Habitat Suitability and Establishment Limitations of a Problematic Liana
Abstract
1. Introduction
2. Results
2.1. Drupe Predation
2.2. Seedling Establishment of Poison Ivy in Forest Interior and Edge Habitats
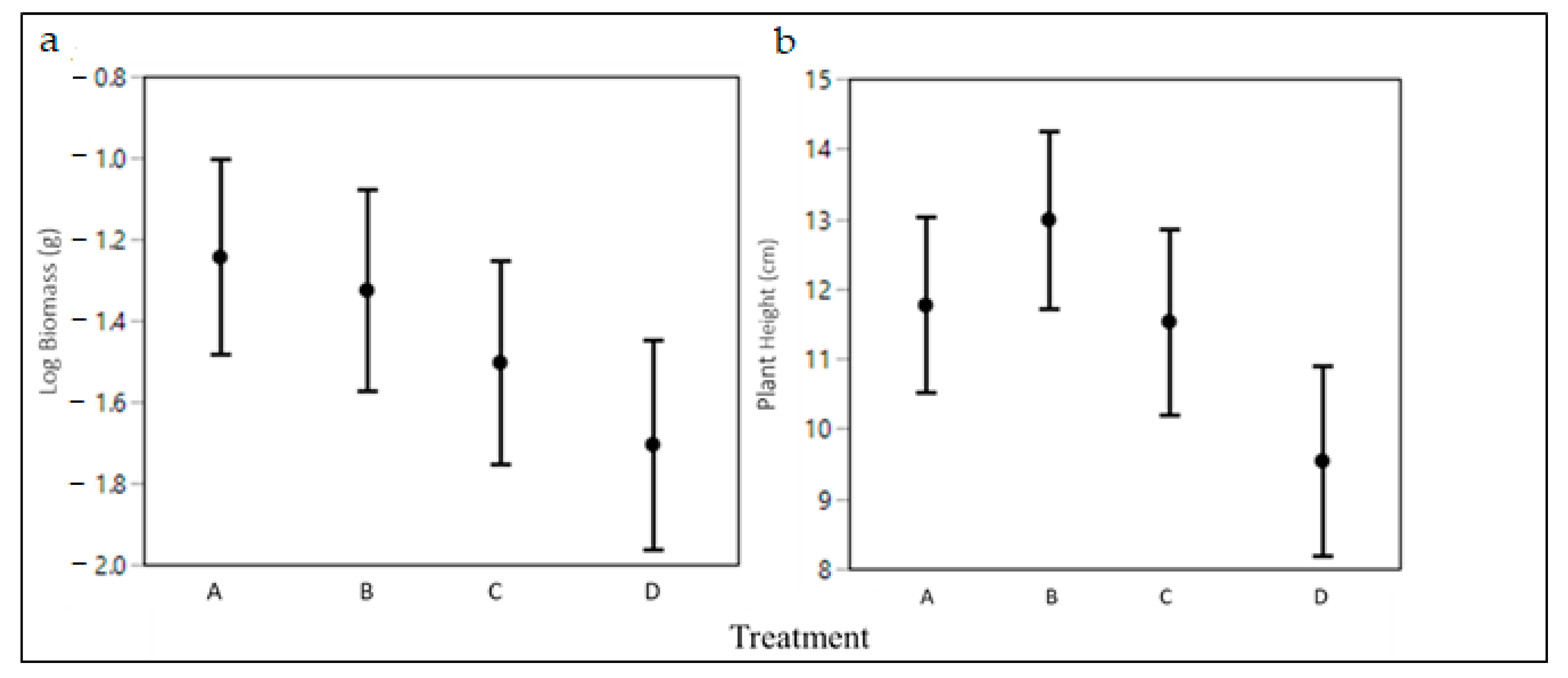
2.3. Seedling Herbivory Tolerance
3. Discussion
4. Materials and Methods
4.1. Post-Dispersal Drupe Predation
Analysis
4.2. Poison Ivy Seedling Establishment in Forest Interior and Edge Habitats
4.2.1. Plant Material
4.2.2. Sowing
4.2.3. Data Collection
4.2.4. Analysis
4.3. Seedling Herbivory Tolerance
4.3.1. Field Sites
4.3.2. Transplanting
4.3.3. Data Collection
4.3.4. Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnitzer, S.A.; Bongers, F. Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecol. Lett. 2011, 14, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, G.M.; Powers, J.S.; Schnitzer, S.A. Lianas reduce carbon accumulation and storage in tropical forests. Proc. Natl. Acad. Sci. USA 2015, 112, 13267–13271. [Google Scholar] [CrossRef] [PubMed]
- Putz, F.E. Vines in Treetops: Consequences of Mechanical Dependence. In Forest Canopies; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Schnitzer, S.A.; Carson, W.P. Lianas suppress tree regeneration and diversity in treefall gaps. Ecol. Lett. 2010, 13, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Condon, M.A.; Sasek, T.W.; Strain, B.R. Allocation Patterns in Two Tropical Vines in Response to Increased Atmospheric CO2. Funct. Ecol. 1992, 6, 680–685. [Google Scholar] [CrossRef]
- Mohan, J.E.; Ziska, L.H.; Schlesinger, W.H.; Thomas, R.B.; Sicher, R.C.; George, K.; Clark, J.S. Biomass and toxicity responses of poison ivy(Toxicodendron radicans) to elevated atmospheric CO2. Proc. Natl. Acad. Sci. USA 2006, 103, 9086–9089. [Google Scholar] [CrossRef]
