Nano Zinc Oxide Green-Synthesized from Plumbago auriculata Lam. Alcoholic Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Investigation of Phenolic and Flavonoid Compounds
2.2. Characterization of ZnO Nanoparticles
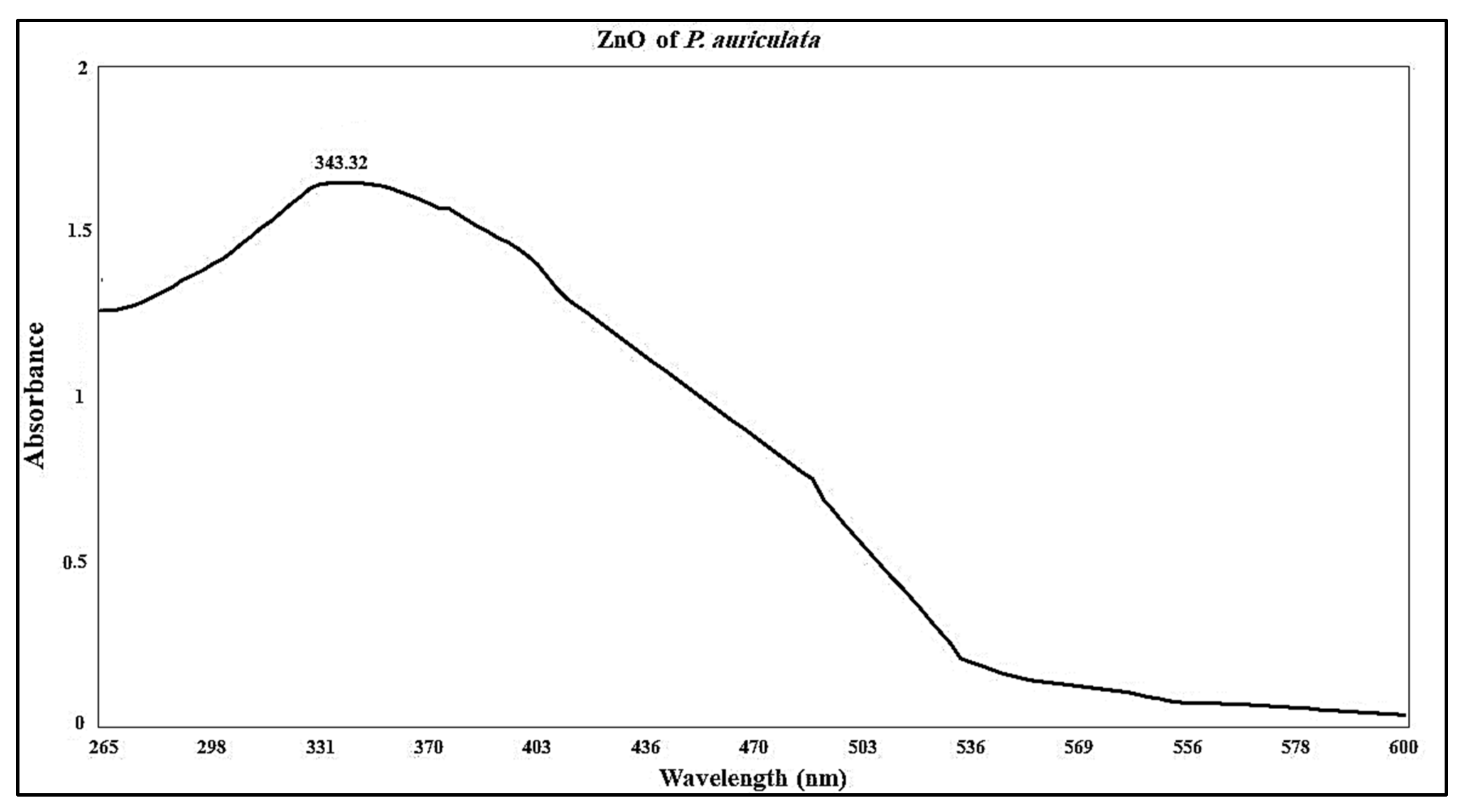
