1. Introduction
Global warming has two dangerous global consequences for agriculture: drought, due to water scarcity, and salinization, due to the prolonged use of water containing high concentrations of salts. Thus, it is important to identify plants that can cope with drought and salt stress to increase crop resilience under different climatic changes. Olive (
Olea europaea L.) tree belonging to family Oleaceae, is one of the most popular species of the genus
Olea which is used for food purpose [
1,
2]. Olive is widely grown all over the world, with a great potential for expansion because of its ability to cope with unfavorable conditions [
3,
4,
5]. However, most of the olive production (about 98%) comes from the Mediterranean basin [
1]. Therefore, climate change will have a strong impact on olive cultivation as this area is warming up to 20% faster than the rest of the world average.
Olive is known as a glycophytic species presenting medium tolerance to salts [
6] with marked differences among cultivars [
7,
8,
9]. One of the major effects of salt stress is oxidative stress, resulting in increased levels of reactive oxygen species (ROS) [
10]. ROS have the potential to interfere with many cellular components, causing cell membrane and other cellular structures damage, metabolic disorders, inhibited photosynthesis, and impaired nutrient uptake [
11]. To face increased ROS production, plants have developed defensive strategies, including enzymatic and non-enzymatic antioxidant systems. Thiol compounds, and in particular glutathione (GSH) [
12], act as antioxidants that protect plants against the damaging effects of increased ROS levels under salt stress conditions [
13]. Furthermore, plant responses to salinity include the synthesis of harmonious osmolytes, and stimulation of phytohormones production [
14]. The molecular mechanism involves the overexpression of particular genes triggering different stress-related proteins and play a pivotal role in stress adaptation mechanisms [
15].
Among the several stress-related proteins, osmotin is known to be induced in response to both biotic and abiotic stresses in plants [
16]. Osmotin is a cationic protein belonging to the PR5 family proteins [
17,
18]. It has been suggested that osmotin confers the tolerance to salinity stress by increasing the accumulation of osmolytes, such as proline and glycine betaine [
15,
17,
19,
20].
The main objective of this study was to understand whether osmotin is involved or not, to show resistance against salt stress in olive shoots. This goal was achieved by using a salt-tolerant (cv Canino), with two of its transgenic lines (Canino AT17-1 and Canino AT17-2), overexpressing tobacco osmotin gene, and a salt-sensitive (cv Sirole) olive cultivar, exposed to NaCl solutions (ranging from 0 to 200 mM). We investigated whether the mechanisms of proline accumulation and lipid peroxidation (as an estimate of oxidative damage) were affected by the presence of the osmotin gene.
Furthermore, as GSH plays an active role in the process of response to salinity, we tested the hypothesis that a close relationship might exist between salt tolerance and sulfur (S) assimilation rate. Thus, changes in thiols levels as well as in extractable activities of ATP sulfurylase (ATPS) and O-acetyl serine(thiol)lyase (OASTL), the first and the last enzyme of the S assimilation pathway, respectively, have been estimated in plants exposed to salt stress.
3. Discussion
Owing to their sessile lifestyle, plants are continuously challenged with a broad range of environmental stresses, among which salinity stress is recognized as one of the most negatively impacting stress on plant growth and crop yield [
23]. Plant response to stress commonly rely on expression of specific genes and synthesis of a large number of stress-related proteins, which plays a crucial role in stress adaptation [
24]. Thus, the expression of genes involved in signaling and regulatory pathways could represent one of the most promising approach for improving stress tolerance in plants. In the past two decades, overexpression of osmotin gene in tobacco [
18,
25], tomato [
26], strawberry [
20,
27], and chili pepper [
24] have been reported to enhance tolerance under NaCl-mediated salinity stress.
Olive (
Olea europaea L.) is a typical crop of the Mediterranean basin, where soil salinization is projected to worsen because of global climate change. In this study, the contribution of osmotin has been investigated in olive in response to salinity by using four different olive genotypes: a salt-tolerant (cv Canino) with its two transgenic lines (Canino AT17-1 and Canino AT17-2), overexpressing tobacco osmotin gene, and a salt-sensitive (cv Sirole). Since one of the major effects of salt stress is oxidative stress, resulting in increased levels of reactive oxygen species (ROS) [
10], and being GSH actively involved as antioxidant in the process of response to salinity, we also tested the hypothesis that a close relationship might exist between salt tolerance and S assimilation rate.
