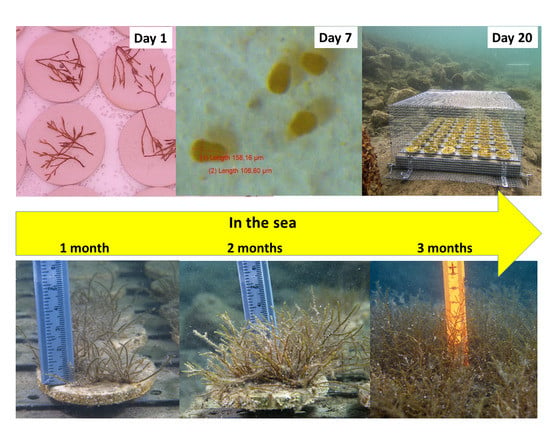
First Restoration Experiment for Gongolaria barbata in Slovenian Coastal Waters. What Can Go Wrong?
Abstract
1. Introduction
2. Materials and Methods
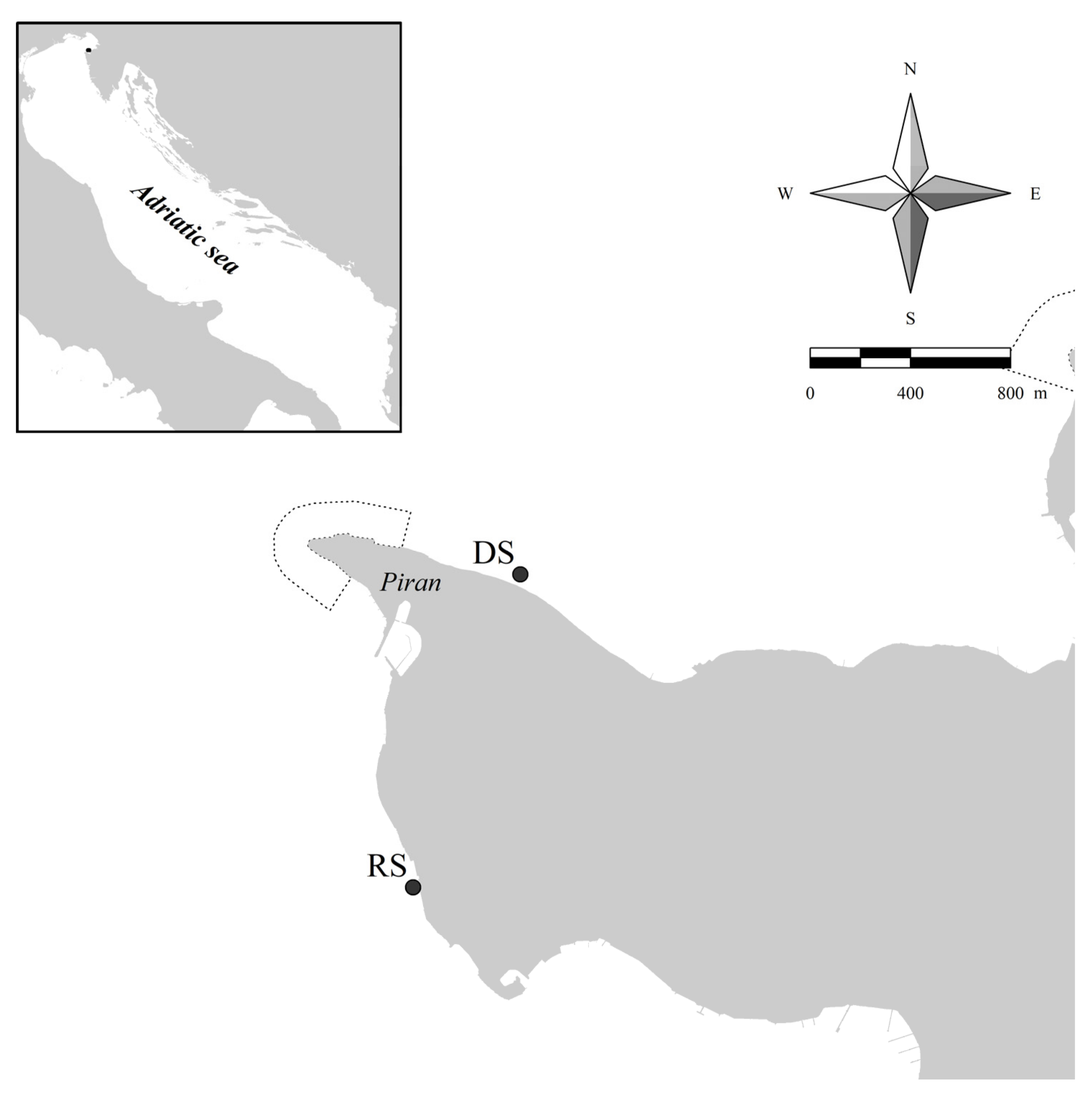
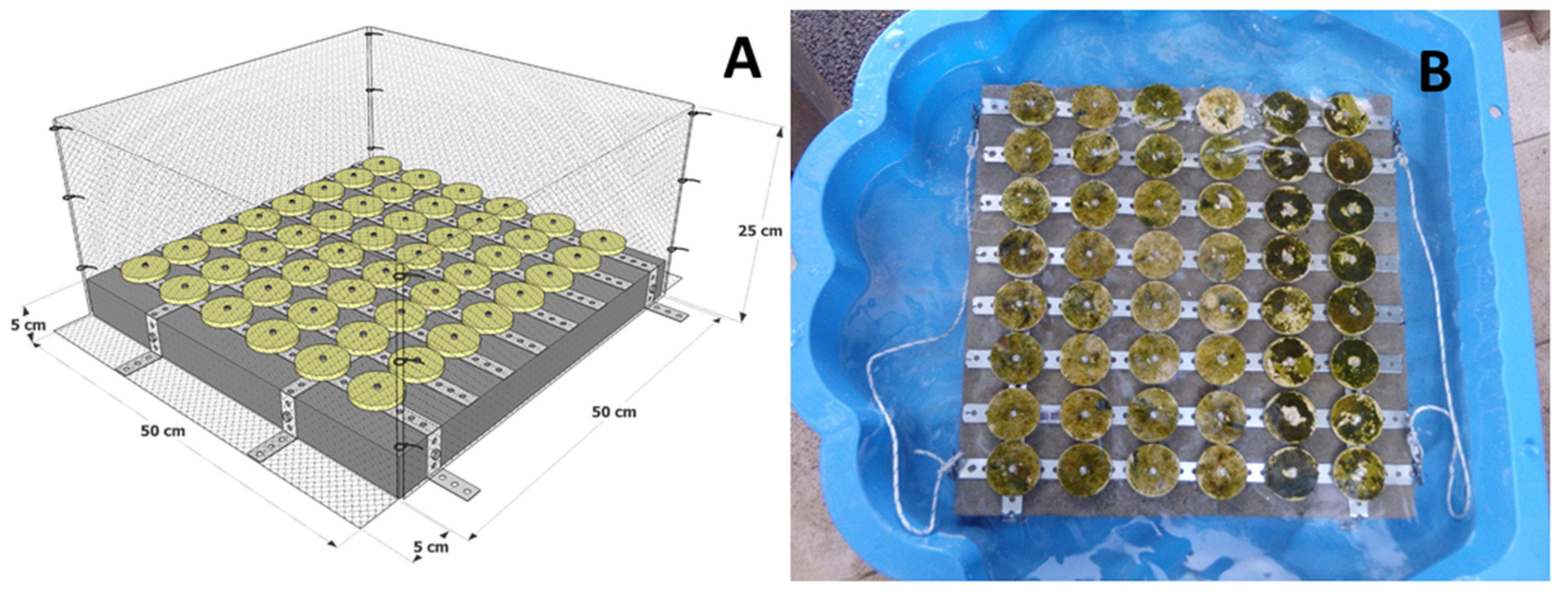
2.1. Fieldwork and Laboratory Work
2.2. Outplanting and Monitoring in the Field
2.3. Data Analysis
3. Results
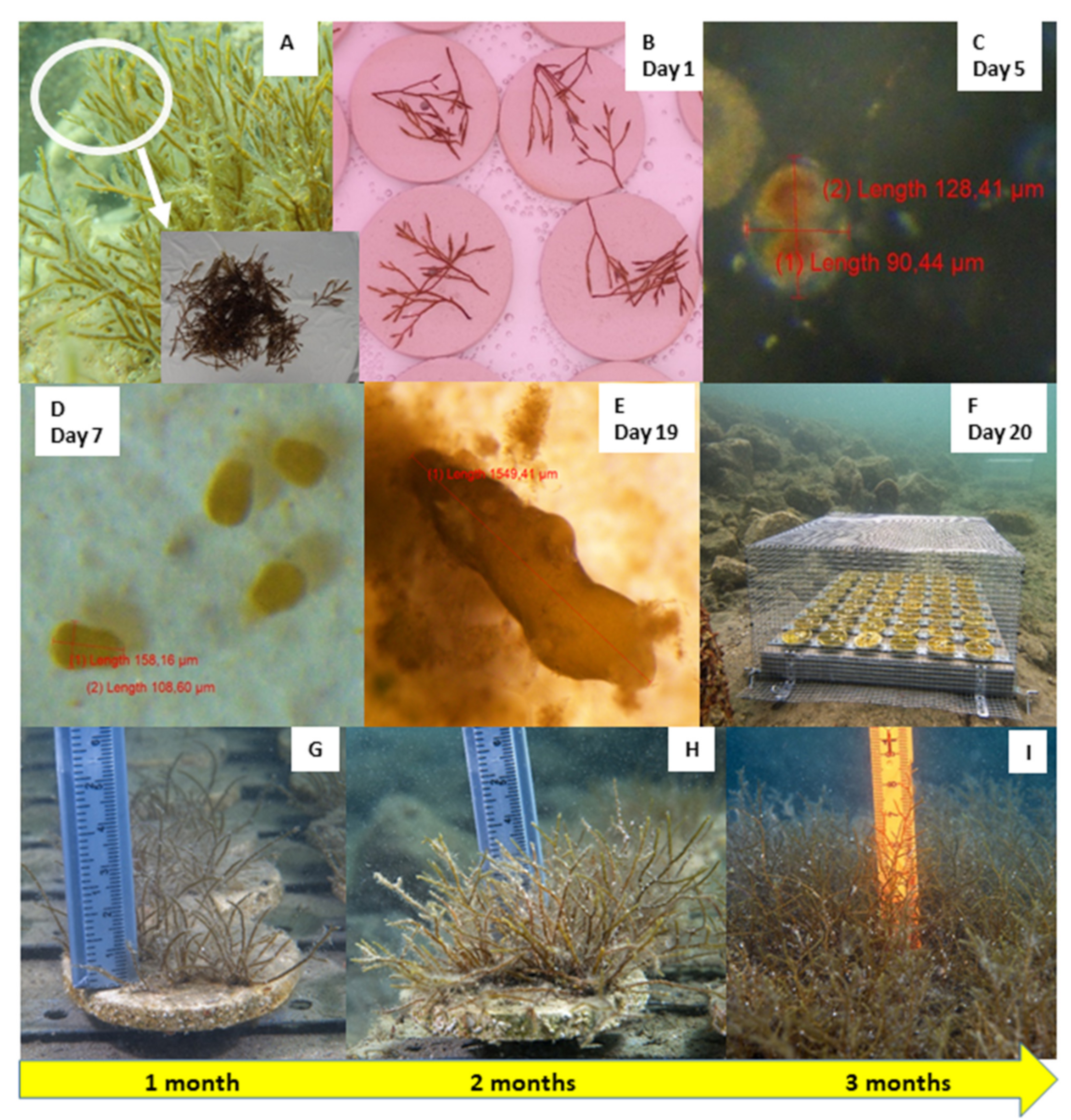
3.1. Laboratory (Ex Situ) Cultivation
3.2. Outplanting and Monitoring in the Field
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orellana, S.; Hernández, M.; Sansón, M. Diversity of Cystoseira sensu lato (Fucales, Phaeophyceae) in the eastern Atlantic and Mediterranean based on morphological and DNA evidence, including Carpodesmia gen. emend. and Treptacantha gen. emend. Eur. J. Phycol. 2019, 54, 447–465. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Ballesteros, E.; Francour, P.; Guidetti, P.; Meinesz, A.; Thibaut, T.; Mangialajo, L. Conservation and Restoration of Marine Forests in the Mediterranean Sea and the Potential Role of Marine Protected Areas. AIOL 2013, 4, 83–101. [Google Scholar] [CrossRef]
- Steneck, R.S.; Johnson, C.R. Kelp Forests: Dynamic Patterns, Processes, and Feedbacks. In Marine Community Ecology and Conservation; Bertness, M.D., Bruno, J.F., Silliman, B.R., Stachowicz, J.J., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2014; pp. 315–336. [Google Scholar]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Blanfuné, A. The ups and downs of a canopy-forming seaweed over a span of more than one century. Sci. Rep. 2019, 9, 5250. