The Role of Tetrapyrrole- and GUN1-Dependent Signaling on Chloroplast Biogenesis
Abstract
1. Introduction
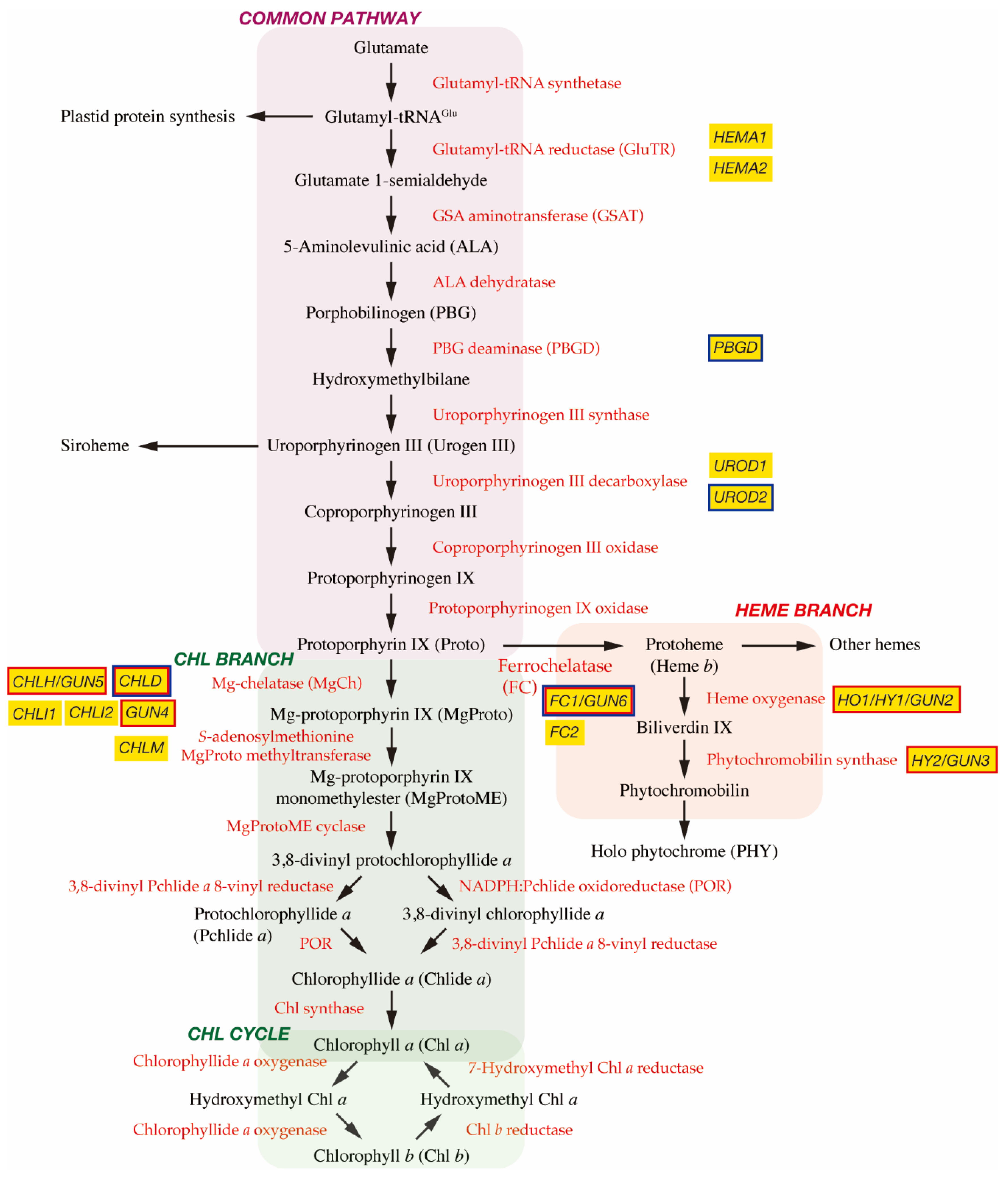
2. Biosynthesis of Tetrapyrroles in Plants
2.1. The C5 Pathway and the Common Pathway
2.2. Chl Branch and Chl Cycle
2.3. Heme Branch
3. Coupling of Two Genomes Is Required for Chloroplast Biogenesis
4. Identification of Gun Mutants
4.1. The Function of MgProto as a Negative Mobile Signal
4.2. Function of Heme as a Positive Mobile Signal
4.3. Function of Other Signaling Components
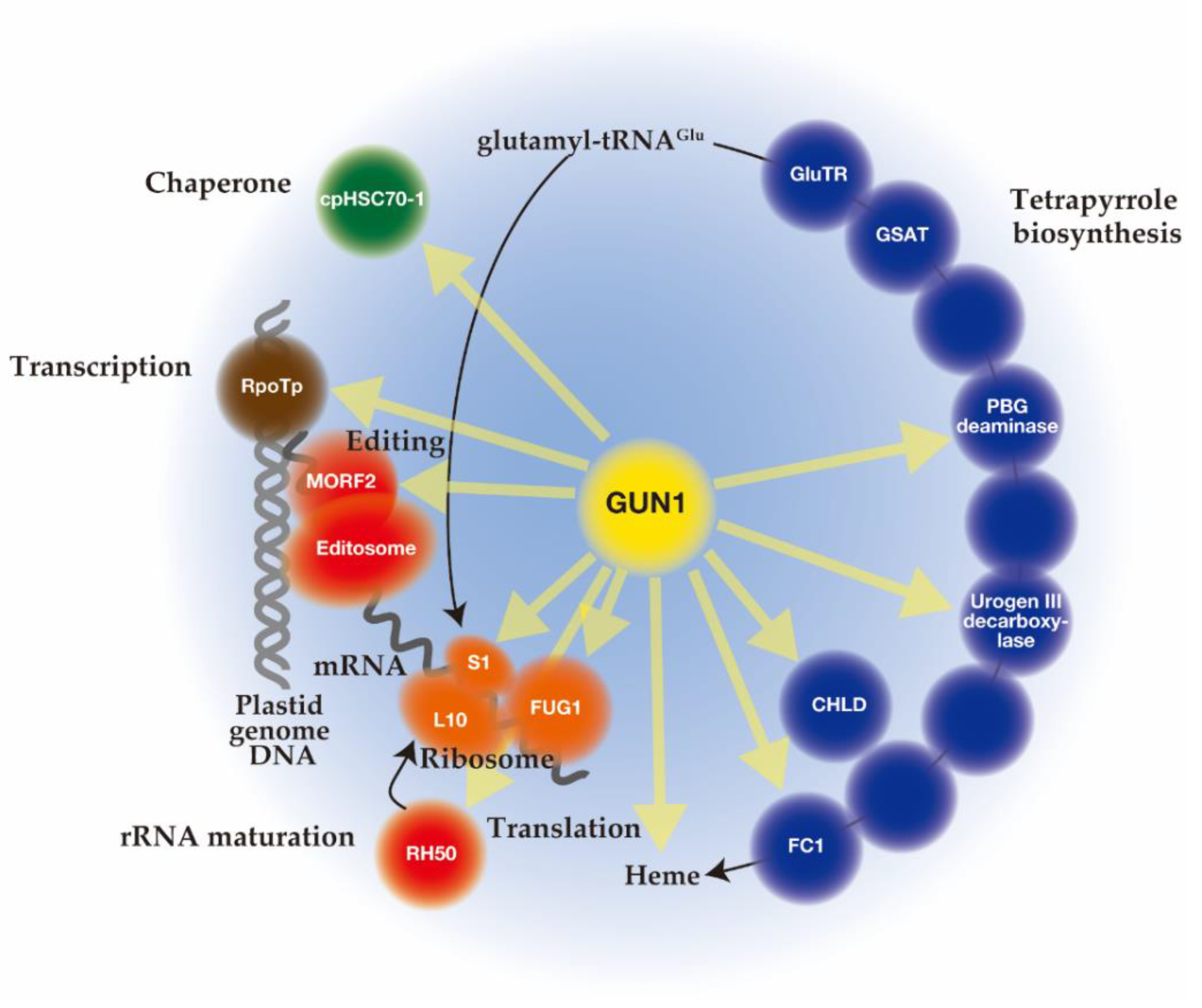
5. The Function of GUN1
5.1. Function of GUN1 on Transcription and Editing of Plastid Genes
5.2. The Function of GUN1 on Translation of Plastid Genome
5.3. The Function of GUN1 on Protein Import into the Plastid
5.4. The Link between GUN1 and Tetrapyrrole Biosynthesis
5.5. The Link between GUN1 and Other Processes
6. Outlook
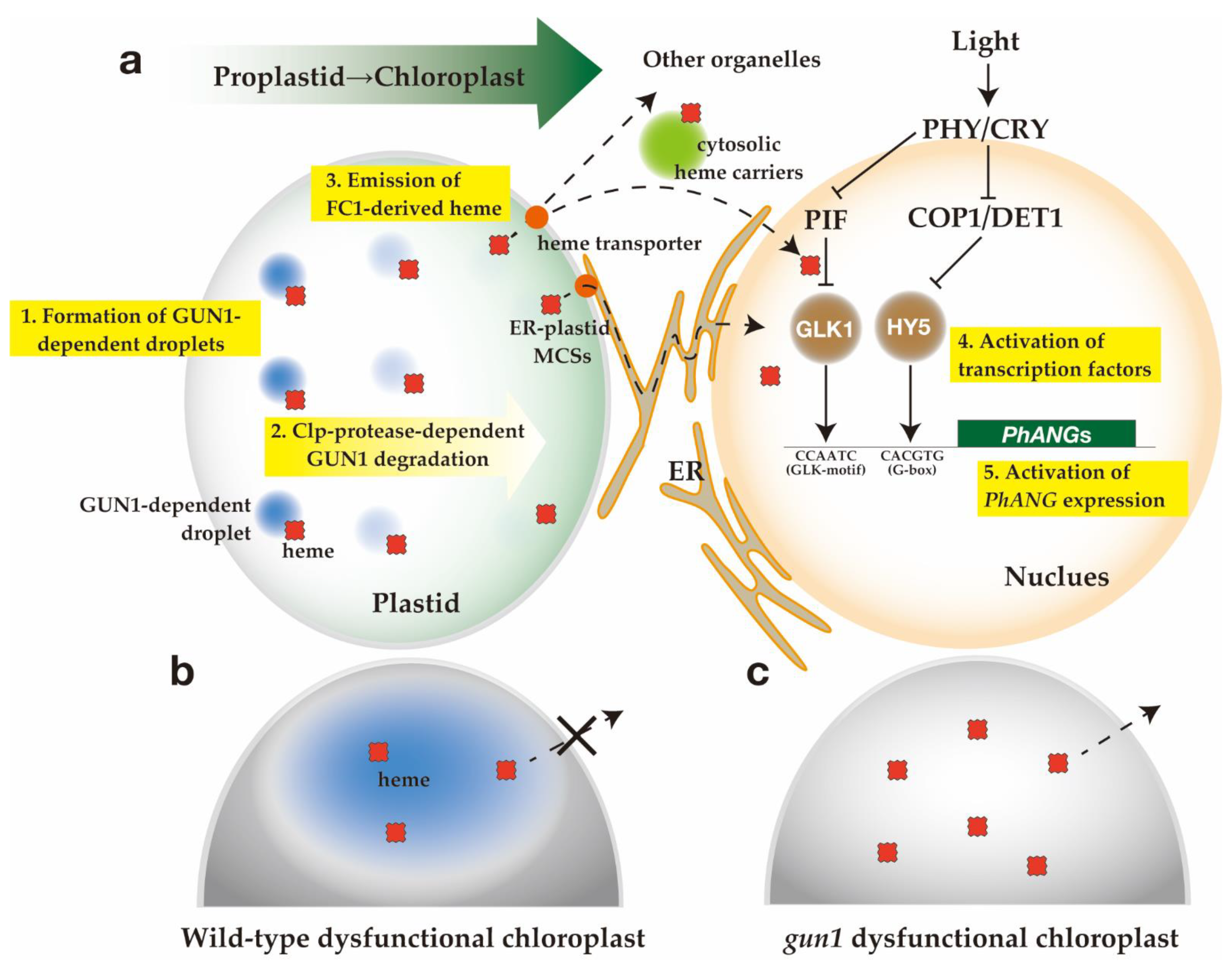
6.1. Proposed Functions of GUN1
6.2. Transfer of FC1-Derived Heme to Nucleus
6.3. A Hypothesis of the Heme Biogenic Plastid-to-Nucleus Retrograde Signaling Pathway
6.4. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Battersby, A. Tetrapyrroles: The pigments of life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Battersby, A.R.; Fookes, C.J.; Matcham, G.W.; McDonald, E. Biosynthesis of the pigments of life: Formation of the macrocycle. Nature 1980, 285, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Op den Camp, R.G.L.; Przybyla, D.; Ochsenbein, C.; Laloi, C.; Kim, C.; Danon, A.; Wagner, D.; Hideg, E.; Göbel, C.; Feussner, I.; et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 2003, 15, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, N.; Tanaka, R.; Grimm, B.; Masuda, T.; Moulin, M.; Smith, A.G.; Tanaka, A.; Terry, M.J. The cell biology of tetrapyrroles: A life and death struggle. Trends Plant Sci. 2010, 15, 488–498. [Google Scholar] [CrossRef]
- Terry, M.J.; Smith, A.G. A model for tetrapyrrole synthesis as the primary mechanism for plastid-to-nucleus signaling during chloroplast biogenesis. Front. Plant Sci. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.M. Tetrapyrrole Signaling in Plants. Front. Plant Sci. 2016, 7, 1586. [Google Scholar] [CrossRef]
- Castelfranco, P.A.; Beale, S.I. Chlorophyll Biosynthesis: Recent Advances and Areas of Current Interest. Annu. Rev. Plant Physiol. 1983, 34, 241–276. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrapyrrole Metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0145. [Google Scholar] [CrossRef]
- Ilag, L.; Kumar, A.; Soll, D. Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. Plant Cell 1994, 6, 265–275. [Google Scholar] [CrossRef]
- McCormac, A.C.; Fischer, A.; Kumar, A.M.; Soll, D.; Terry, M.J. Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in Arabidopsis thaliana. Plant J. 2001, 25, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Koncz, C.; Mayerhofer, R.; Koncz-Kalman, Z.; Nawrath, C.; Reiss, B.; Redei, G.; Schell, J. Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana. EMBO J. 1990, 9, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Rissler, H.M.; Collakova, E.; DellaPenna, D.; Whelan, J.; Pogson, B.J. Chlorophyll biosynthesis. Expression of a second chl I gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol. 2002, 128, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Mochizuki, N.; Yoshimura, N.; Motohashi, K.; Hisabori, T.; Masuda, T. Functional analysis of Arabidopsis thaliana isoforms of the Mg-chelatase CHLI subunit. Photochem. Photobiol. Sci. 2008, 7, 1188–1195. [Google Scholar] [CrossRef]
- Larkin, R.; Alonso, J.; Ecker, J.; Chory, J. GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 2003, 299, 902–906. [Google Scholar] [CrossRef]