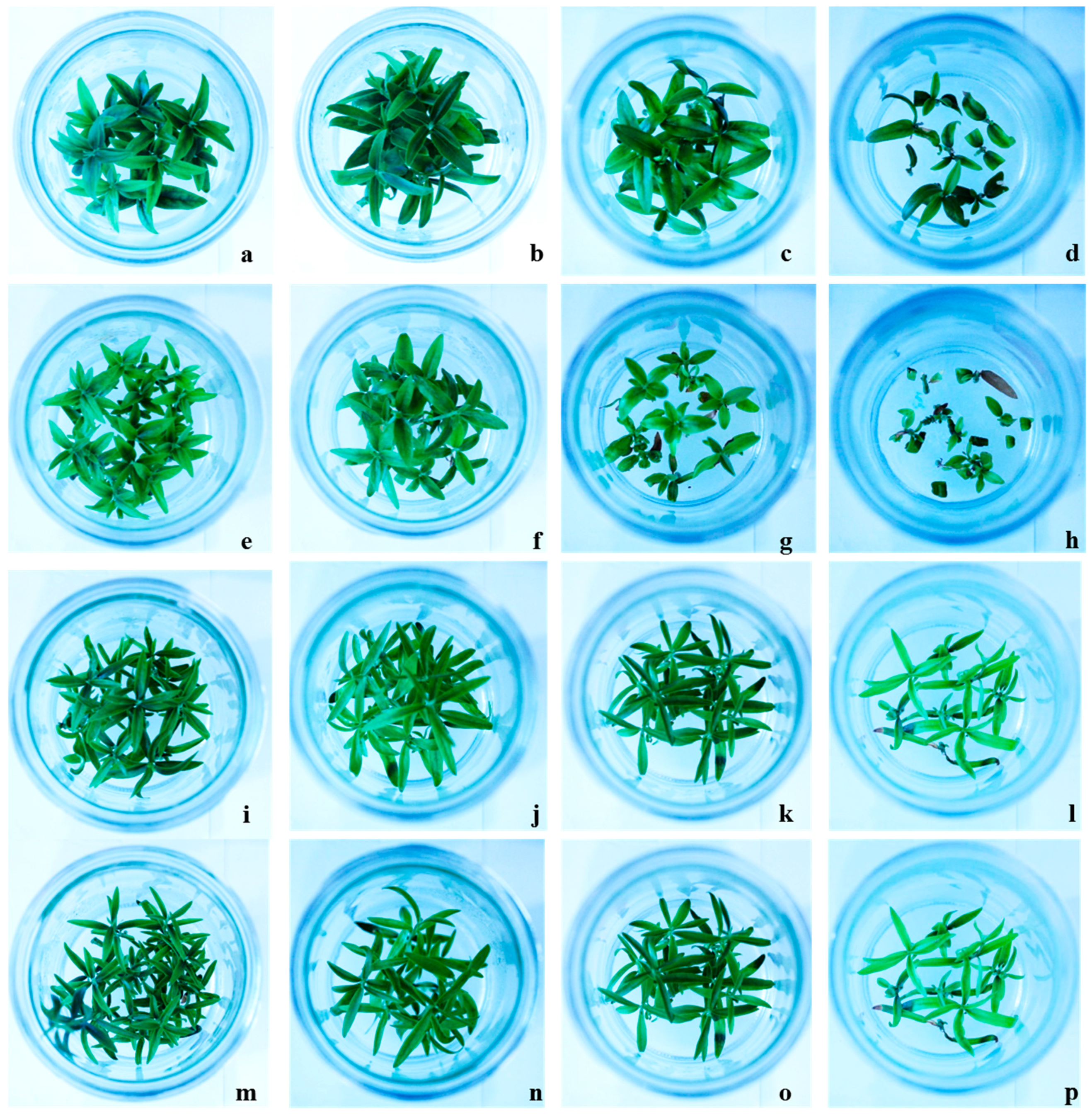
The evaluation of olive resistance to salinity stress in terms of the McKinney index (MKI) and tolerance index (TI), indicating the level of shoot chlorosis and necrosis, respectively, we demonstrated that the lower effects of salt-induced stress were observed in both transgenic lines (
Table 2). Zero MKI values, corresponding to green leaves and stems, were observed in all tested genotypes grown in control condition (0 mM NaCl) until the end of the experiment. Both Canino and Sirole showed significantly increased chlorosis, with increasing salt concentration in the growth medium, while the transgenic lines showed normal growth even at highest salt stress level (
Figure 1). This is a clear indication that both transgenic lines responded better to exogenous NaCl treatments, showing lower MKI values as well as higher TI values, than Canino and Sirole (
Table 2). These findings are consistent with previous study showing that
Myrtus communis L. plants exposed to 150 and 250 mM NaCl showed high chlorosis levels, with highest MKI values found in shoots exposed to 250 mM NaCl for 30 days [
28].
