Insight into the Biological Activity of Hennosides—Glucosides Isolated from Lawsonia inermis (henna): Could They Be Regarded as Active Constituents Instead
Abstract
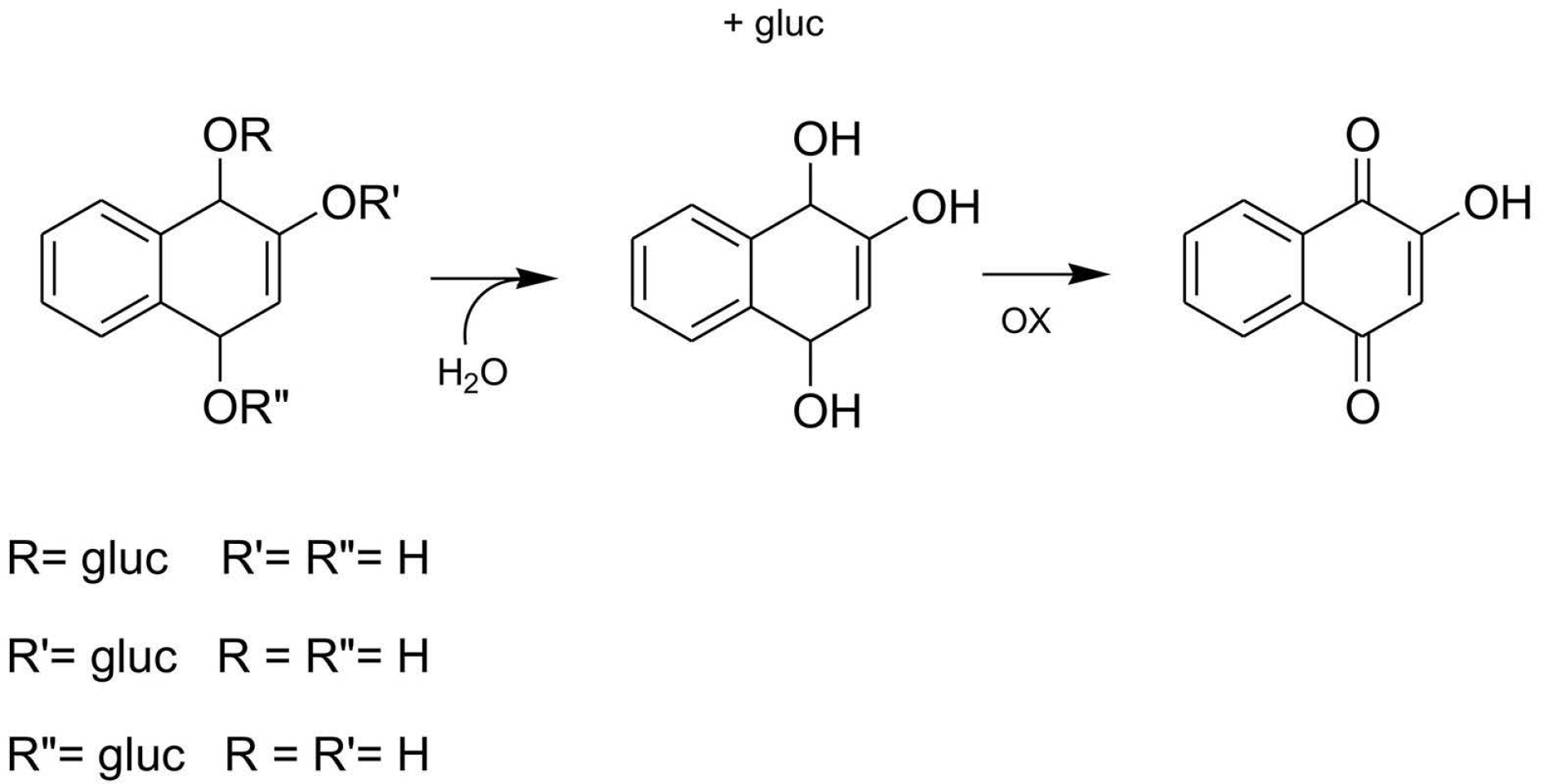
:1. Introduction
2. Results
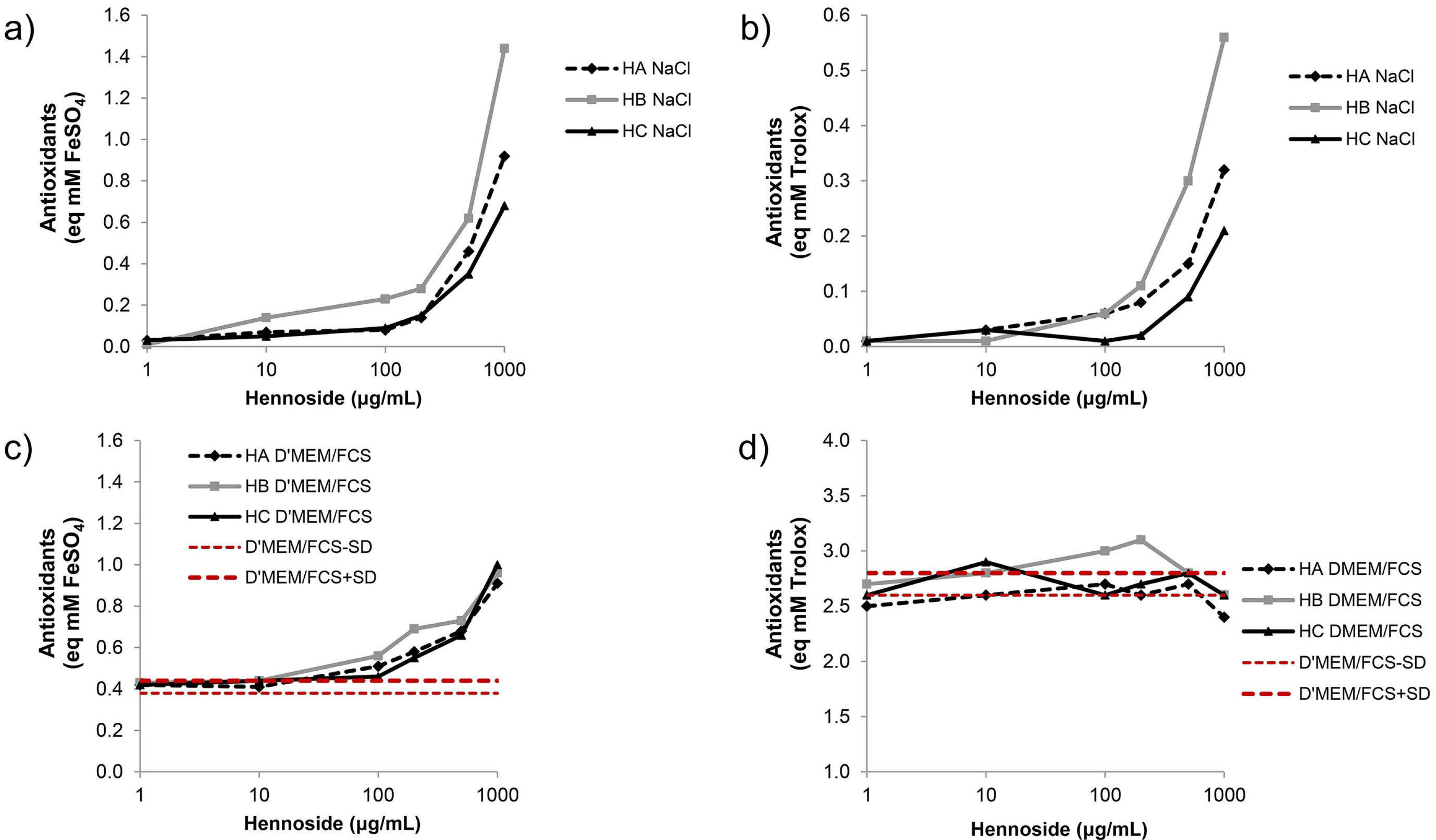
2.1. Antioxidant Activity of Hennosides
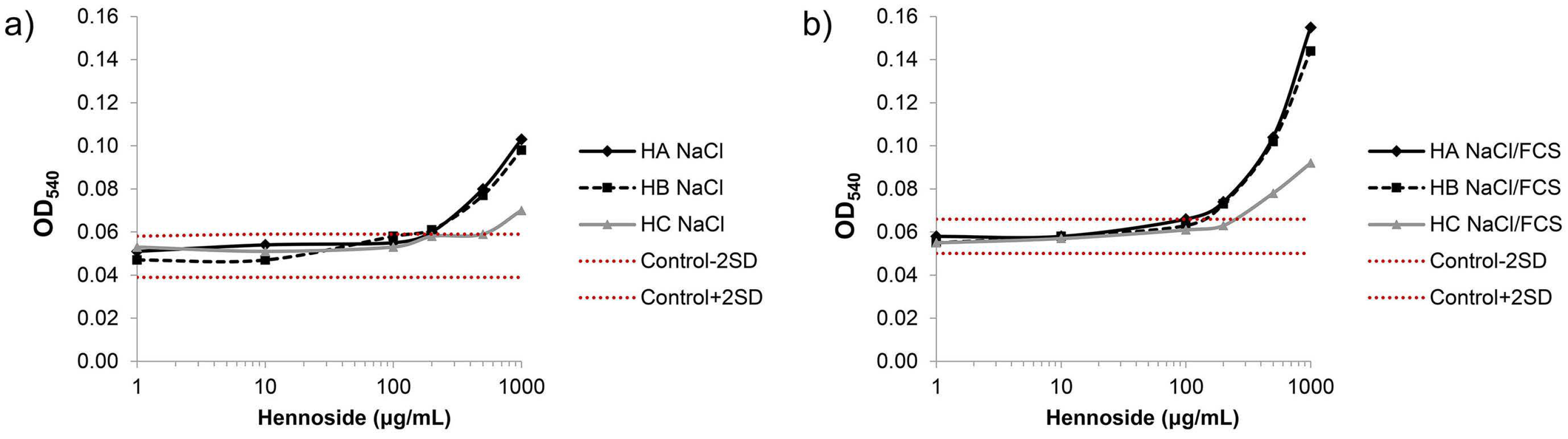
2.2. Effect of Hennosides on Erythrocyte lysis
2.3. Effect of Hennosides on Hemoglobin
2.4. Effect of Hennosides on Cell Viability
2.5. Antioxidant Activity of Culture Supernatant of Hennoside-Treated Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material, Authentication