- Adhikari, N.D.; Froehlich, J.E.; Strand, D.D.; Buck, S.M.; Kramer, D.M.; Larkin, R.M. GUN4-Porphyrin Complexes Bind the ChlH/GUN5 Subunit of Mg-Chelatase and Promote Chlorophyll Biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1449–1467. [Google Scholar] [CrossRef]
- Davison, P.; Schubert, H.; Reid, J.; Iorg, C.; Heroux, A.; Hill, C.; Hunter, C. Structural and biochemical characterization of Gun4 suggests a mechanism for its role in chlorophyll biosynthesis. Biochemistry 2005, 44, 7603–7612. [Google Scholar] [CrossRef]
- Schneider, S.; Marles-Wright, J.; Sharp, K.H.; Paoli, M. Diversity and conservation of interactions for binding heme in b-type heme proteins. Nat. Prod. Rep. 2007, 24, 621–630. [Google Scholar] [CrossRef]
- Chow, K.-S.; Singh, D.P.; Walker, A.R.; Smith, A.G. Two different genes encode ferrochelatase in Arabidopsis: Mapping, expression and subcellular targeting of the precursor proteins. Plant J. 1998, 15, 531–541. [Google Scholar] [CrossRef]
- Nagai, S.; Koide, M.; Takahashi, S.; Kikuta, A.; Aono, M.; Sasaki-Sekimoto, Y.; Ohta, H.; Takamiya, K.-i.; Masuda, T. Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis. Plant Physiol. 2007, 144, 1039–1051. [Google Scholar] [CrossRef]
- Terry, M.J.; Linley, P.J.; Kohchi, T. Making light of it: The role of plant haem oxygenases in phytochrome chromophore synthesis. Biochem. Soc. Trans. 2002, 30, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Pogson, B.J.; Ganguly, D.; Albrecht-Borth, V. Insights into chloroplast biogenesis and development. Biochim. Biophys. Acta 2015, 1847, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Pribil, M.; Labs, M.; Leister, D. Structure and dynamics of thylakoids in land plants. J. Exp. Bot. 2014, 65, 1955–1972. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Takamiya, K.-i. Novel Insights into the Enzymology, Regulation and Physiological Functions of Light-dependent Protochlorophyllide Oxidoreductase in Angiosperms. Photosynth. Res. 2004, 81, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Mereschkowsky, C. Über Natur und Ursprung der Chromatophore im Pflanzenreiche. Biol. Cent. 1905, 25, 593–604. [Google Scholar]
- Archibald, J.M. The puzzle of plastid evolution. Curr. Biol. 2009, 19, R81–R88. [Google Scholar] [CrossRef]
- Keeling, P.J. The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu. Rev. Plant Biol. 2013, 64, 583–607. [Google Scholar] [CrossRef]
- Criscuolo, A.; Gribaldo, S. Large-scale phylogenomic analyses indicate a deep origin of primary plastids within cyanobacteria. Mol. Biol. Evol. 2011, 28, 3019–3032. [Google Scholar] [CrossRef]
- Abdallah, F.; Salamini, F.; Leister, D. A prediction of the size and evolutionary origin of the proteome of chloroplasts of Arabidopsis. Trends Plant Sci. 2000, 5, 141–142. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Retrograde signaling between plastid and nucleus: A review. J. Plant Physiol. 2015, 181, 55–66. [Google Scholar] [CrossRef]
- Pogson, B.J.; Woo, N.S.; Forster, B.; Small, I.D. Plastid signalling to the nucleus and beyond. Trends Plant Sci. 2008, 13, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Nott, A.; Jung, H.; Koussevitzky, S.; Chory, J. Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 2006, 57, 739–759. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.X.; Phua, S.Y.; Crisp, P.; McQuinn, R.; Pogson, B.J. Learning the Languages of the Chloroplast: Retrograde Signaling and Beyond. Annu. Rev. Plant Biol. 2016, 67, 25–53. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.; Wang, J.Z.; Dehesh, K. Retrograde Signals: Integrators of Interorganellar Communication and Orchestrators of Plant Development. Annu. Rev. Plant Biol. 2017, 68, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Susek, J.; Chory, J. A tale of two genomes: Role of a chloroplast signal in coordinating nuclear and plastid genome expression. Aust. J. Plant Physiol. 1992, 19, 387–399. [Google Scholar] [CrossRef]