2.2.1. UV Analysis
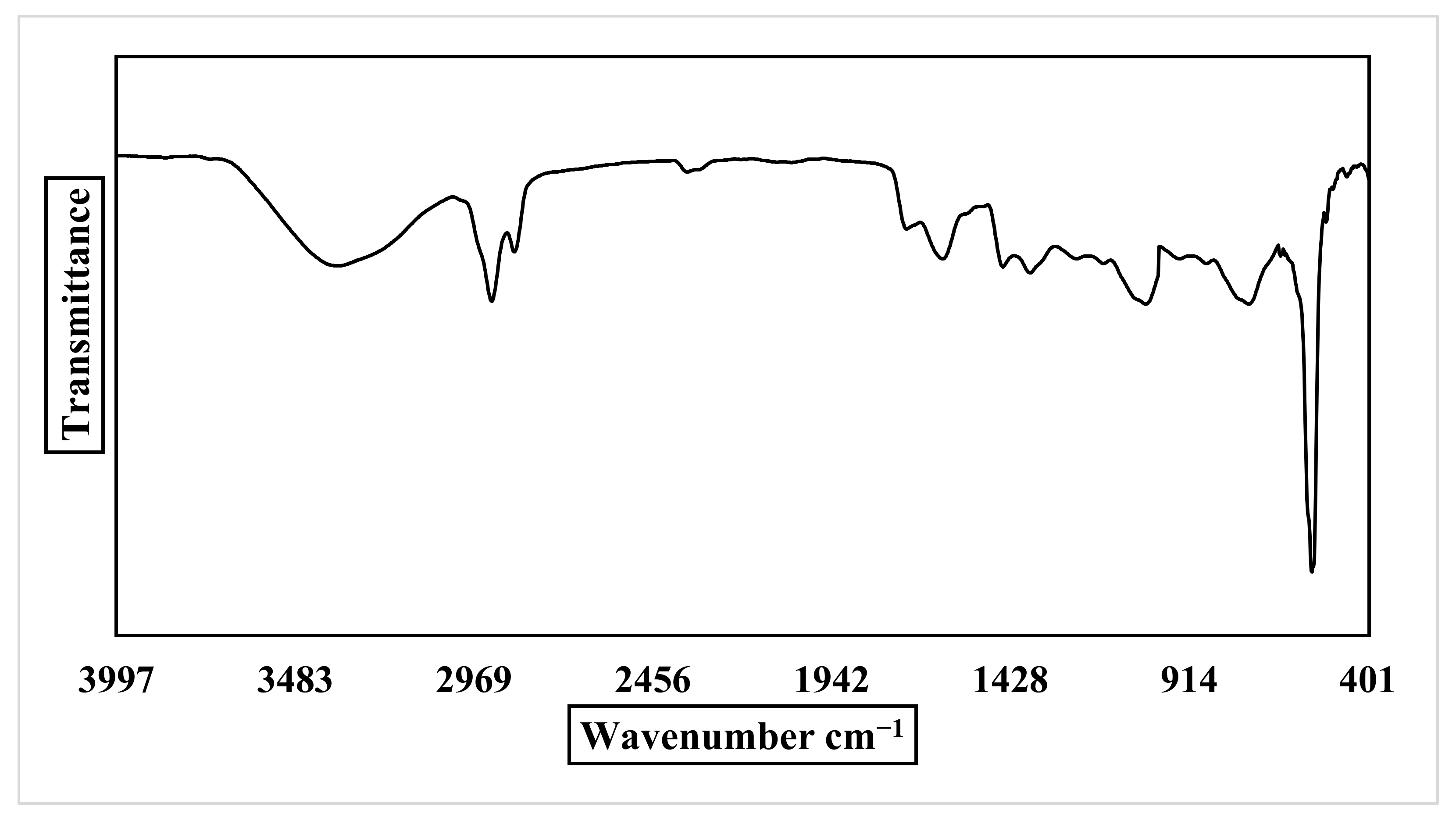
2.2.2. FT-IR Analysis of Nano-ZnO and P. auriculata Lam.