4.2. Sample Preparation
4.3. Antioxidant Capacity Estimation
4.3.1. Ferric-Reducing Antioxidant Power Assay
4.3.2. ABTS Radical Cation Decolorization Assay
4.4. In Vitro Erythrocyte Lysis
4.5. VIS Spectroscopy of Hemoglobin
4.6. MTT (Thiazolyl Blue Tetrazolium Bromide) Assay
4.7. Antioxidant Activity in the Cell Culture Supernatants
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Cartwright-Jones, C.; Viljoen, A. Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J. Ethnopharmacol. 2014, 155, 80–133. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Anderson, R. Body piercing, tattooing, self-esteem, and body investment in adolescent girls. Adolescence 2002, 37, 627–637. [Google Scholar] [PubMed]
- Dweck, A.C. Natural ingredients for colouring and styling. Int. J. Cosmet. Sci. 2002, 24, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Temporary Tattoos & Henna/Mehndi. Available online: https://www.fda.gov/cosmetics/cosmetic-products/temporary-tattoos-hennamehndi-and-black-henna-fact-sheet (accessed on 10 December 2020).
- FDA, Food and Drug Administration. Color Additives Permitted for Use in Cosmetics. Available online: https://www.fda.gov/cosmetics/cosmetic-ingredient-names/color-additives-permitted-use-cosmetics (accessed on 10 December 2020).