- Zotz, G.; Cueni, N.; Körner, C. In situ growth stimulation of a temperate zone liana (Hedera helix) in elevated CO2. Funct. Ecol. 2006, 20, 763–769. [Google Scholar] [CrossRef]
- Laurance, W.F.; Andrade, A.S.; Magrach, A.; Camargo, J.L.C.; Valsko, J.J.; Campbell, M.; Fearnside, P.M.; Edwards, W.; Lovejoy, T.E.; Laurance, S.G. Long-term changes in liana abundance and forest dynamics in undisturbed Amazonian forests. Ecology 2014, 95, 1604–1611. [Google Scholar] [CrossRef]
- Mitich, L.W. Kudzu [Pueraria lobata (Willd.) Ohwi]. Weed Technol. 2000, 14, 231–235. [Google Scholar] [CrossRef]
- Weaver, M.A.; Hoagland, R.E.; Boyette, C.D. Kudzu response to foliar applied herbicides. Am. J. Plant Sci. 2015, 6, 856. [Google Scholar] [CrossRef][Green Version]
- Malhi, Y. The productivity, metabolism and carbon cycle of tropical forest vegetation. J. Ecol. 2012, 100, 65–75. [Google Scholar] [CrossRef]
- Van der Heijden, G.M.; Schnitzer, S.A.; Powers, J.S.; Phillips, O.L. Liana Impacts on Carbon Cycling, Storage and Sequestration in Tropical Forests. Biotropica 2013, 45, 682–692. [Google Scholar] [CrossRef]
- Carey, M.P.; Sanderson, B.L.; Barnas, K.A.; Olden, J.D. Native invaders—Challenges for science, management, policy, and society. Front. Ecol. Environ. 2012, 10, 373–381. [Google Scholar] [CrossRef]
- Simberloff, D.; Souza, L.; Nunez, M.A.; Barrios-Garcia, M.N.; Bunn, W. The natives are restless, but not often and mostly when disturbed. Ecology 2012, 93, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Epstein, W.L. Plant-induced dermatitis. Ann. Emerg. Med. 1987, 16, 950–955. [Google Scholar] [CrossRef]
- Epstein, W.L. Occupational poison ivy and oak dermatitis. Dermatol. Clin. 1994, 3, 511–516. [Google Scholar] [CrossRef]
- USDA. The PLANTS Database. Toxicodendron Radicans [Online Database]. 2017. Available online: http://plants.usda.gov/core/profile?symbol=TORA2 (accessed on 21 October 2015).