- Gray, J.C.; Sullivan, J.A.; Wang, J.H.; Jerome, C.A.; MacLean, D. Coordination of plastid and nuclear gene expression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 135–144, discussion 144–135. [Google Scholar] [CrossRef] [PubMed]
- Susek, R.; Ausubel, F.; Chory, J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 1993, 74, 787–799. [Google Scholar] [CrossRef]
- Mochizuki, N.; Brusslan, J.; Larkin, R.; Nagatani, A.; Chory, J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. USA 2001, 98, 2053–2058. [Google Scholar] [CrossRef]
- Woodson, J.D.; Perez-Ruiz, J.M.; Chory, J. Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Curr. Biol. 2011, 21, 897–903. [Google Scholar] [CrossRef]
- Ruckle, M.E.; DeMarco, S.M.; Larkin, R.M. Plastid signals remodel light signaling networks and are essential for efficient chloroplast biogenesis in Arabidopsis. Plant Cell 2007, 19, 3944–3960. [Google Scholar] [CrossRef]
- Kim, C.; Apel, K. 1O2-mediated and EXECUTER-dependent retrograde plastid-to-nucleus signaling in norflurazon-treated seedlings of Arabidopsis thaliana. Mol. Plant 2013, 6, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.; Oster, U.; Börnke, F.; Jahns, P.; Dietz, K.-J.; Leister, D.; Kleine, T. In-depth analysis of the distinctive effects of norflurazon implies that tetrapyrrole biosynthesis, organellar gene expression and ABA cooperate in the GUN-type of plastid signalling. Physiol. Plant 2010, 138, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.M. Influence of plastids on light signalling and development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, K.; Hanaoka, M. The early days of plastid retrograde signaling with respect to replication and transcription. Front. Plant Sci. 2012, 3, 301. [Google Scholar] [CrossRef] [PubMed]
- Kropat, J.; Oster, U.; Rudiger, W.; Beck, C.F. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc. Natl. Acad. Sci. USA 1997, 94, 14168–14172. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kanesaki, Y.; Tanaka, A.; Kuroiwa, H.; Kuroiwa, T.; Tanaka, K. Tetrapyrrole signal as a cell-cycle coordinator from organelle to nuclear DNA replication in plant cells. Proc. Natl. Acad. Sci. USA 2009, 106, 803–807. [Google Scholar] [CrossRef]
- Strand, A.; Asami, T.; Alonso, J.; Ecker, J.R.; Chory, J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 2003, 421, 79–83. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Li, H.-M. Arabidopsis CHLI2 can substitute for CHLI1. Plant Physiol. 2009, 150, 636–645. [Google Scholar] [CrossRef]
- Ankele, E.; Kindgren, P.; Pesquet, E.; Strand, A. In vivo visualization of Mg-protoporphyrin IX, a coordinator of photosynthetic gene expression in the nucleus and the chloroplast. Plant Cell 2007, 19, 1964–1979. [Google Scholar] [CrossRef]
- Pontier, D.; Albrieux, C.; Joyard, J.; Lagrange, T.; Block, M. Knock-out of the magnesium protoporphyrin IX methyltransferase gene in Arabidopsis. Effects on chloroplast development and on chloroplast-to-nucleus signaling. J. Biol. Chem. 2007, 282, 2297–2304. [Google Scholar] [CrossRef]
- Gadjieva, R.; Axelsson, E.; Olsson, U.; Hansson, M. Analysis of gun phenotype in barley magnesium chelatase and Mg-protoporphyrin IX monomethyl ester cyclase mutants. Plant Physiol. Biochem. 2005, 43, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, N.; Tanaka, R.; Tanaka, A.; Masuda, T.; Nagatani, A. The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 15184–15189. [Google Scholar] [CrossRef] [PubMed]
- Moulin, M.; McCormac, A.C.; Terry, M.J.; Smith, A.G. Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc. Natl. Acad. Sci. USA 2008, 105, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Kindgren, P.; Eriksson, M.-J.; Benedict, C.; Mohapatra, A.; Gough, S.P.; Hansson, M.; Kieselbach, T.; Strand, A. A novel proteomic approach reveals a role for Mg-protoporphyrin IX in response to oxidative stress. Physiol. Plant 2011, 141, 310–320. [Google Scholar] [CrossRef]