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, E. Production of seaweeds in Northwestern Mediterranean marine communities: Its relation with environmental factors. Sci. Mar. 1989, 53, 357–364. [Google Scholar]
- Gianni, F.; Bartolini, F.; Pey, A.; Laurent, N.; Martins, G.M.; Airoldi, L.; Mangialajo, L. Threats to large brown algal forests in temperate seas: The overlooked role of native herbivorous fish. Sci. Rep. 2017, 7, 6012. [Google Scholar] [CrossRef] [PubMed]
- Orlando-Bonaca, M.; Lipej, L. Factors affecting habitat occupancy of fish assemblage in the Gulf of Trieste (Northern Adriatic Sea). Mar. Ecol. 2005, 26, 42–53. [Google Scholar] [CrossRef]
- Ballesteros, E.; Garrabou, J.; Hereu, B.; Zabala, M.; Cebrian, E.; Sala, E. Deep-water stands of Cystoseira zosteroides C. Agardh (Fucales, Ochrophyta) in the Northwestern Mediterranean: Insights into assemblage structure and population dynamics. Estuar. Coast. Shelf. Sci. 2009, 82, 477–484. [Google Scholar] [CrossRef]
- Cheminée, A.; Sala, E.; Pastor, J.; Bodilis, P.; Thiriet, P.; Mangialajo, L.; Cottalorda, J.-M.; Francour, P. Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Mar. Biol. Ecol. 2013, 442, 70–79. [Google Scholar] [CrossRef]
- Pitacco, V.; Orlando-Bonaca, M.; Mavrič, B.; Popovič, A.; Lipej, L. Mollusc fauna associated with the Cystoseira algal associations in the Gulf of Trieste (northern Adriatic Sea). Mediterr. Mar. Sci. 2014, 15, 225–238. [Google Scholar] [CrossRef]