- OMAFRA. Available online: http://www.omafra.gov.on.ca/english/crops/facts/99-015.htm (accessed on 23 April 2019).
- Allen, B.P.; Sharitz, R.R.; Goebel, P.C. Twelve years post-hurricane liana dynamics in an old-growth southeastern floodplain forest. For. Ecol. Manag. 2005, 218, 259–269. [Google Scholar] [CrossRef]
- Fraver, S. Vegetation Responses along Edge-to-Interior Gradients in the Mixed Hardwood Forests of the Roanoke River Basin, North Carolina. Conserv. Biol. 1994, 8, 822–832. [Google Scholar] [CrossRef]
- Londré, R.A.; Schnitzer, S.A. The distribution of lianas and their change in abundance in temperate forests over the past 45 years. Ecology 2006, 87, 2973–2978. [Google Scholar] [CrossRef]
- Murcia, C. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef]
- Essl, F.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Katsanevakis, S.; Kühn, I.; Lenzner, B.; Pauchard, A.; Pyšek, P.; et al. A Conceptual Framework for Range-Expanding Species that Track Human-Induced Environmental Change. BioScience 2019. [Google Scholar] [CrossRef]
- Senchina, D.S. Fungal and animal associates of Toxicodendron spp.(Anacardiaceae) in North America. Perspect. Plant Ecol. Evol. Syst. 2008, 10, 197–216. [Google Scholar] [CrossRef]
- Suthers, H.B.; Bickal, J.M.; Rodewald, P.G. Use of successional habitat and fruit resources by songbirds during autumn migration in central New Jersey. Wilson Bull. 2000, 112, 249–260. [Google Scholar] [CrossRef]
- Benhase, E.B.; Jelesko, J.G. Germinating and culturing axenic poison ivy seedlings. HortScience 2013, 48, 1525–1529. [Google Scholar] [CrossRef]
- Penner, R.; Moodie, G.E.E.; Staniforth, R.J. The dispersal of fruits and seeds of Poison-ivy, Toxicodendron radicans, by Ruffed Grouse, Bonasa umbellus, and squirrels, Tamiasciurus hudsonicus and Sciurus carolinensis. Can. Field Nat. 1999, 113, 616–620. [Google Scholar]
- Angelo, A.; Ory, R.L. Lipid degradation during seed deterioration. Phytopathology 1983, 73. [Google Scholar] [CrossRef]
- Schafer, M.; Kotanen, P.M. The influence of soil moisture on losses of buried seeds to fungi. Acta Oecol. 2003, 24, 255–263. [Google Scholar] [CrossRef]
- Boman, J.S.; Casper, B.B. Differential postdispersal seed predation in disturbed and intact temperate forest. Am. Midl. Nat. 1995, 107–116. [Google Scholar] [CrossRef]
- Vander Wall, S.B.; Kuhn, K.M.; Beck, M.J. Seed removal, seed predation, and secondary dispersal. Ecology 2005, 86, 801–806. [Google Scholar] [CrossRef]
- Gillis, W.T. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora 1971, 73, 72–159. [Google Scholar]
- Talley, S.M.; Lawton, R.O.; Setzer, W.N. Host preferences of Rhus radicans (Anacardiaceae) in a southern deciduous hardwood forest. Ecology 1996, 77, 1271–1276. [Google Scholar] [CrossRef]
- Buron, J.; Lavigne, D.; Grote, K.; Takis, R.; Sholes, O. Association of Vines and Trees in Second-Growth Forest. Northeast. Nat. 1998, 5, 359–362. [Google Scholar] [CrossRef]
- Mulligan, G.A.; Junkins, B.E. The biology of canadian weeds: 23. Rhus radicans L. Can. J. Plant Sci. 1977, 57, 515–523. [Google Scholar] [CrossRef]