- Kindgren, P.; Norén, L.; López, J.d.D.B.; Shaikhali, J.; Strand, A. Interplay between Heat Shock Protein 90 and HY5 controls PhANG expression in response to the GUN5 plastid signal. Mol. Plant 2012, 5, 901–913. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Yuan, S.; Feng, H.; Xu, F.; Cheng, J.; Shang, J.; Zhang, D.-W.; Lin, H.-H. Transient accumulation of Mg-protoporphyrin IX regulates expression of PhANGs—New evidence for the signaling role of tetrapyrroles in mature Arabidopsis plants. J. Plant Physiol. 2011, 168, 714–721. [Google Scholar] [CrossRef]
- Schlicke, H.; Hartwig, A.S.; Firtzlaff, V.; Richter, A.S.; Gläßer, C.; Maier, K.; Finkemeier, I.; Grimm, B. Induced deactivation of genes encoding chlorophyll biosynthesis enzymes disentangles tetrapyrrole-mediated retrograde signaling. Mol. Plant 2014, 7, 1211–1227. [Google Scholar] [CrossRef]
- Mense, S.M.; Zhang, L. Heme: A versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006, 16, 681–692. [Google Scholar] [CrossRef]
- Tsiftsoglou, A.; Tsamadou, A.; Papadopoulou, L. Heme as key regulator of major mammalian cellular functions: Molecular, cellular, and pharmacological aspects. Pharmacol. Ther. 2006, 111, 327–345. [Google Scholar] [CrossRef]
- Von Gromoff, E.; Alawady, A.; Meinecke, L.; Grimm, B.; Beck, C. Heme, a plastid-derived regulator of nuclear gene expression in Chlamydomonas. Plant Cell 2008, 20, 552–567. [Google Scholar] [CrossRef]
- Voss, B.; Meinecke, L.; Kurz, T.; Al-Babili, S.; Beck, C.F.; Hess, W.R. Hemin and Magnesium-Protoporphyrin IX Induce Global Changes in Gene Expression in Chlamydomonas reinhardtii. Plant Physiol. 2011, 155, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tanaka, K. Transcriptional Regulation of Tetrapyrrole Biosynthetic Genes Explains Abscisic Acid-Induced Heme Accumulation in the Unicellular Red Alga Cyanidioschyzon merolae. Front. Plant Sci. 2016, 7, 1300. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ando, H.; Hanaoka, M.; Tanaka, K. Abscisic Acid Participates in the Control of Cell Cycle Initiation Through Heme Homeostasis in the Unicellular Red Alga Cyanidioschyzon merolae. Plant Cell Physiol. 2016, 57, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, C.; Zapotoczny, G.; Masquelier, D.; Ghislain, M.; Batoko, H. The Arabidopsis multistress regulator TSPO is a heme binding membrane protein and a potential scavenger of porphyrins via an autophagy-dependent degradation mechanism. Plant Cell 2011, 23, 785–805. [Google Scholar] [CrossRef]
- Cornah, J.; Roper, J.; Pal Singh, D.; Smith, A. Measurement of ferrochelatase activity using a novel assay suggests that plastids are the major site of haem biosynthesis in both photosynthetic and non-photosynthetic cells of pea (Pisum sativum L.). Biochem. J. 2002, 362, 423–432. [Google Scholar] [CrossRef]
- Masuda, T.; Suzuki, T.; Shimada, H.; Ohta, H.; Takamiya, K. Subcellular localization of two types of ferrochelatase in cucumber. Planta 2003, 217, 602–609. [Google Scholar] [CrossRef]
- Hey, D.; Ortega-Rodes, P.; Fan, T.; Schnurrer, F.; Brings, L.; Hedtke, B.; Grimm, B. Transgenic Tobacco Lines Expressing Sense or Antisense FERROCHELATASE 1 RNA Show Modified Ferrochelatase Activity in Roots and Provide Experimental Evidence for Dual Localization of Ferrochelatase 1. Plant Cell Physiol. 2016, 57, 171–2585. [Google Scholar] [CrossRef]
- Espinas, N.A.; Kobayashi, K.; Sato, Y.; Mochizuki, N.; Takahashi, K.; Tanaka, R.; Masuda, T. Allocation of Heme Is Differentially Regulated by Ferrochelatase Isoforms in Arabidopsis Cells. Front. Plant Sci. 2016, 7, 69. [Google Scholar] [CrossRef]