- Kapadia, G.J.; Fayez, M.B.E.; Sethi, M.L. Hennosides, the primary glycosidic constituents of henna. J. Nat. Prod. Lloydia 1969, 32, 523. [Google Scholar]
- Gallo, F.R.; Multari, G.; Palazzino, G.; Pagliuca, G.; Majid Majd Zadeh, S.; Prosper Cabral Nya, B.; Nicoletti, M. Henna through the centuries: A quick HPTLC analysis proposal to check henna identity. Rev. Bras. De Farmacogn. 2014, 24, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, M.; Petitto, V.; Gallo, F.R.; Multari, G.; Federici, E.; Palazzino, G. The modern analytical determination of botanicals and similar novel natural products by the HPTLC fingerprint approach. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 37, pp. 217–258. [Google Scholar]
- Anonymous. Papyrus Ebers. Calif. State J. Med. 1912, 19, 219. [Google Scholar]
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef]
- Sherif, S.M.; Amal, E.I. Analysis of retinal b-wave by fourier transformation due to ammonia exposure and the role of blood erythrocytes. Rom. J. Biophys. 2010, 20, 269–281. [Google Scholar]
- Hanson, E.K.; Ballantyne, J. A blue spectral shift of the hemoglobin Soret band correlates with the age (time since deposition) of dried bloodstains. PLoS ONE 2010, 5, e12830. [Google Scholar] [CrossRef] [Green Version]
- Kukolj, T.; Trivanović, D.; Djordjević, I.O.; Mojsilović, S.; Krstić, J.; Obradović, H.; Janković, S.; Santibanez, J.F.; Jauković, A.; Bugarski, D. Lipopolysaccharide can modify differentiation and immunomodulatory potential of periodontal ligament stem cells via ERK1,2 signaling. J. Cell. Physiol. 2018, 233, 447–462. [Google Scholar] [CrossRef]
- Duke, J.A. Database of Biologically Active Phytochemicals and Their Activity; eBook; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Kök, N.; Ertekin, M.V.; Ertekin, V.; Avci, B. Henna (Lawsonia inermis Linn.) induced haemolytic anaemia in siblings. Int. J. Clin. Pract. 2004, 58, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Raupp, P.; Hassan, J.A.; Varughese, M.; Kristiansson, B. Henna causes life threatening hemolysis in glucose-6-phosphate dehydrogenase deficiency. Arch. Dis. Child. 2001, 85, 411–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameneva, M.V.; Antaki, J.F.; Yeleswarapu, K.K.; Watach, M.J.; Griffith, B.P.; Borovetz, H.S. Plasma protective effect on red blood cells exposed to mechanical stress. ASAIO J. 1997, 43, M571–M575. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; Sarvate, S.D.; Oatis, J.E., Jr.; Jollow, D.J. Role of oxidant stress in lawsone-induced hemolytic anemia. Toxicol. Sci. 2004, 82, 647–655. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Lewinska, A.; Wnuk, M.; Slota, E.; Bartosz, G. Total anti-oxidant capacity of cell culture media. Clin. Exp. Pharmacol. Physiol. 2007, 34, 781–786. [Google Scholar] [CrossRef]
- López López, L.I.; Flores, N.; Daniel, S.; Silva Belmares, S.; Sáenz Galindo, A. Naphthoquinones: Biological properties and synthesis of lawsone and derivatives—A structured review. Vitae 2014, 21, 248–258. [Google Scholar]
- Chaudhary, G.; Goyal, S.; Poonia, P. Lawsonia inermis Linnaeus: A phytopharmacological review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 91–98. Available online: https://ijpsdr.com/index.php/ijpsdr/article/view/89/77 (accessed on 10 December 2020).