- Habeck, D.H. Insects associated with poison ivy and their potential as biological control agents. In Proceedings of the VII international Symposium on Biological Control of Weeds, Kruger National Park, South Africa, 2–7 March 2014; pp. 6–11. [Google Scholar]
- Averill, K.M.; Mortensen, D.A.; Smithwick, E.A.H.; Kalisz, S.; McShea, W.J.; Bourg, N.A.; Parker, J.D.; Royo, A.A.; Abrams, M.D.; Apsley, D.K.; et al. A regional assessment of white-tailed deer effects on plant invasion. AoB Plants 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Senchina, D.S. Beetle Interactions with Poison Ivy and Poison Oak (Toxicodendron P. Mill. sect. Toxicodendron, Anacardiaceae). Coleopt. Bull. 2005, 59, 328–334. [Google Scholar] [CrossRef]


| Emergence | Survival | Percent Emergence | |||||
|---|---|---|---|---|---|---|---|
| X2 | p | X2 | p | SS | F | p | |
| Habitat | 105.3 | <0.001 | 11.5 | <0.001 | – | – | – |
| Treatment | 90.4 | <0.001 | 3.2 | 0.076 | 1.03 | 31.24 | <0.001 |
| Site | 60.2 | <0.001 | 17 | 0.002 | 0.53 | 4.05 | 0.005 |
| Habitat x Treatment | 2.5 | 0.116 | 0.6 | 0.446 | – | – | – |
| % Bare Ground | – | – | – | – | 0.4 | 12.04 | <0.001 |
| % Soil Moisture | – | – | – | – | 0.06 | 1.71 | 0.194 |
| Soil pH | – | – | – | – | 0.23 | 7.02 | 0.01 |
| Incidence of Herbivory | Incidence of Disease | Survival | ||||
|---|---|---|---|---|---|---|
| X2 | p | X2 | p | X2 | p | |
| Treatment | 1.13 | 0.770 | 0.71 | 0.871 | 3.59 | 0.309 |
| Block [Site] | 6.83 | 0.337 | 14.49 | 0.025 | 8.55 | 0.200 |
| Site | 27.26 | <0.001 | 5.29 | 0.071 | 5.84 | 0.054 |
| Initial Height | 9.80 | 0.002 | 0.57 | 0.450 | 0.54 | 0.461 |
| Herbivory in 2017 | 0.14 | 0.706 | – | – | – | – |
| Herbivory Extent | |||
|---|---|---|---|
| B ± se | z | p | |
| No Exclusion | 1.0 ± 0.4 | 2.31 | 0.021 |
| Large Vertebrate Exclusion | 0.6 ± 0.4 | 1.29 | 0.198 |
| Large/Small Vertebrate Exclusion | 0.4 ± 0.4 | 0.8 | 0.426 |
| Log Biomass | Height | |||||
|---|---|---|---|---|---|---|
| SS | F | p | SS | F | p | |
| Treatment | 4.41 | 2.6 | 0.055 | 213.7 | 4.59 | 0.004 |
| Site | 1.9 | 1.68 | 0.191 | 154.53 | 4.97 | 0.008 |
| Initial Height | 9.09 | 16.04 | <0.001 | 702.76 | 45.21 | <0.001 |
| Block [Site] | 3.75 | 1.1 | 0.363 | 141.81 | 1.52 | 0.176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickinson, C.C.; Jelesko, J.G.; Barney, J.N. Habitat Suitability and Establishment Limitations of a Problematic Liana. Plants 2021, 10, 263. https://doi.org/10.3390/plants10020263
Dickinson CC, Jelesko JG, Barney JN. Habitat Suitability and Establishment Limitations of a Problematic Liana. Plants. 2021; 10(2):263. https://doi.org/10.3390/plants10020263
Chicago/Turabian StyleDickinson, Christopher C., John G. Jelesko, and Jacob N. Barney. 2021. "Habitat Suitability and Establishment Limitations of a Problematic Liana" Plants 10, no. 2: 263. https://doi.org/10.3390/plants10020263
APA StyleDickinson, C. C., Jelesko, J. G., & Barney, J. N. (2021). Habitat Suitability and Establishment Limitations of a Problematic Liana. Plants, 10(2), 263. https://doi.org/10.3390/plants10020263