- Fan, T.; Roling, L.; Meiers, A.; Brings, L.; Ortega-Rodes, P.; Hedtke, B.; Grimm, B. Complementation studies of the Arabidopsis fc1 mutant substantiate essential functions of ferrochelatase 1 during embryogenesis and salt stress. Plant Cell Environ. 2019, 42, 618–632. [Google Scholar] [CrossRef]
- Page, M.T.; Garcia-Becerra, T.; Smith, A.G.; Terry, M.J. Overexpression of chloroplast-targeted ferrochelatase 1 results in a genomes uncoupled chloroplast-to-nucleus retrograde signalling phenotype. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190401. [Google Scholar] [CrossRef]
- Espinas, N.A.; Kobayashi, K.; Takahashi, S.; Mochizuki, N.; Masuda, T. Evaluation of Unbound Free Heme in Plant Cells by Differential Acetone Extraction. Plant Cell Physiol. 2012, 53, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yasuda, R.; Mukai, Y.; Tanoue, R.; Shimada, T.; Imamura, S.; Tanaka, K.; Watanabe, S.; Masuda, T. Proteomic analysis of haem-binding protein from Arabidopsis thaliana and Cyanidioschyzon merolae. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190488. [Google Scholar] [CrossRef] [PubMed]
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Lim, J.; Mittler, R.; Chory, J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007, 316, 715–719. [Google Scholar] [CrossRef]
- Brunkard, J.O.; Burch-Smith, T.M. Ties that bind: The integration of plastid signalling pathways in plant cell metabolism. Essays Biochem. 2018, 62, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Verdeja, T.; Strand, A. Retrograde Signals Navigate the Path to Chloroplast Development. Plant Physiol. 2018, 176, 967–976. [Google Scholar] [CrossRef]
- Kacprzak, S.M.; Mochizuki, N.; Naranjo, B.; Xu, D.; Leister, D.; Kleine, T.; Okamoto, H.; Terry, M.J. Plastid-to-Nucleus Retrograde Signalling during Chloroplast Biogenesis Does Not Require ABI4. Plant Physiol. 2019, 179, 18–23. [Google Scholar] [CrossRef]
- Cottage, A.; Gray, J.C. Timing the switch to phototrophic growth: A possible role of GUN1. Plant Signal. Behav. 2011, 6, 578–582. [Google Scholar] [CrossRef]
- Martin, G.; Leivar, P.; Ludevid, D.; Tepperman, J.M.; Quail, P.H.; Monte, E. Phytochrome and retrograde signalling pathways converge to antagonistically regulate a light-induced transcriptional network. Nat. Commun. 2016, 7, 11431. [Google Scholar] [CrossRef]
- Mhamdi, A.; Gommers, C.M.M. Another gun Dismantled: ABSCISIC ACID INSENSITIVE4 Is Not a Target of Retrograde Signaling. Plant Physiol. 2019, 179, 13–14. [Google Scholar] [CrossRef]
- Sun, X.; Feng, P.; Xu, X.; Guo, H.; Ma, J.; Chi, W.; Lin, R.; Lu, C.; Zhang, L. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2011, 2, 477. [Google Scholar] [CrossRef]
- Page, M.T.; Kacprzak, S.M.; Mochizuki, N.; Okamoto, H.; Smith, A.G.; Terry, M.J. Seedlings Lacking the PTM Protein Do Not Show a genomes uncoupled (gun) Mutant Phenotype. Plant Physiol. 2017, 174, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Verdeja, T.; Vuorijoki, L.; Strand, A. Emerging from the darkness: Interplay between light and plastid signaling during chloroplast biogenesis. Physiol. Plant 2020, 169, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.; Ruckle, M. Integration of light and plastid signals. Curr. Opin. Plant Biol. 2008, 11, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Choi, G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef] [PubMed]
- Lau, O.S.; Deng, X.-W. Plant hormone signaling lightens up: Integrators of light and hormones. Curr. Opin. Plant Biol. 2010, 13, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Fitter, D.W.; Martin, D.J.; Copley, M.J.; Scotland, R.W.; Langdale, J.A. GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 2002, 31, 713–727. [Google Scholar] [CrossRef]
- Waters, M.; Moylan, E.; Langdale, J. GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J. 2008, 56, 432–444. [Google Scholar] [CrossRef]