- Zinkham, W.H.; Oski, F.A. Henna: A potential cause of oxidative hemolysis and neonatal hyperbilirubinemia. Pediatrics 1996, 97, 707–709. [Google Scholar]
- Das, D.; Samanta, D.; Banerjee, R.; Sinha, S.; Mallick, B.; Ganguli, S.; Roy, D. Insights into the phytochemical potential of Lawsonia inermis L. for future small molecule based therapeutic applications. Review. Int. Res. J. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Matulich, J.; Sullivan, J. A temporary henna tattoo causing hair and clothing dye allergy. Contact Dermat. 2005, 53, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 2017, 60, 3221–3240. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Bar-Or, R.; Rael, L.T.; Brody, E.N. Oxidative stress in severe acute illness. Redox Biol. 2015, 4, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, D.L.; Slavkoviv-Jovanovic, M.R. Allergic contact dermatitis from temporary henna tattoo. J. Dermatol. 2009, 36, 63–65. [Google Scholar] [CrossRef]
- Lamchahab, F.-Z.; Guerrouj, B.; Benomar, S.; Ait Ourhroui, M.; Senouci, K.; Hassam, B.; Benzekri, L. Henna symbolic tattoo and real dermatitis. Arch. Pediatr. 2011, 18, 653–656. [Google Scholar] [CrossRef]
- Putzbach, K.; Rimmer, C.A.; Sharpless, K.; Wise, S.A.; Sander, L.C. Determination of bitter orange alkaloids in dietary supplement Standard Reference Materials by liquid chromatography with atmospheric-pressure ionization mass spectrometry. Anal. Bioanal. Chem. 2007, 389, 197–205. [Google Scholar] [CrossRef]
- Jin, W.; Ge, R.L.; Wei, Q.J.; Bao, T.Y.; Shi, H.M.; Tu, P.F. Development of high-performance liquid chromatographic fingerprint for the quality control of Rheum tanguticum Maxim. ex Balf. J. Chromatogr. A 2006, 1132, 320–324. [Google Scholar] [CrossRef]
- Nicoletti, M.; Frezza, C.; Tomassini, L.; Toniolo, C.; Bianco, A. Detection of picramic acid and picramate in henné products by NMR Spectroscopy. Nat. Prod. Res. 2018, 33, 2073–2078. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drvenica, I.; Stančić, A.; Kalušević, A.; Marković, S.; Maksimović, J.D.; Nedović, V.; Bugarski, B.; Ilić, V. Maltose-mediated long-term stabilization of freeze-and spray-dried forms of bovine and porcine hemoglobin. J. Serb. Chem. Soc. 2019, 84, 1105–1117. [Google Scholar] [CrossRef] [Green Version]