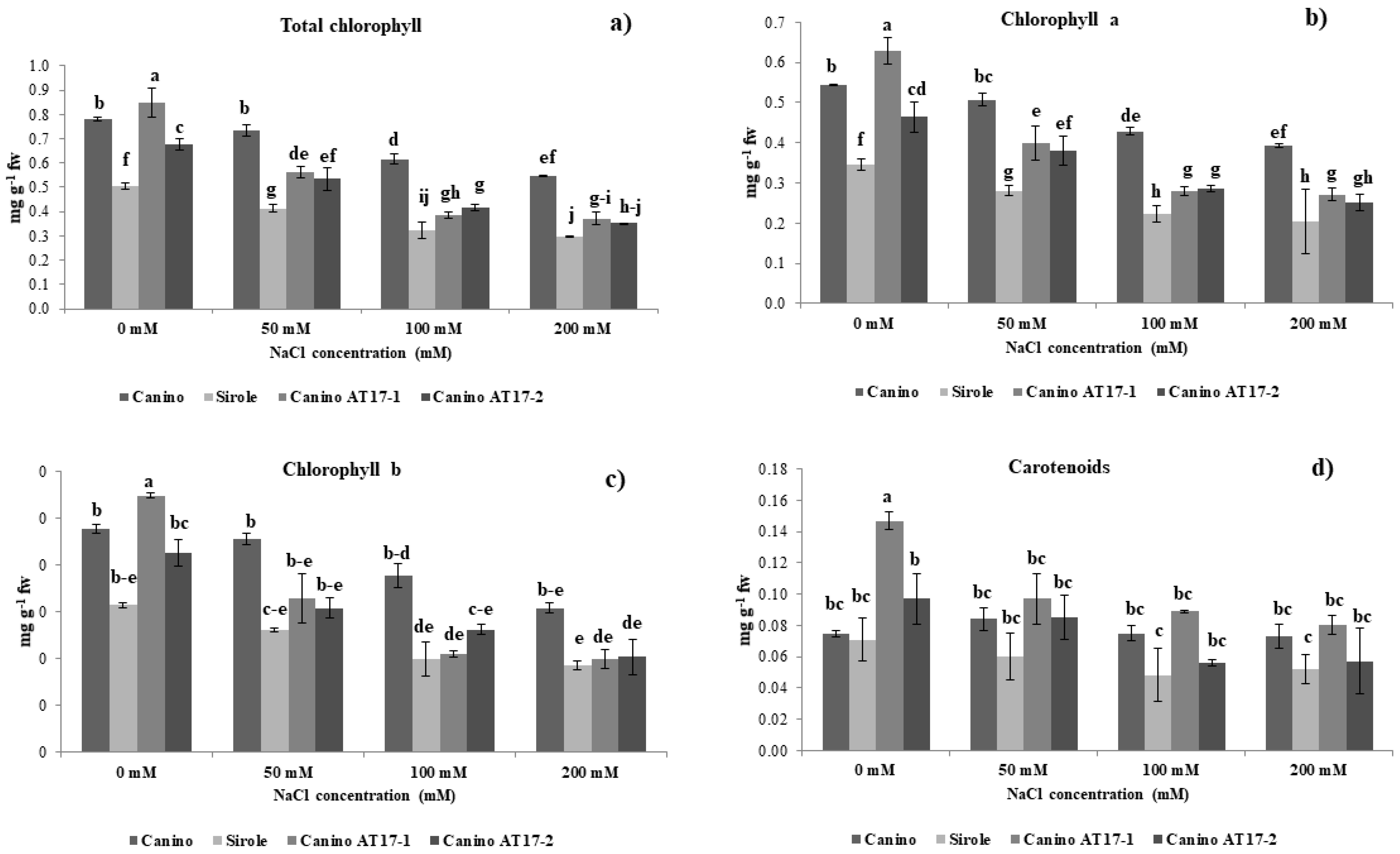
Nevertheless, this effect seemed not to be directly linked to total chlorophyll content. In fact, in our experimental conditions, we observed that the treatment with NaCl was more negatively impacting on Sirole and both transgenic lines, which showed a higher rate of chlorophyll loss from shoots, as compared to Canino (
Figure 2). In fact, in this latter case concentration of chlorophyll remained relatively stable from 0 to 50 mM NaCl, to later decrease further increasing NaCl concentration (
Figure 2). Salinity effect on chlorophyll content is often ambiguous. For instance, it has been recently demonstrated that salt stress increased photosynthetic pigments in both tolerant and sensitive alfalfa ecotypes [
29]. This could also be the case for olive, which has not been previously reported and suggests that chlorophyll content alone do not to provide an indication of the extent of salt stress.
It is well known that several abiotic stresses influence the accumulation of carotenoids, whose regulation is suggested to lead to stress tolerant phenotypes. In our experimental conditions, we found that both transgenic lines showed higher carotenoid contents as compared to Canino and Sirole in control condition (0 mM NaCl), whereas Sirole and transgenic lines exhibited a net reduction of carotenoids level under salt stress (
Figure 2d). This data was in consistent with those results obtained by [
30], which showed that β-carotene content decreases in pepper under oxidative-induced stress.
Our findings under salt stress resulted in similar decrease rate of carotenoids for genotypes with contrasted tolerance (Sirole and Canino transgenic lines), suggesting that carotenoids metabolism is not directly involved in the stress tolerance mechanism, and changes in carotenoid accumulation are rather due to plant general metabolic modifications occurring during adaptation to stress conditions.
Although it has not been clearly demonstrated that the mechanism by which osmotin improves plant response under abiotic stress, a few studies have shown that under salinity stress osmotin allows the reduction of Na
+ ions uptake into the cytoplasm though a Na
+/H
+ anti-porter [
31], in addition that osmotin could be involved in osmotic adjustment of plants under stress either by facilitating the accumulation or compartmentation of different solutes in intracellular spaces [
18]. As salinity results in water stress, plants have to accomplish an osmotic adjustment as well to maintain their metabolic activities. Osmotic adjustment is achieved by the accumulation of the compatible solutes such as free proline. It has been reported that stress tolerance increases with increasing proline accumulation [
32] and, furthermore, that proline can induce the expression of several genes involved in stress tolerance [
33], suggesting a role for this metabolite not only to low water potential but also in plant protection from osmotic stress [
34]. Thus, the transgenic approach could represent a promising strategy to increase proline production and improve stress tolerance in plants.