- Waters, M.T.; Wang, P.; Korkaric, M.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. GLK Transcription Factors Coordinate Expression of the Photosynthetic Apparatus in Arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar] [CrossRef]
- Kakizaki, T.; Matsumura, H.; Nakayama, K.; Che, F.-S.; Terauchi, R.; Inaba, T. Coordination of plastid protein import and nuclear gene expression by plastid-to-nucleus retrograde signaling. Plant Physiol. 2009, 151, 1339–1353. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Wang, Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef]
- Matsumoto, F.; Obayashi, T.; Sasaki-Sekimoto, Y.; Ohta, H.; Takamiya, K.-i.; Masuda, T. Gene expression profiling of the tetrapyrrole metabolic pathway in Arabidopsis with a mini-array system. Plant Physiol. 2004, 135, 2379–2391. [Google Scholar] [CrossRef]
- Masuda, T.; Fujita, Y. Regulation and evolution of chlorophyll metabolism. Photochem. Photobiol. Sci. 2008, 7, 1131. [Google Scholar] [CrossRef]
- Kobayashi, K.; Obayashi, T.; Masuda, T. Role of the G-box element in regulation of chlorophyll biosynthesis in Arabidopsis roots. Plant Signal. Behav. 2012, 7, 922–926. [Google Scholar] [CrossRef]
- Kobayashi, K.; Baba, S.; Obayashi, T.; Sato, M.; Toyooka, K.; Keränen, M.; Aro, E.-M.; Fukaki, H.; Ohta, H.; Sugimoto, K.; et al. Regulation of Root Greening by Light and Auxin/Cytokinin Signaling in Arabidopsis. Plant Cell 2012, 24, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Leister, D.; Kleine, T. Definition of a core module for the nuclear retrograde response to altered organellar gene expression identifies GLK overexpressors as gun mutants. Physiol. Plant 2016, 157, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, J.; Chory, J. Genome uncoupled 1 mutants are hypersensitive to norflurazon and lincomycin. Plant Physiol. 2018, 178. [Google Scholar] [CrossRef] [PubMed]
- Tadini, L.; Peracchio, C.; Trotta, A.; Colombo, M.; Mancini, I.; Jeran, N.; Costa, A.; Faoro, F.; Marsoni, M.; Vannini, C.; et al. GUN1 influences the accumulation of NEP-dependent transcripts and chloroplast protein import in Arabidopsis cotyledons upon perturbation of chloroplast protein homeostasis. Plant J. 2020, 101, 1198–1220. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, Z.; Larkin, R.M. The genomes uncoupled mutants are more sensitive to norflurazon than wild type. Plant Physiol. 2018, 178. [Google Scholar] [CrossRef]
- Kotera, E.; Tasaka, M.; Shikanai, T. A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 2005, 433, 326–330. [Google Scholar] [CrossRef]
- Moreira, D.; Philippe, H. Smr: A bacterial and eukaryotic homologue of the C-terminal region of the MutS2 family. Trends Biochem. Sci. 1999, 24, 298–300. [Google Scholar] [CrossRef]
- Tadini, L.; Pesaresi, P.; Kleine, T.; Rossi, F.; Guljamow, A.; Sommer, F.; Mühlhaus, T.; Schroda, M.; Masiero, S.; Pribil, M.; et al. GUN1 Controls Accumulation of the Plastid Ribosomal Protein S1 at the Protein Level and Interacts with Proteins Involved in Plastid Protein Homeostasis. Plant Physiol. 2016, 170, 1817–1830. [Google Scholar] [CrossRef]
- Jia, Y.; Tian, H.; Zhang, S.; Ding, Z.; Ma, C. GUN1-Interacting Proteins Open the Door for Retrograde Signaling. Trends Plant Sci. 2019, 24, 884–887. [Google Scholar] [CrossRef]
- Wu, G.-Z.; Chalvin, C.; Hoelscher, M.; Meyer, E.H.; Wu, X.N.; Bock, R. Control of Retrograde Signaling by Rapid Turnover of GENOMES UNCOUPLED1. Plant Physiol. 2018, 176, 2472–2495. [Google Scholar] [CrossRef]
- Shimizu, T.; Kacprzak, S.M.; Mochizuki, N.; Nagatani, A.; Watanabe, S.; Shimada, T.; Tanaka, K.; Hayashi, Y.; Arai, M.; Leister, D.; et al. The retrograde signaling protein GUN1 regulates tetrapyrrole biosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 24900–24906. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Intrinsically disordered proteins from A to Z. Int. J. Biochem. Cell Biol. 2011, 43, 1090–1103. [Google Scholar] [CrossRef]