- Bruno de Sousa, C.; Gangadhar, K.N.; Macridachis, J.; Pavão, M.; Morais, T.R.; Campino, L.; Varela, J.; Lago, J.H.G. Cystoseira algae (Fucaceae): Update on their chemical entities and biological activities. Tetrahedron Asymmetry 2017, 28, 1486–1505. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Laroche-Clary, A.; Robert, J.; Bouraoui, A. Anti-inflammatory, antiproliferative and antioxidant activities of organic extracts from the Mediterranean seaweed, Cystoseira crinita. Afr. J. Biotechnol. 2011, 10, 16682–16690. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Custódio, L.; Acosta, G.; Lago, J.H.G.; Morais, T.R.; Bruno de Sousa, C.; Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Lima, R.T.; et al. Can macroalgae provide promising anti-tumoral compounds? A closer look at Cystoseira tamariscifolia as a source for antioxidant and anti-hepatocarcinoma compounds. PeerJ 2016, 4, e1704. [Google Scholar] [CrossRef] [PubMed]
- Bruno de Sousa, C.; Gangadhar, K.N.; Morais, T.R.; Conserva, G.A.; Vizetto-Duarte, C.; Pereira, H.; Laurenti, M.D.; Campino, L.; Levy, D.; Uemi, M.; et al. Antileishmanial activity of meroditerpenoids from the macroalgae Cystoseira baccata. Exp. Parasitol. 2017, 174, 1–9. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme (UNEP). Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and Its Protocols. Mediterranean Action Plan-Barcelona Convention Secretariat. 2019, p. 153. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/31970/bcp2019_web_eng.pdf (accessed on 3 November 2020).
