CrossRef]
- Roger-Estrade, J.; Anger, C.; Bertrand, M.; Richard, G. Tillage and soil ecology: Partners for sustainable agriculture. Soil Till. Res. 2010, 111, 33–40. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H.; Stark, J.M. Soil history as a primary control on plant invasion in abandoned agricultural fields. J. App. Ecol. 2006, 43, 868–876. [Google Scholar] [CrossRef]
- Kallimanis, A.S.; Tsiafouli, M.A.; Pantis, J.D.; Mazaris, A.D.; Matsinos, Y.; Sgardelis, S.P. Arable land and habitat diversity in Natura 2000 sites in Greece. JBRT 2008, 9, 55–66. [Google Scholar]
- Tsiafouli, M.A.; Apostolopoulou, E.; Mazaris, A.D.; Kallimanis, A.S.; Drakou, E.G.; Pantis, J.D. Human activities in Natura 2000 sites: A highly diversified conservation network. Environ. Manag. 2013, 51, 1025–1033. [Google Scholar] [CrossRef]
- Milton, S.J.; Dean, W.R.J. Alien plant assemblages near roads in arid and semi-arid South Africa. Divers. Distribut. 1998, 4, 175–187. [Google Scholar] [CrossRef]
- Krigas, N.; Mouflis, G.; Grigoriadou, K.; Maloupa, E. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodivers. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Krigas, N.; Papadimitriou, K.; Mazaris, A.D. GIS and ex situ plant conservation. In Application of Geographic Information Systems; Alam, B.M., Ed.; InTechopen.com: Rjieka, Croatia, 2012; pp. 153–174. [Google Scholar]
- Brundu, G. A review of geomatic tools for assessing, inventorying and mapping alien plant invasions in the Mediterranean Basin. In Proceedings of the International Workshop “Invasive Plants of the Mediterranean Type Regions of the World”, Environmental Encounter Series 59th ed.; Brunel, S., Ed.; Council of Europe Publishing: Strasbourg, France, 2006; pp. 65–87. [Google Scholar]
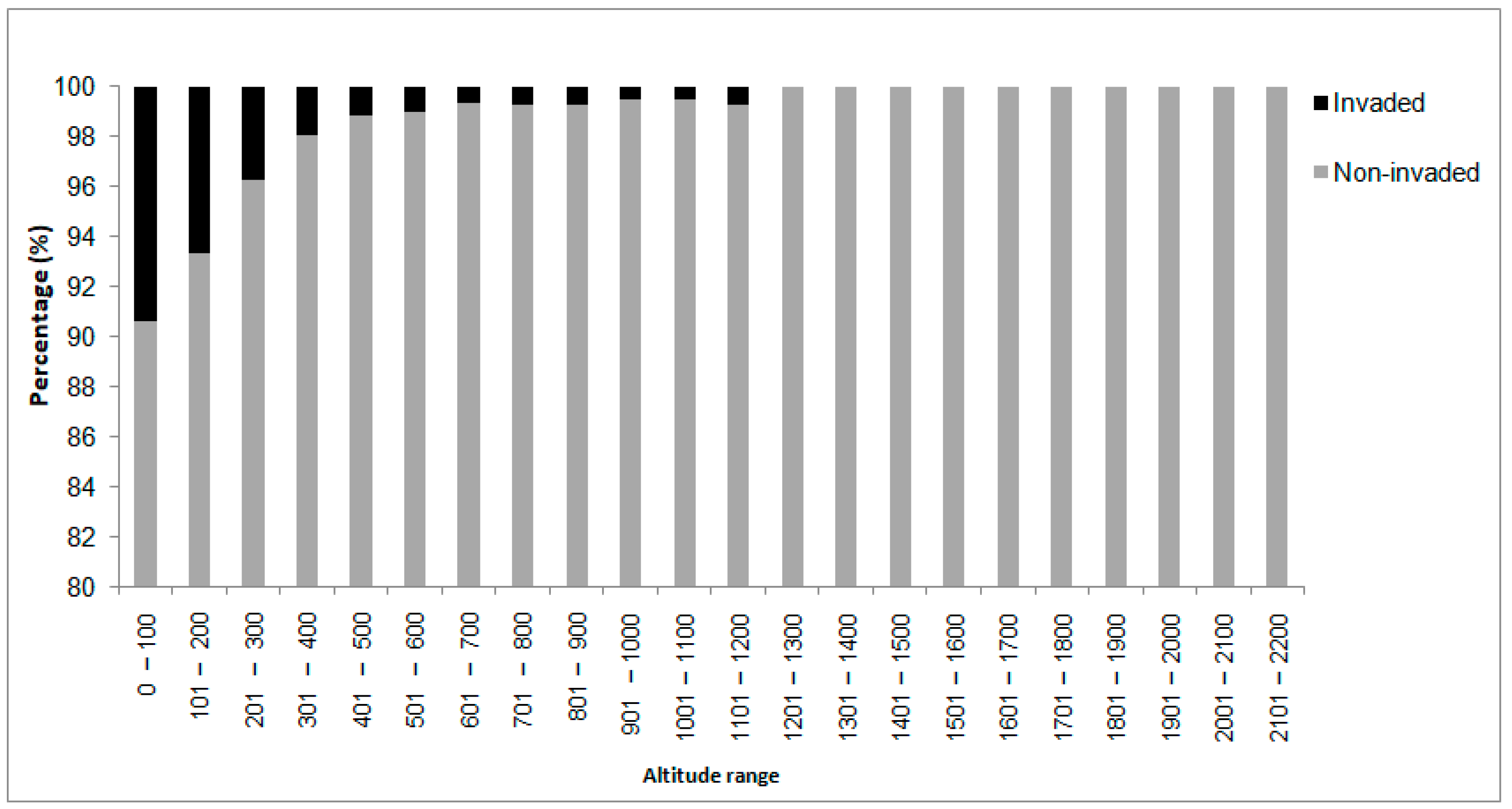



| Altitude Class (m) | Invaded Areas (% of Cells) | Non-Invaded Areas (% of Cells) |
|---|---|---|
| 0–100 | 60.09 | 32.84 |
| 101–200 | 24.58 | 19.46 |
| 201–300 | 8.53 | 12.48 |
| 301–400 | 2.96 | 8.49 |