- Marino, G.; Naranjo, B.; Wang, J.; Penzler, J.F.; Kleine, T.; Leister, D. Relationship of GUN1 to FUG1 in chloroplast protein homeostasis. Plant J. 2019, 99, 521–535. [Google Scholar] [CrossRef]
- Wu, G.Z.; Meyer, E.H.; Richter, A.S.; Schuster, M.; Ling, Q.; Schottler, M.A.; Walther, D.; Zoschke, R.; Grimm, B.; Jarvis, R.P.; et al. Control of retrograde signalling by protein import and cytosolic folding stress. Nat. Plants 2019, 5, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Tadini, L.; Peracchio, C.; Ferrari, R.; Pesaresi, P. GUN1, a Jack-Of-All-Trades in Chloroplast Protein Homeostasis and Signaling. Front. Plant Sci. 2016, 7, 1449. [Google Scholar] [CrossRef] [PubMed]
- Tadini, L.; Jeran, N.; Pesaresi, P. GUN1 and Plastid RNA Metabolism: Learning from Genetics. Cells 2020, 9, 2307. [Google Scholar] [CrossRef]
- Tadini, L.; Jeran, N.; Peracchio, C.; Masiero, S.; Colombo, M.; Pesaresi, P. The plastid transcription machinery and its coordination with the expression of nuclear genome: Plastid-Encoded Polymerase, Nuclear-Encoded Polymerase and the Genomes Uncoupled 1-mediated retrograde communication. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190399. [Google Scholar] [CrossRef]
- Huang, X.Q.; Wang, L.J.; Kong, M.J.; Huang, N.; Liu, X.Y.; Liang, H.Y.; Zhang, J.X.; Lu, S. At3g53630 encodes a GUN1-interacting protein under norflurazon treatment. Protoplasma 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liebers, M.; Grubler, B.; Chevalier, F.; Lerbs-Mache, S.; Merendino, L.; Blanvillain, R.; Pfannschmidt, T. Regulatory Shifts in Plastid Transcription Play a Key Role in Morphological Conversions of Plastids during Plant Development. Front. Plant Sci. 2017, 8, 23. [Google Scholar] [CrossRef]
- Pfannschmidt, T.; Blanvillain, R.; Merendino, L.; Courtois, F.; Chevalier, F.; Liebers, M.; Grubler, B.; Hommel, E.; Lerbs-Mache, S. Plastid RNA polymerases: Orchestration of enzymes with different evolutionary origins controls chloroplast biogenesis during the plant life cycle. J. Exp. Bot. 2015, 66, 6957–6973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, J.; Chory, J. GUN1 interacts with MORF2 to regulate plastid RNA editing during retrograde signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 10162–10167. [Google Scholar] [CrossRef]
- Zhao, X.B.; Huang, J.Y.; Chory, J. Unraveling the Linkage between Retrograde Signaling and RNA Metabolism in Plants. Trends Plant Sci. 2020, 25, 141–147. [Google Scholar] [CrossRef]
- Paieri, F.; Tadini, L.; Manavski, N.; Kleine, T.; Ferrari, R.; Morandini, P.; Pesaresi, P.; Meurer, J.; Leister, D. The DEAD-box RNA Helicase RH50 Is a 23S-4.5S rRNA Maturation Factor that Functionally Overlaps with the Plastid Signaling Factor GUN1. Plant Physiol. 2018, 176, 634–648. [Google Scholar] [CrossRef]
- Bischof, S.; Baerenfaller, K.; Wildhaber, T.; Troesch, R.; Vidi, P.A.; Roschitzki, B.; Hirsch-Hoffmann, M.; Hennig, L.; Kessler, F.; Gruissem, W.; et al. Plastid proteome assembly without Toc159: Photosynthetic protein import and accumulation of N-acetylated plastid precursor proteins. Plant Cell 2011, 23, 3911–3928. [Google Scholar] [CrossRef]
- Woodson, J.D.; Perez-Ruiz, J.M.; Schmitz, R.J.; Ecker, J.R.; Chory, J. Sigma factor-mediated plastid retrograde signals control nuclear gene expression. Plant J. 2012, 73, 1–13. [Google Scholar] [CrossRef]
- Xu, X.; Chi, W.; Sun, X.; Feng, P.; Guo, H.; Li, J.; Lin, R.; Lu, C.; Wang, H.; Leister, D.; et al. Convergence of light and chloroplast signals for de-etiolation through ABI4-HY5 and COP1. Nat. Plants 2016, 2, 16066. [Google Scholar] [CrossRef]