- Council of Europe. Bern Convention/Convention de Berne: Convention on the Conservation of European Wildlife and Natural Habitats/Convention Relative à la Conservation Dela Vie Sauvage et du Milieu Naturel de l’Europe; Appendix/Annexe I, 19.IX.1979; Council of Europe: Strasbourg, France, 1979. [Google Scholar]
- Directive, H. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- Airoldi, L.; Bulleri, F. Anthropogenic Disturbance Can Determine the Magnitude of Opportunistic Species Responses on Marine Urban Infrastructures. PLoS ONE 2011, 6, e22985. [Google Scholar] [CrossRef]
- Rinne, H.; Salovius-Laurén, S.; Mattila, J. The occurrence and depth penetration of macroalgae along environmental gradients in the northern Baltic Sea. Estuar. Coast. Shelf. Sci. 2011, 94, 182–191. [Google Scholar] [CrossRef]
- Orfanidis, S.; Dencheva, K.; Nakou, K.; Tsioli, S.; Papathanasiou, V.; Rosati, I. Benthic macrophyte metrics as bioindicators of water quality: Towards overcoming typological boundaries and methodological tradition in Mediterranean and Black Seas. Hydrobiologia 2014, 740, 61–78. [Google Scholar] [CrossRef]
- Catra, M.; Alongi, G.; Leonardi, R.; Negri, M.; Sanfilippo, R.; Sciuto, F.; Serio, D.; Viola, A.; Rosso, A. Degradation of a photophilic algal community and its associated fauna from eastern Sicily (Mediterranean Sea). Mediterr. Mar. Sci. 2019, 20, 74–89. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Verlaque, M. Decline and local extinction of Fucales in French Riviera: The harbinger of future extinctions? Mediterr. Mar. Sci. 2015, 16, 206–224. [Google Scholar] [CrossRef]
- Iveša, L.; Djakovac, T.; Devescovi, M. Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Pollut. Bull. 2016, 106, 162–173. [Google Scholar] [CrossRef]
- Rindi, L.; Bello, M.; Dai, L.; Gore, J.; Benedetti-Cecchi, L. Direct observation of increasing recovery length before collapse of a marine benthic ecosystem. Nat. Ecol. Evol. 2017, 1, 0153. [Google Scholar] [CrossRef] [PubMed]
- Rindi, L.; Dal Bello, M.; Benedetti-Cecchi, L. Experimental evidence of spatial signatures of approaching regime shifts in macroalgal canopies. Ecology 2018, 99, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Perkol-Finkel, S.; Airoldi, L. Loss and Recovery Potential of Marine Habitats: An Experimental Study of Factors Maintaining Resilience in Subtidal Algal Forests at the Adriatic Sea. PLoS ONE 2010, 5, e10791. [Google Scholar] [CrossRef] [PubMed]
- Gorman, D.; Russell, B.D.; Connell, S.D. Land-to-sea connectivity: Linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts. Ecol. Appl. 2009, 19, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Connell, S.D.; Foster, M.S.; Airoldi, L. What Are Algal Turfs? Towards a Better Description of Turfs. Mar. Ecol. Prog. Ser. 2014, 495, 299–307. [Google Scholar] [CrossRef]
- Strain, E.M.; Thomson, R.J.; Micheli, F.; Mancuso, F.P.; Airoldi, L. Identifying the Interacting Roles of Stressors in Driving the Global Loss of Canopy-Forming to Mat-Forming Algae in Marine Ecosystems. Glob. Chang. Biol. 2014, 20, 3300–3312. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Tamburello, L.; Bulleri, F.; Maggi, E.; Gennusa, V.; Miller, M. Linking patterns and processes across scales: The application of scale-transition theory to algal dynamics on rocky shores. J. Exp. Biol. 2012, 215, 977–985. [Google Scholar] [CrossRef]
- Tsiamis, K.; Panayotidis, P.; Salomidi, M.; Pavlidou, A.; Kleinteich, J.; Balanika, K.; Küpper, F.C. Macroalgal community response to re-oligotrophication in Saronikos Gulf. Mar. Ecol. Prog. Ser. 2013, 472, 73–85. [Google Scholar] [CrossRef]
- De La Fuente, G.; Chiantore, M.; Gaino, F.; Asnaghi, V. Ecological status improvement over a decade along the Ligurian coast according to a macroalgae based index (CARLIT). PLoS ONE 2018, 13, e0206826. [Google Scholar] [CrossRef]
- Rilov, G.; Peleg, O.; Yeruham, E.; Garval, T.; Vichik, A.; Raveh, O. Alien turf: Overfishing, overgrazing and invader domination in south-eastern Levant reef ecosystems. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 28, 351–369. [Google Scholar] [CrossRef]
- Grbec, B.; Matić, F.; Beg Paklar, G.; Morović, M.; Popović, R.; Vilibić, I. Long-term trends, variability and extremes of in situ sea surface temperature measured along the Eastern Adriatic Coast and its relationship to hemispheric processes. Pure Appl. Geophys. 2018, 175, 4031–4046. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Kim, H.; Watson, W.; Di Lorenzo, E.; Sugihara, G. Climate-driven changes in abundance and distribution of larvae of oceanic fishes in the southern California region. Glob. Chang. Biol. 2009, 15, 2137–2152. [Google Scholar] [CrossRef]
- Nikolić, V.; Žuljević, A.; Mangialajo, L.; Antolić, B.; Kušpilić, G.; Ballesteros, E. Cartography of littoral rocky-shore communities (CARLIT) as a tool for ecological quality assessment of coastal waters in the Eastern Adriatic Sea. Ecol. Indic. 2013, 34, 87–93. [Google Scholar] [CrossRef]
- Agnetta, D.; Badalamenti, F.; Ceccherelli, G.; Di Trapani, F.; Bonaviri, C.; Gianguzza, P. Role of two co-occurring Mediterranean Sea urchins in the formation of barren from Cystoseira canopy. Estuar. Coast. Shelf Sci. 2015, 152, 73–77. [Google Scholar] [CrossRef]
- Medrano, A.; Linares, C.; Aspillaga, E.; Capdevila, P.; Montero-Serra, I.; Pagès-Escolà, M.; Hereu, B. No-take marine reserves control the recovery of sea urchin populations after mass mortality events. Mar. Environ. Res. 2019, 145, 147–154. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Blanfuné, A.; Pergent, G.; Pergent-Martini, C.; Perret-Boudouresque, M.; Thibaut, T. Impacts of Marine and Lagoon Aquaculture on Macrophytes in Mediterranean Benthic Ecosystems. Front. Mar. Sci. 2020, 7, 218. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Mangialajo, L. Reduction of herbivorous fish pressure can facilitate focal algal species forestation on artificial structures. Mar. Environ. Res. 2018, 138, 102–109. [Google Scholar] [CrossRef]