| 401–500 | 1.28 | 6.36 |
| 501–600 | 0.97 | 5.24 |
| 601–700 | 0.51 | 4.53 |
| 701–800 | 0.41 | 3.04 |
| 801–900 | 0.31 | 2.34 |
| 901–1000 | 0.15 | 1.70 |
| 1001–1100 | 0.10 | 1.13 |
| 1101–1200 | 0.10 | 0.76 |
| 1201–2200 | - | 1.62 |
| Road Type | Road Length (km) with Occurrences (% of the Total Invaded) | Surveyed Length (km) (% of the Total Surveyed) | Total Length (km) in Greece (% of the Extant Network) |
|---|---|---|---|
| Multilane highways | 674 (12.79) | 922 (5.86) | 2789 (3.82) |
| Old interstate roads | 1276 (24.21) | 4589 (29.16) | 7316 (10.02) |
| Rural roads | 1922 (36.47) | 3977 (25.27) | 19,003 (26.02) |
| Local roads | 1399 (26.53) | 6248 (39.71) | 43,921 (60.14) |
| Total | 5270 (100) | 15736 (100) | 73029 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krigas, N.; Tsiafouli, M.A.; Katsoulis, G.; Votsi, N.-E.; van Kleunen, M. Investigating the Invasion Pattern of the Alien Plant Solanum elaeagnifolium Cav. (Silverleaf Nightshade): Environmental and Human-Induced Drivers. Plants 2021, 10, 805. https://doi.org/10.3390/plants10040805
Krigas N, Tsiafouli MA, Katsoulis G, Votsi N-E, van Kleunen M. Investigating the Invasion Pattern of the Alien Plant Solanum elaeagnifolium Cav. (Silverleaf Nightshade): Environmental and Human-Induced Drivers. Plants. 2021; 10(4):805. https://doi.org/10.3390/plants10040805
Chicago/Turabian StyleKrigas, Nikos, Maria A. Tsiafouli, Georgios Katsoulis, Nefta-Eleftheria Votsi, and Mark van Kleunen. 2021. "Investigating the Invasion Pattern of the Alien Plant Solanum elaeagnifolium Cav. (Silverleaf Nightshade): Environmental and Human-Induced Drivers" Plants 10, no. 4: 805. https://doi.org/10.3390/plants10040805
APA StyleKrigas, N., Tsiafouli, M. A., Katsoulis, G., Votsi, N.-E., & van Kleunen, M. (2021). Investigating the Invasion Pattern of the Alien Plant Solanum elaeagnifolium Cav. (Silverleaf Nightshade): Environmental and Human-Induced Drivers. Plants, 10(4), 805. https://doi.org/10.3390/plants10040805