- Trivanović, D.; Kocić, J.; Mojsilović, S.; Krstić, A.; Ilić, V.; Djordjević, I.O.; Santibanez, J.F.; Jovcić, G.; Terzić, M.; Bugarski, D. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp. Arh. Celok. Lek. 2013, 141, 178–186. [Google Scholar] [CrossRef]
- Miletić, M.; Mojsilović, S.; Okić Đorđević, I.; Kukolj, T.; Jauković, A.; Santibañez, J.F.; Jovčić, G.; Bugarski, D. Mesenchymal stem cells isolated from human periodontal ligament. Arch. Biol. Sci. 2014, 66, 261–271. [Google Scholar] [CrossRef]
- Kocić, J.; Santibañez, J.F.; Krstić, A.; Mojsilović, S.; Ilić, V.; Bugarski, D. Interleukin-17 modulates myoblast cell migration by inhibiting urokinase type plasminogen activator expression through p38 mitogen-activated protein kinase. Int. J. Biochem. Cell Biol. 2013, 45, 464–475. [Google Scholar] [CrossRef]

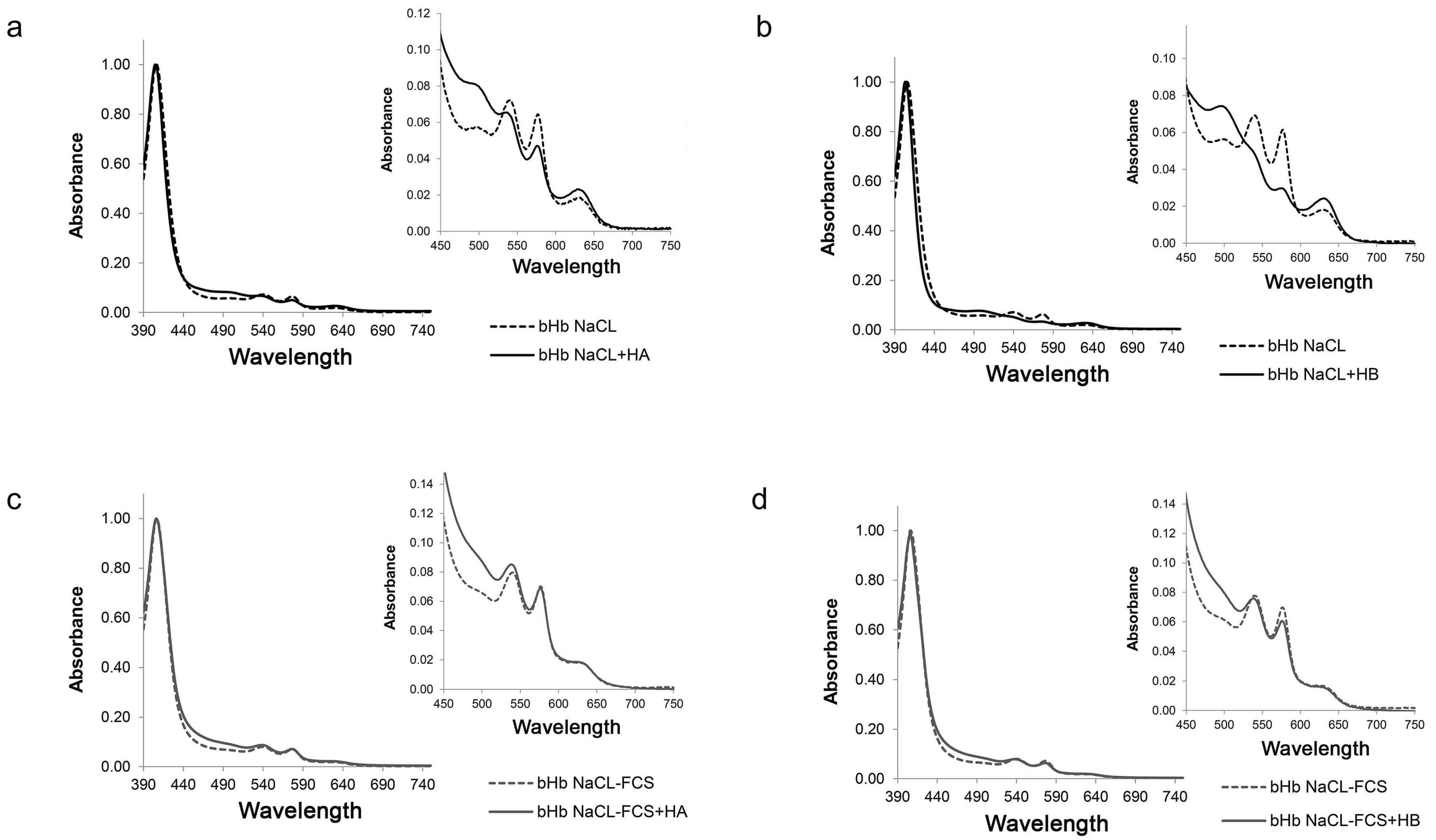



| Soret Band | Δα/Δβ | ODSoret/OD575 | OD630 | |
|---|---|---|---|---|
| Hb in NaCl | 406 | 0.70 | 15.6 | 0.018 |
| Hb in NaCl + HA | 405 | 0.29 | 20.4 | 0.026 |
| Hb in NaCl-FCS | 407 | 0.64 | 14.1 | 0.019 |
| Hb in NaCl-FCS + HA | 406 | 0.54 | 14.1 | 0.022 |
| Hb in NaCl | 406 | 0.69 | 15.9 | 0.020 |
| Hb in NaCl + HB | 403 | 0.00 | 30.3 | 0.028 |
| Hb in NaCl-FCS | 406 | 0.71 | 13.9 | 0.019 |
| Hb in NaCl-FCS + HB | 406 | 0.46 | 15.6 | 0.020 |
| Relative Viability (%) | ||||
|---|---|---|---|---|
| Hennoside | 24 h | 48 h | 72 h | |
| (μg/mL) | ||||
| MDA 231 | ||||
| HA | 0 | 100 | 100 | 100 |
| 100 | 116 | 101 | 107 | |
| HB | 0 | 100 | 100 | 100 |
| 100 | 101 | 95 | 104 | |
| HC | 0 | 100 | 100 | 100 |
| 100 | 109 | 95 | 105 | |
| MCF 7 | ||||
| HA | 0 | 100 | 100 | 100 |
| 100 | 118 | 117 | 118 | |
| HB | 0 | 100 | 100 | 100 |
| 100 | 116 | 111 | 114 | |
| HC | 0 | 100 | 100 | 100 |
| 100 | 91 | 90 | 116 | |
| PDL-MSC | ||||
| HA | 0 | 100 | 100 | 100 |
| 100 | 98 | 99 | 88 | |
| HB | 0 | 100 | 100 | 100 |
| 100 | 104 | 107 | 92 | |
| HC | 0 | 100 | 100 | 100 |
| 100 | 95 | 99 | 83 | |
| PB-MSC | ||||