- Gianni, F.; Mačić, V.; Bartolini, F.; Pey, A.; Laurent, M.; Mangialajo, L. Optimizing canopy-forming algae conservation and restoration with a new herbivorous Fish Deterrent device (DeFish). Restor. Ecol. 2020, 28, 750–756. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-Term Decline of the Populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Alberes Coast (France, North-Western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Falace, A.; Kaleb, S.; De La Fuente, G.; Asnaghi, V.; Chiantore, M. Ex Situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PLoS ONE 2018, 13, e0193011. [Google Scholar] [CrossRef]
- Verdura, J.; Sales, M.; Ballesteros, E.; Cefalì, M.E.; Cebrian, E. Restoration of a Canopy-Forming Alga Based on Recruitment Enhancement: Methods and Long-Term Success Assessment. Front. Plant Sci. 2018, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- Savonitto, G.; De La Fuente, G.; Tordoni, E.; Ciriaco, S.; Srijemsi, M.; Bacaro, G.; Chiantore, M.; Falace, A. Addressing reproductive stochasticity and grazing impacts in the restoration of a canopy-forming brown alga by implementing mitigation solutions. Aquat. Conserv. 2021, in press. [Google Scholar]
- Hobbs, R.J.; Davis, M.A.; Slobodkin, L.B.; Lackey, R.T.; Halvorson, W.; Throop, W. Restoration ecology: The challenge of social values and expectations. Front. Ecol. Environ. 2004, 2, 43–48. [Google Scholar] [CrossRef]
- Abelson, A.; Reed, D.C.; Edgar, G.J.; Smith, C.S.; Kendrick, G.A.; Orth, R.J.; Airoldi, L.; Silliman, B.; Beck, M.W.; Krause, G.; et al. Challenges for Restoration of Coastal Marine Ecosystems in the Anthropocene. Front. Mar. Sci. 2020, 7, 544105. [Google Scholar] [CrossRef]
- Falace, A.; Zanelli, E.; Bressan, G. Algal transplantation as a potential tool for artificial reef management and environmental mitigation. Bull. Mar. Sci. 2006, 8, 161–166. [Google Scholar]
- Susini, M.L.; Mangialajo, L.; Thibaut, T.; Meinesz, A. Development of a transplantation technique of Cystoseira amentacea var. stricta and Cystoseira compressa. Hydrobiologia 2007, 580, 241–244. [Google Scholar] [CrossRef]
- Medrano, A.; Hereu, B.; Cleminson, M.; Pagès-Escolà, M.; la Rovira, G.; Solà, J.; Linares, C. From marine deserts to algal beds: Treptacantha elegans revegetation to reverse stable degraded ecosystems inside and outside a no-take marine reserve. Restor. Ecol. 2020, 28, 632–644. [Google Scholar] [CrossRef]
- Sales, M.; Cebrian, E.; Tomas, F.; Ballesteros, E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf Sci. 2011, 92, 347–357. [Google Scholar] [CrossRef]
- De La Fuente, G.; Chiantore, M.; Asnaghi, V.; Kaleb, S.; Falace, A. First ex situ outplanting of the habitat-forming seaweed Cystoseira amentacea var. stricta from a restoration perspective. PeerJ 2019, 7, e7290. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Susini, M.-L.; Meinesz, A.; Cattaneo-Vietti, R.; Thibaut, T. Zonation patterns and interspecific relationships of fucoids in microtidal environments. J. Exp. Mar. Biol. Ecol. 2012, 412, 72–80. [Google Scholar] [CrossRef]
- Water Framework Directive. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060 (accessed on 10 October 2020).
- Marine Strategy Framework Directive. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0056 (accessed on 15 October 2020).
- Orlando-Bonaca, M.; Lipej, L.; Orfanidis, S. Benthic macrophytes as a tool for delineating, monitoring and assessing ecological status: The case of Slovenian coastal waters. Mar. Pollut. Bull. 2008, 56, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Orlando-Bonaca, M.; Rotter, A. Any signs of replacement of canopy-forming algae by turf-forming algae in the northern Adriatic Sea? Ecol. Indic. 2018, 87, 272–284. [Google Scholar] [CrossRef]
- Mariani, S.; Cefalì, M.E.; Chappuis, E.; Terradas, M.; Pinedo, S.; Torras, X.; Jordana, E.; Medrano, A.; Verdura, J.; Ballesteros, E. Past and present of Fucales from shallow and sheltered shores in Catalonia. Reg. Stud. Mar. Sci. 2019, 32, 100824. [Google Scholar] [CrossRef]
- Iveša, L. Effects of increased seawater temperature and benthic mucilage formation on shallow Cystoseira forests of the West Istrian coast (northern Adriatic Sea). Seventh European Phycological Congress. Eur. J. Phycol. 2019, 54, 96. [Google Scholar] [CrossRef]
- Mozetič, P.; Francé, J.; Kogovšek, T.; Talaber, I.; Malej, A. Plankton trends and community changes in a coastal sea (northern Adriatic): Bottom-up vs. top-down control in relation to environmental drivers. Estuar. Coast. Shelf. Sci. 2012, 115, 138–148. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Mavrič, B. Recurrence of Sargassum vulgare C. Agardh in Slovenian coastal waters (Adriatic Sea). Ann. Ser. Hist. Nat. 2014, 24, 109–114. [Google Scholar]
- Talaber, I.; Francé, J.; Flander-Putrle, V.; Mozetič, P. Primary production and community structure of coastal phytoplankton in the Adriatic Sea: Insights on taxon-specific productivity. Mar. Ecol. Prog. Ser. 2018, 604, 65–81. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: https://www.R-project.org/ (accessed on 15 October 2020).
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’ Create Elegant Data Visualisations Using the Grammar of Graphics; Version 2. 2016, pp. 1–189. Available online: https://github.com/tidyverse/ggplot2 (accessed on 15 October 2020).