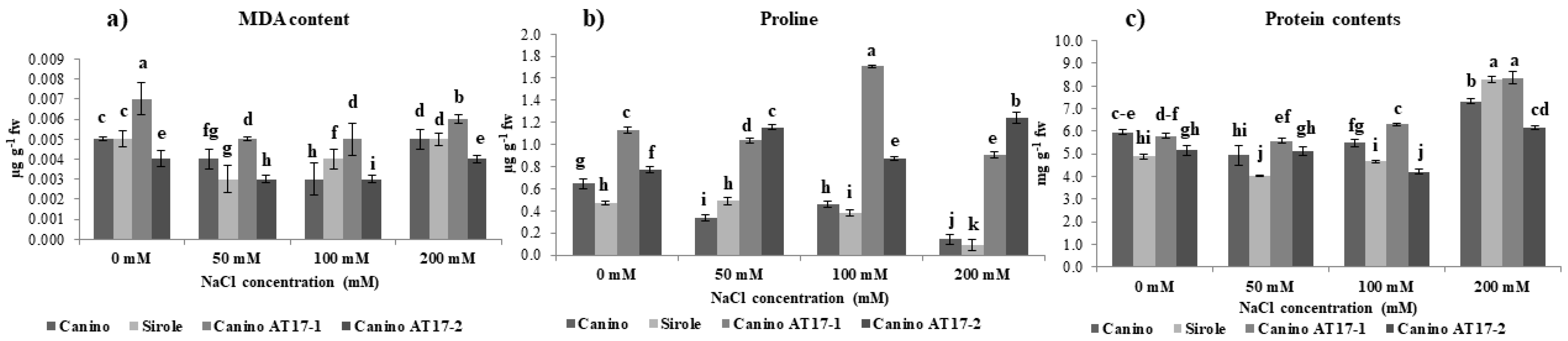
Interestingly, our results showed that the salt-tolerant cv Canino accumulated more proline as compared to the salt-sensitive one (cv Sirole) (
Figure 3b), confirming the previous study which suggested that proline accumulation is genotype-dependent [
35]. Moreover, we found that shoots of both transgenic lines were able to increase proline accumulation with increasing NaCl concentrations in the medium and when they were exposed to the highest salt concentration (200 mM), proline content accumulated substantially (almost 7- and 9-fold higher in Canino AT17-1 and Canino AT17-2, respectively, compared to those found in Canino wt in the same condition) (
Figure 3b). This result reasonably suggests that the presence of osmotin gene could play an important role in the regulation of the mechanism of proline accumulation under salt stress. Proline synthesis is commonly induced under stressed conditions through an intercellular signal pathway mediated by ROS accumulation [
34]. Certainly, in plants exposed to environmental constraints, the accumulation of ROS is generally associated to proline accumulation [
34].
Salt stress provokes an excessive ROS production resulting in oxidative damage leading to cell injury and death [
36]. Therefore, we compared the changes in MDA concentration (as an estimate of lipid peroxidation) to assess the degree of oxidative damage induced by NaCl treatment [
37]. Our data showed that all tested genotypes were able to maintain on average a lower MDA content than that found in control condition (
Figure 3a). This result might be explained by assuming a significant role of proline in modulating both ROS generation and scavenging of ions [
38], but also to high efficiency of the antioxidant system leading to an increased stress tolerance. This result meant that the olive shoots had a proper membrane stability even under salt stress.
According to the literature, plants possess some strategies to cope with oxidative stress, such as enzymatic and non-enzymatic antioxidant systems. Non-enzymatic systems include thiol compounds such as glutathione and cysteine, playing an important role in improving the tolerance to oxidative stress induced by NaCl toxicity [
13,
34,
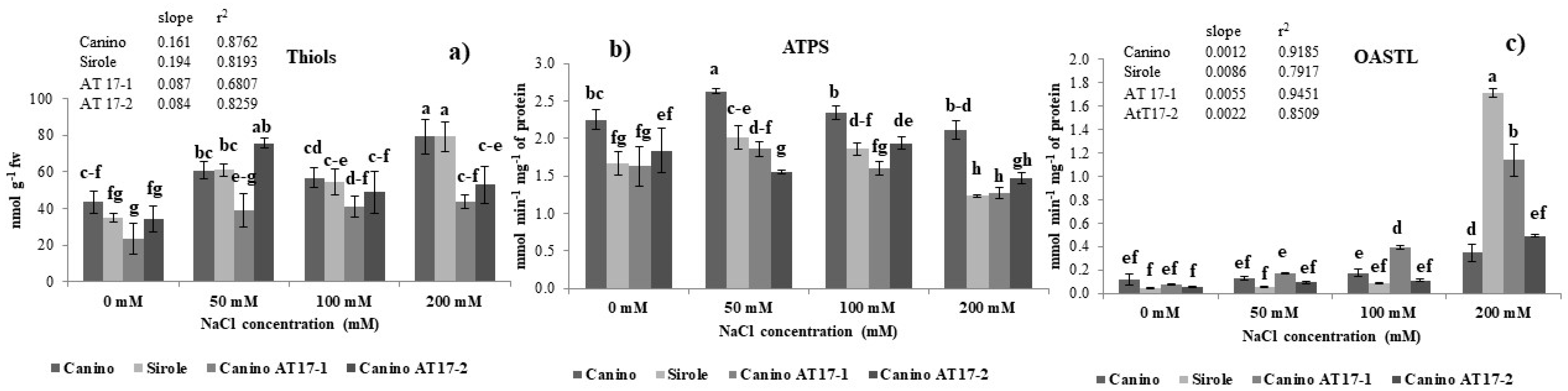
39]. Our results showed that non-protein thiols production and NaCl concentration in the growth medium were significantly positively correlated in all four studied genotypes (Canino r
2 = 0.8762; Sirole r
2 = 0.8193; Canino AT17-1 r
2 = 0.6807; Canino AT17-2 r
2 = 0.8259) (
Figure 4a) and Sirole cv exhibited the highest slope of the increase of thiols accumulation with increasing salt concentration (
Figure 4a). Thus, based on our results, we suggest that in Sirole, being a salt-sensitive cv, a large amount of ROS is likely produced due to salt stress, leading to increased pressure on ROS scavenging activity and thus to accumulate increased amounts of antioxidant compounds to cope with stress condition. Moreover, this response was well in line with the lowest proline contents found in Sirole.