- Richter, A.S.; Banse, C.; Grimm, B. The GluTR-binding protein is the heme-binding factor for feedback control of glutamyl-tRNA reductase. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Cottage, A.; Mott, E.K.; Kempster, J.A.; Gray, J.C. The Arabidopsis plastid-signalling mutant gun1 (genomes uncoupled1) shows altered sensitivity to sucrose and abscisic acid and alterations in early seedling development. J. Exp. Bot. 2010, 61, 3773–3786. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.S.; Tohge, T.; Fernie, A.R.; Grimm, B. The genomes uncoupled-dependent signalling pathway coordinates plastid biogenesis with the synthesis of anthocyanins. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X.; Rivas, G.; Minton, A.P. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Delarue, M.; Brittingham, G.P.; Pfeffer, S.; Surovtsev, I.V.; Pinglay, S.; Kennedy, K.J.; Schaffer, M.; Gutierrez, J.I.; Sang, D.; Poterewicz, G.; et al. mTORC1 Controls Phase Separation and the Biophysical Properties of the Cytoplasm by Tuning Crowding. Cell 2018, 174, 338–349.e320. [Google Scholar] [CrossRef]
- Hara, K.; Yonezawa, K.; Weng, Q.P.; Kozlowski, M.T.; Belham, C.; Avruch, J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef]
- Levican, G.; Katz, A.; Valenzuela, P.; Soll, D.; Orellana, O. A tRNA(Glu) that uncouples protein and tetrapyrrole biosynthesis. FEBS Lett. 2005, 579, 6383–6387. [Google Scholar] [CrossRef]
- Agrawal, S.; Karcher, D.; Ruf, S.; Bock, R. The Functions of Chloroplast Glutamyl-tRNA in Translation and Tetrapyrrole Biosynthesis. Plant Physiol. 2020, 183, 263–276. [Google Scholar] [CrossRef]
- Hanna, D.A.; Harvey, R.M.; Martinez-Guzman, O.; Yuan, X.; Chandrasekharan, B.; Raju, G.; Outten, F.W.; Hamza, I.; Reddi, A.R. Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc. Natl. Acad. Sci. USA 2016, 113, 7539–7544. [Google Scholar] [CrossRef]
- Martinez-Guzman, O.; Willoughby, M.M.; Saini, A.; Dietz, J.V.; Bohovych, I.; Medlock, A.E.; Khalimonchuk, O.; Reddi, A.R. Mitochondrial-nuclear heme trafficking in budding yeast is regulated by GTPases that control mitochondrial dynamics and ER contact sites. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Li, T.; Xiao, Z.; Li, H.; Liu, C.; Shen, W.; Gao, C. A Combinatorial Reporter Set to Visualize the Membrane Contact Sites Between Endoplasmic Reticulum and Other Organelles in Plant Cell. Front. Plant Sci. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Takahashi, S.; Ogawa, T.; Inoue, K.; Masuda, T. Characterization of cytosolic tetrapyrrole-binding proteins in Arabidopsis thaliana. Photochem. Photobiol. Sci. 2008, 7, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre-Gonon, E.; Schwartz, M.; Girardet, J.M.; Hecker, A.; Rouhier, N. Is there a role for tau glutathione transferases in tetrapyrrole metabolism and retrograde signalling in plants? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190404. [Google Scholar] [CrossRef] [PubMed]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Branon, T.C.; Bosch, J.A.; Sanchez, A.D.; Udeshi, N.D.; Svinkina, T.; Carr, S.A.; Feldman, J.L.; Perrimon, N.; Ting, A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018, 36, 880–887. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, T.; Masuda, T. The Role of Tetrapyrrole- and GUN1-Dependent Signaling on Chloroplast Biogenesis. Plants 2021, 10, 196. https://doi.org/10.3390/plants10020196
Shimizu T, Masuda T. The Role of Tetrapyrrole- and GUN1-Dependent Signaling on Chloroplast Biogenesis. Plants. 2021; 10(2):196. https://doi.org/10.3390/plants10020196
Chicago/Turabian StyleShimizu, Takayuki, and Tatsuru Masuda. 2021. "The Role of Tetrapyrrole- and GUN1-Dependent Signaling on Chloroplast Biogenesis" Plants 10, no. 2: 196. https://doi.org/10.3390/plants10020196
APA StyleShimizu, T., & Masuda, T. (2021). The Role of Tetrapyrrole- and GUN1-Dependent Signaling on Chloroplast Biogenesis. Plants, 10(2), 196. https://doi.org/10.3390/plants10020196