- Solidoro, C.; Bastianini, M.; Bandelj, V.; Codermatz, R.; Cossarini, G.; Melaku Canu, D.; Ravagnan, E.; Salon, S.; Trevisani, S. Current state, scales of variability, and trends of biogeochemical properties in the northern Adriatic Sea. J. Geophys. Res. 2009, 114, C07S91. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Savonitto, G.; Lipizer, M.; Mancuso, P.; Ciriaco, S.; Srijemsi, M.; Falace, A. Climatic anomalies may create a long-lasting ecological phase shift by altering the reproduction of a foundation species. Ecology 2019, 100, e02838. [Google Scholar] [CrossRef] [PubMed]
- Bulleri, F.; Eriksson, B.K.; Queirós, A.; Airoldi, L.; Arenas, F.; Arvanitidis, C.; Bouma, T.J.; Crowe, T.P.; Davoult, D.; Guizien, K.; et al. Harnessing positive species interactions as a tool against climate-driven loss of coastal biodiversity. PLoS Biol. 2018, 16, e2006852. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Martins, I.; Rosa, R.; Matos, A.R.; Roleda, M.Y.; Reusch, T.B.H.; Engelen, A.H.; Serrão, E.A.; Pearson, G.A.; Marques, J.C.; et al. Climate change impacts on seagrass meadows and macroalgal forests: An integrative perspective on acclimation and adaptation potential. Front. Mar. Sci. 2018, 5, 190. [Google Scholar] [CrossRef]
- Verdura, J.; Verges, A.; Santamaría, J.; de Caralt, S.; Ballesteros, E.; Cebrian, E. Drastic effects of climate change on Mediterranean marine forests. PeerJ. Prepr. 2018, 6, e26804v2. [Google Scholar] [CrossRef][Green Version]
- Assis, J.; Fragkopoulou, E.; Frade, D.; Neiva, J.; Oliveira, A.; Abecasis, D.; Faugeron, S.; Serrão, E.A. A fine-tuned global distribution dataset of marine forests. Sci. Data 2020, 7, 119. [Google Scholar] [CrossRef]
- Martínez, B.; Arenas, F.; Rubal, M.; Burgués, S.; Esteban, R.; García-Plazaola, I.; Figueroa, F.L.; Pereira, R.; Saldaña, L.; Sousa-Pinto, I.; et al. Physical factors driving intertidal macroalgae distribution: Physiological stress of a dominant fucoid at its southern limit. Oecologia 2012, 170, 341–353. [Google Scholar] [CrossRef]