Changes in thiols production reasonably require an adjustment of sulfate assimilation rate under salt stress, thus the activities of ATPS and OASTL, the first and the last enzyme of S assimilation pathway, respectively, have been estimated in plants exposed to salt stress. In this study, the increase of thiols production (
Figure 4a) was parallel with that of OASTL activity (
Figure 4c), indicating that S assimilation pathway plays an important role in the regulation of plant response to salt stress. As for thiols, OASTL activity increased with increasing salt concentration in the growth medium and, indeed, the correlation was very high in all four studied genotypes (Canino r
2 = 0.9185; Sirole r
2 = 0.7917; Canino AT17-1 r
2 = 0.9451; Canino AT17-2 r
2 = 0.8509) (
Figure 4f), and as for thiols, the highest slope of the linear correlation was found in Sirole cv (
Figure 4c). Interestingly, for both thiols and OASTL activity the lowest slope of the linear correlation was found in both transgenic lines (
Figure 4a,c). The different pattern observed for ATPS and OASTL activity could be explained by assuming that OASTL directly catalyzes the biosynthesis of cysteine [
40], the precursor for glutathione synthesis, and thus its activity could play a more important role in adaptation mechanisms to salt stress condition, than ATPS (
Figure 4b). These results are well in line with previous studies showing ATPS activity increase [
41,
42] as well as decrease [
43] in response to salt treatment.
Summarizing all these findings by the PCA analysis computed with the complete dataset comprising MDA and thiols levels, and enzyme activities (
Figure 5) appeared a clear clustering along the first component (describing 54.11% of the total variance) based on NaCl concentration in the media. Most of samples treated with the highest salt concentrations were found on the negative axis and interestingly with a negative loading for MDA, proline, and OASTL (
Figure 5). In conclusion, our results suggested that both Canino AT17-1 and Canino AT17-2 transgenic lines had better salt tolerance ability as compared to their relative wt (Canino), suggesting that the presence of osmotin could likely improve olive salt stress tolerance. Although the mechanism involved is still not fully understood, our results indicate that osmotin could play an important role in the regulation of proline accumulation, which in turn would exert a protective role preventing or modulating ROS generation.
Furthermore, the data obtained suggests an interesting interaction between salt stress and S metabolism and the involvement of this nutrient during adaptation of olive shoots to salt stress. Indeed, we have revealed that antioxidant response to scavenging excessive ROS requires the stimulation of S assimilation rate to sustain higher demand of reduced S.