| HA | 0 | 100 | 100 | 100 |
| 100 | 107 | 102 | 99 | |
| HB | 0 | 100 | 100 | 100 |
| 100 | 105 | 102 | 93 | |
| HC | 0 | 100 | 100 | 100 |
| 100 | 107 | 110 | 96 | |
| Compound | Hennoside A | Hennoside B | Hennoside C |
|---|---|---|---|
| H-5 | 8.00, d, J = 8.1 Hz | 8.32, d, J = 8.1 Hz | 7.68, d, J = 8.1 Hz |
| H-6 | 7.42, t, J = 8.1 Hz | 6.96, t, J = 8.1 Hz | 7.22, t, J = 8.1 Hz |
| H-7 | 7.22, t, J = 8.1 Hz | 7.42, t, J = 8.1 Hz | 7.24, t, J = 8.1 Hz |
| H-8 | 7.07, d, J = 8.1 Hz | 7.24, d, J = 8.1 Hz | 7.40, d, J = 8.1 Hz |
| H-3 | 6.48, s | 5.87, s | 6.18, s |
| H-1′ | 4.58, d, J = 6.5 Hz | 4.72, d, J = 6.5 Hz | 4.52, d, J = 6.5 Hz |
| H-2′-6′ | 3.80–3.26 | 3.80–3.26 | 3.80–3.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslovarić, I.; Ilić, V.; Drvenica, I.; Stančić, A.; Mojsilović, S.; Kukolj, T.; Bugarski, D.; Saso, L.; Nicoletti, M. Insight into the Biological Activity of Hennosides—Glucosides Isolated from Lawsonia inermis (henna): Could They Be Regarded as Active Constituents Instead. Plants 2021, 10, 237. https://doi.org/10.3390/plants10020237
Maslovarić I, Ilić V, Drvenica I, Stančić A, Mojsilović S, Kukolj T, Bugarski D, Saso L, Nicoletti M. Insight into the Biological Activity of Hennosides—Glucosides Isolated from Lawsonia inermis (henna): Could They Be Regarded as Active Constituents Instead. Plants. 2021; 10(2):237. https://doi.org/10.3390/plants10020237
Chicago/Turabian StyleMaslovarić, Irina, Vesna Ilić, Ivana Drvenica, Ana Stančić, Slavko Mojsilović, Tamara Kukolj, Diana Bugarski, Luciano Saso, and Marcello Nicoletti. 2021. "Insight into the Biological Activity of Hennosides—Glucosides Isolated from Lawsonia inermis (henna): Could They Be Regarded as Active Constituents Instead" Plants 10, no. 2: 237. https://doi.org/10.3390/plants10020237
APA StyleMaslovarić, I., Ilić, V., Drvenica, I., Stančić, A., Mojsilović, S., Kukolj, T., Bugarski, D., Saso, L., & Nicoletti, M. (2021). Insight into the Biological Activity of Hennosides—Glucosides Isolated from Lawsonia inermis (henna): Could They Be Regarded as Active Constituents Instead. Plants, 10(2), 237. https://doi.org/10.3390/plants10020237