- Olischläger, M.; Wild, C. How does the sexual reproduction of marine life respond to ocean acidification? Diversity 2020, 12, 241. [Google Scholar] [CrossRef]
- Coleman, M.A.; Goold, H. Harnessing synthetic biology for kelp forest conservation. J. Phycol. 2019, 55, 745–751. [Google Scholar] [CrossRef]
- Cerino, F.; Fornasaro, D.; Kralj, M.; Giani, M.; Cabrini, M. Phytoplankton temporal dynamics in the coastal waters of the north-eastern Adriatic Sea (Mediterranean Sea) from 2010 to 2017. Nat. Conserv. 2019, 34, 343–372. [Google Scholar] [CrossRef]
- Kralj, M.; Lipizer, M.; Čermelj, B.; Celio, M.; Fabbro, C.; Brunetti, F.; Francé, J.; Mozetič, P.; Giani, M. Hypoxia and dissolved oxygen trends in the northeastern Adriatic Sea (Gulf of Trieste). Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 164, 74–88. [Google Scholar] [CrossRef]
- Giovanardi, F.; France, J.; Mozetič, P.; Precali, R. Development of ecological classification criteria for the Biological Quality Element phytoplankton for Adriatic and Tyrrhenian coastal waters by means of chlorophyll a (2000/60/EC WFD). Ecol. Ind. 2018, 93, 316–332. [Google Scholar] [CrossRef]
- Vergés, A.; Alcoverro, T.; Ballesteros, E. The role of fish herbivory in structuring the vertical distribution of canopy algae (Cystoseira spp.) in the Mediterranean Sea. Mar. Ecol. Progr. Ser. 2009, 375, 1–11. [Google Scholar] [CrossRef]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A.; et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. PNAS 2016, 113, 13791–13796. [Google Scholar] [CrossRef] [PubMed]
- Vukovič, A. Spatial distribution and dynamics of the benthic vegetation in the Gulf of Piran. Sci. Rep. 1976, 7, 73. (In Slovenian) [Google Scholar]
- Turk, R.; Vukovič, A. Preliminary inventory and topography of flora and fauna in the marine part of the Strunjan Nature Reserve. Annales Ser. Hist. Nat. 1994, 4, 101–112. (In Slovenian) [Google Scholar]
- Buñuel, X.; Alcoverro, T.; Pagès, J.F.; Romero, J.; Ruiz, J.M.; Arthur, R. The dominant seagrass herbivore Sarpa salpa shifts its shoaling and feeding strategies as they grow. Sci. Rep. 2020, 10, 10622. [Google Scholar] [CrossRef] [PubMed]
- Grilli, F.; Accoroni, S.; Acri, F.; Bernardi Aubry, F.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and Interannual Trends of Oceanographic Parameters over 40 Years in the Northern Adriatic Sea in Relation to Nutrient Loadings Using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Capdevila, P.; Linares, C.; Aspillaga, E.; Navarro, L.; Kersting, D.; Hereu, B. Recruitment patterns in the Mediterranean deep-water alga Cystoseira zosteroides. Mar. Biol. 2015, 162, 1165–1174. [Google Scholar] [CrossRef]

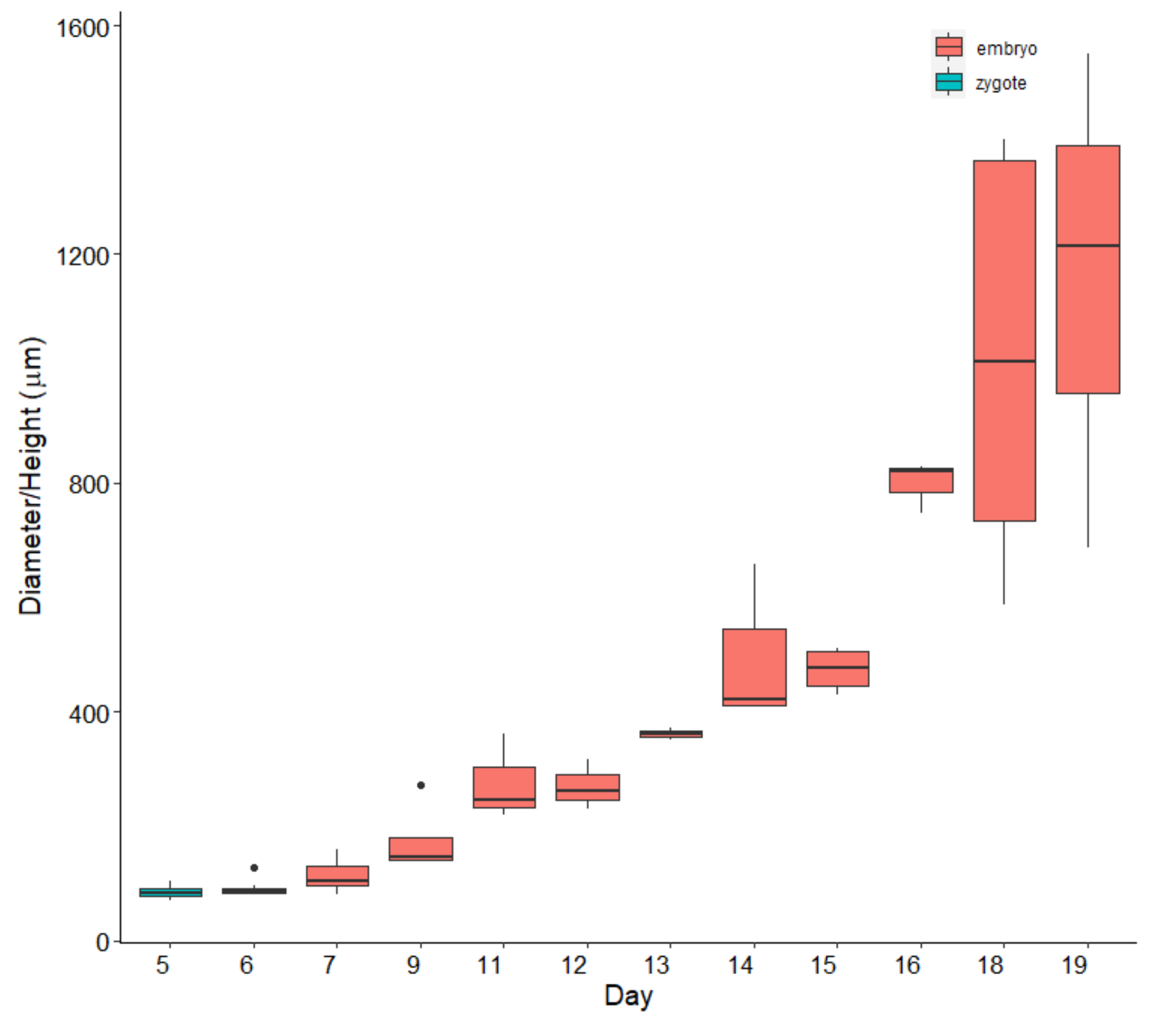
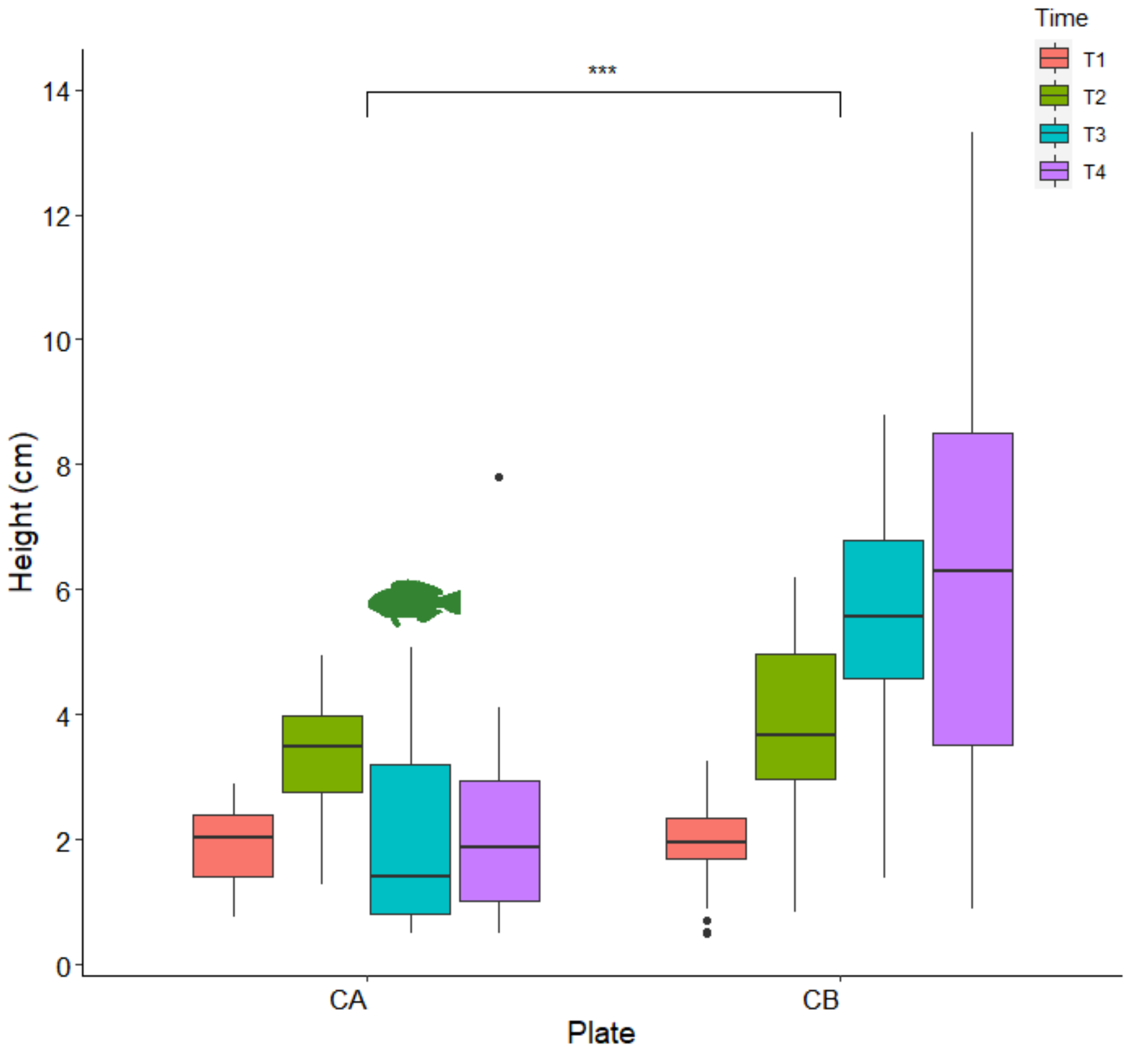
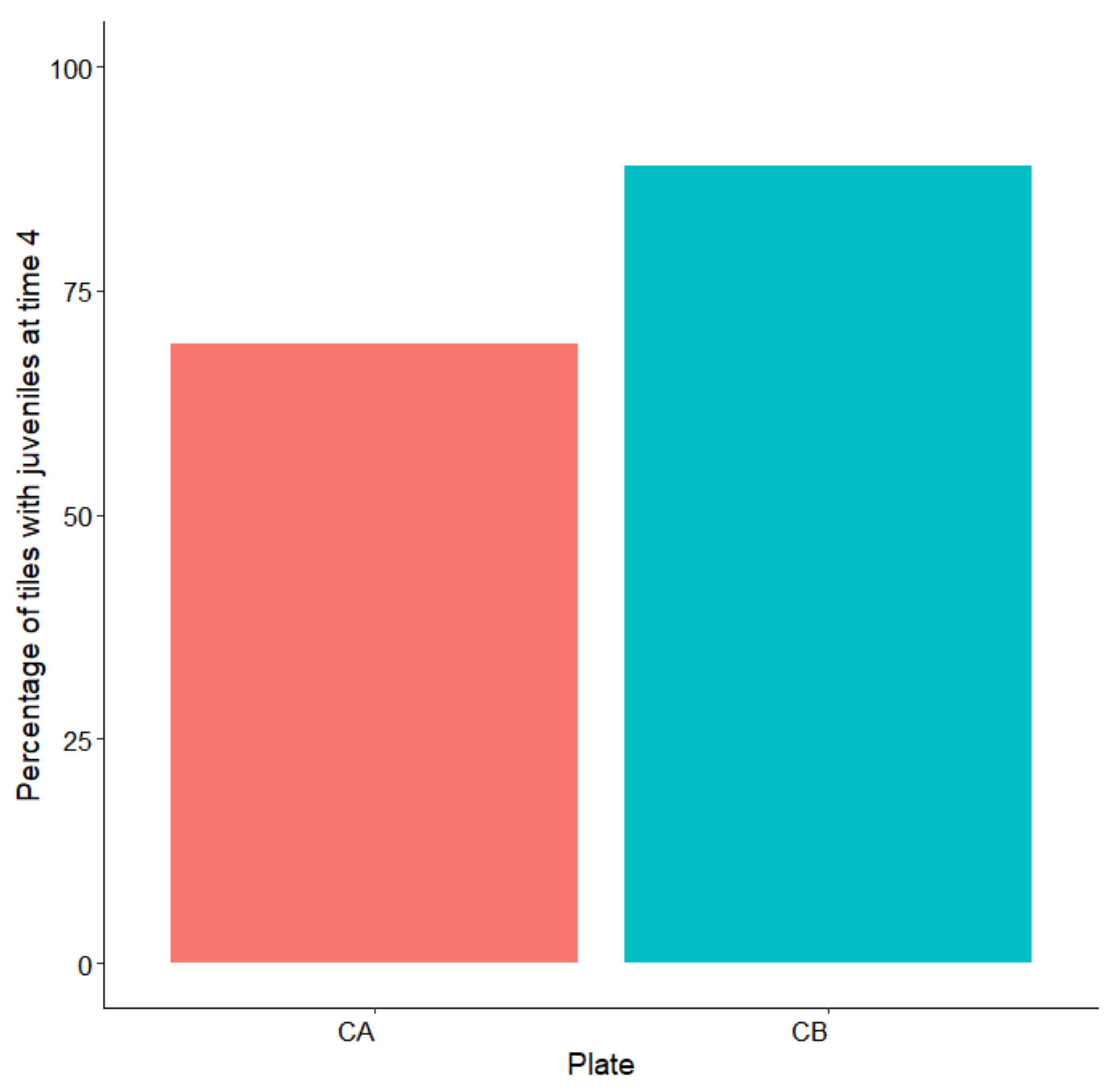
| Factor | df | Sum sq | Mean sq | F-Ratio | p-Value |
|---|---|---|---|---|---|
| Time | 3 | 303.9 | 101.3 | 39.8 | <0.0001 |
| Plate | 1 | 138.7 | 138.7 | 54.5 | <0.0001 |
| Time × Plate | 3 | 215.5 | 71.8 | 28.2 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando-Bonaca, M.; Pitacco, V.; Slavinec, P.; Šiško, M.; Makovec, T.; Falace, A. First Restoration Experiment for Gongolaria barbata in Slovenian Coastal Waters. What Can Go Wrong? Plants 2021, 10, 239. https://doi.org/10.3390/plants10020239
Orlando-Bonaca M, Pitacco V, Slavinec P, Šiško M, Makovec T, Falace A. First Restoration Experiment for Gongolaria barbata in Slovenian Coastal Waters. What Can Go Wrong? Plants. 2021; 10(2):239. https://doi.org/10.3390/plants10020239
Chicago/Turabian StyleOrlando-Bonaca, Martina, Valentina Pitacco, Petra Slavinec, Milijan Šiško, Tihomir Makovec, and Annalisa Falace. 2021. "First Restoration Experiment for Gongolaria barbata in Slovenian Coastal Waters. What Can Go Wrong?" Plants 10, no. 2: 239. https://doi.org/10.3390/plants10020239
APA StyleOrlando-Bonaca, M., Pitacco, V., Slavinec, P., Šiško, M., Makovec, T., & Falace, A. (2021). First Restoration Experiment for Gongolaria barbata in Slovenian Coastal Waters. What Can Go Wrong? Plants, 10(2), 239. https://doi.org/10.3390/plants10020239