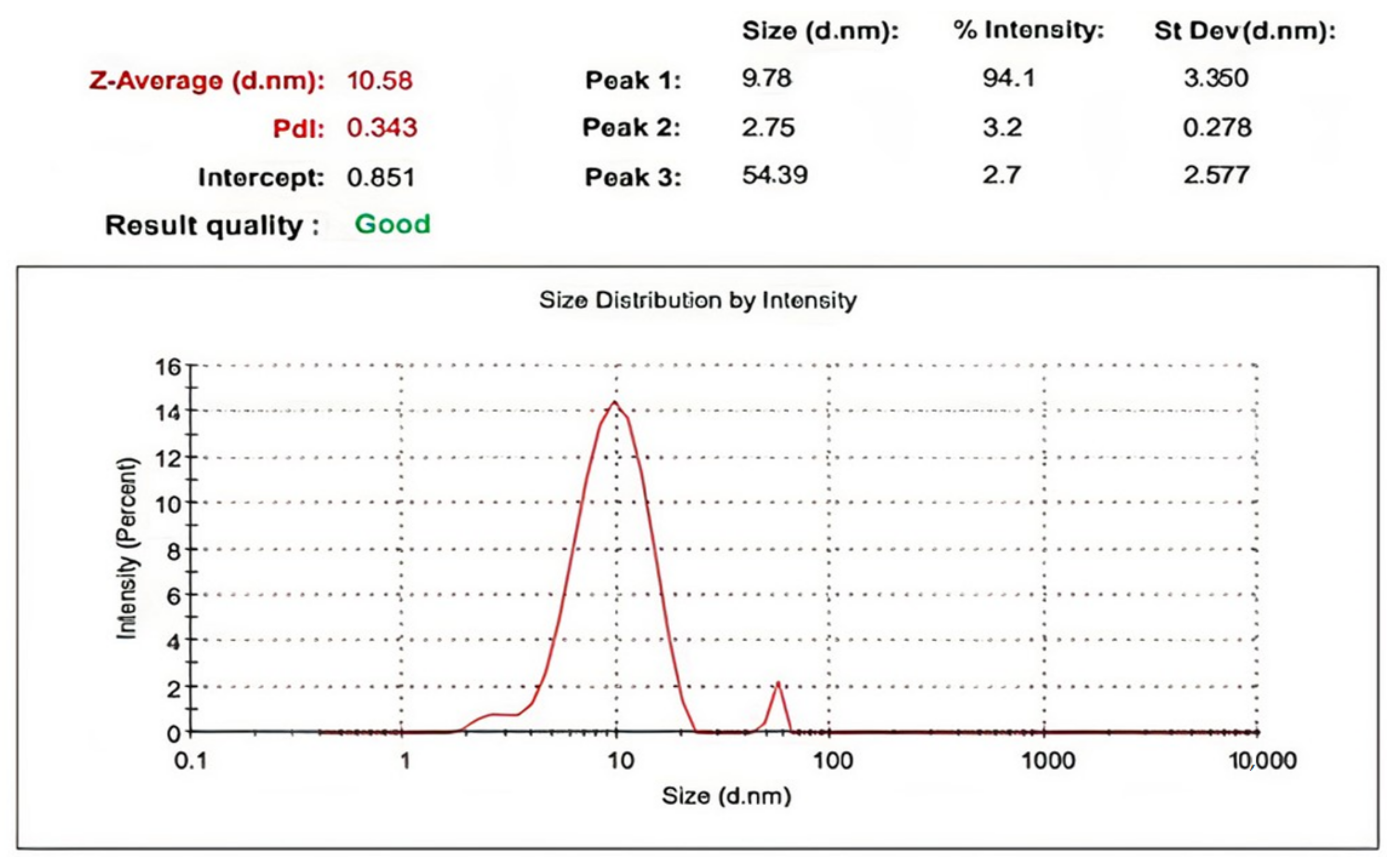
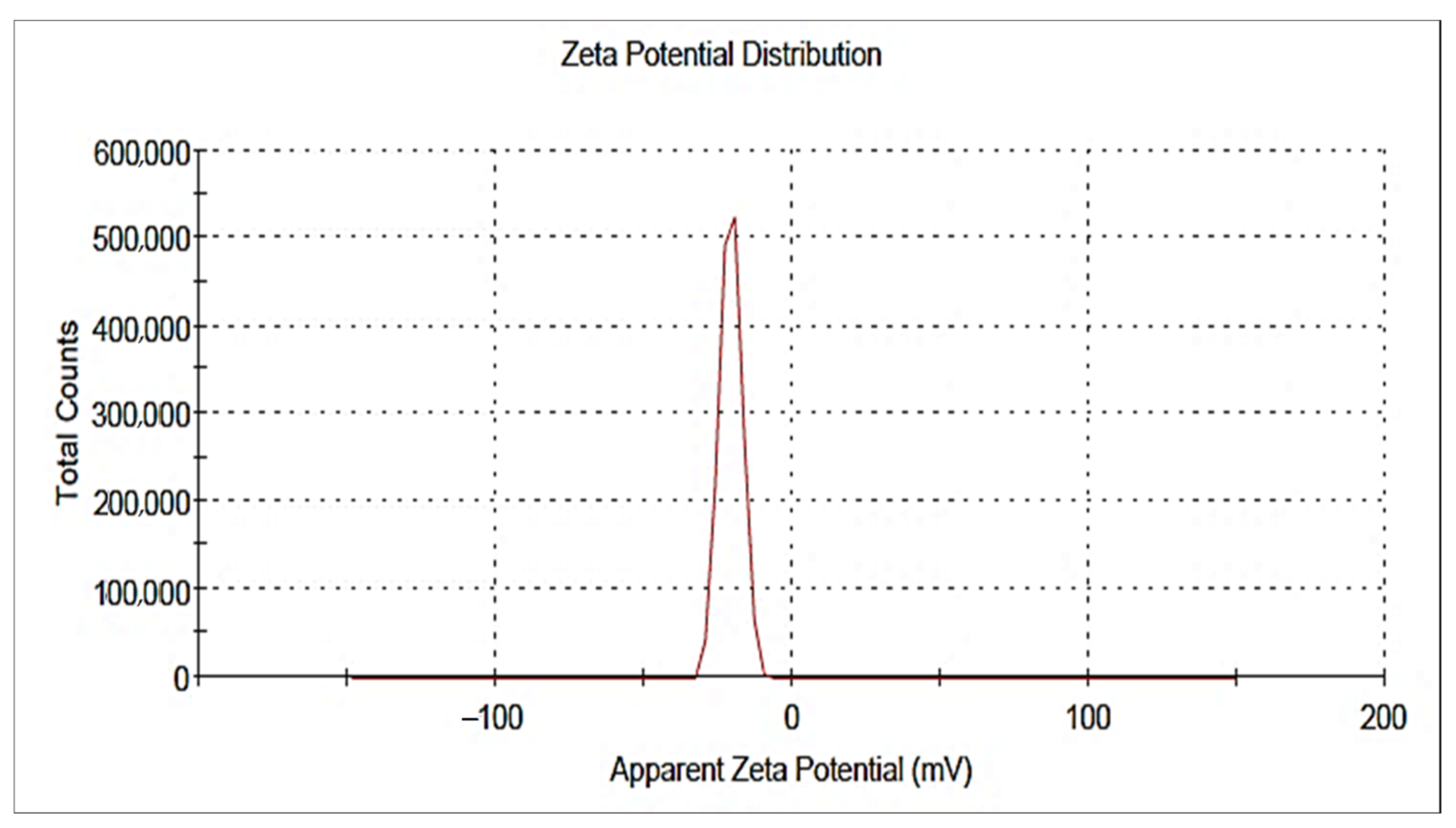
2.2.3. DLS and Zeta Potential
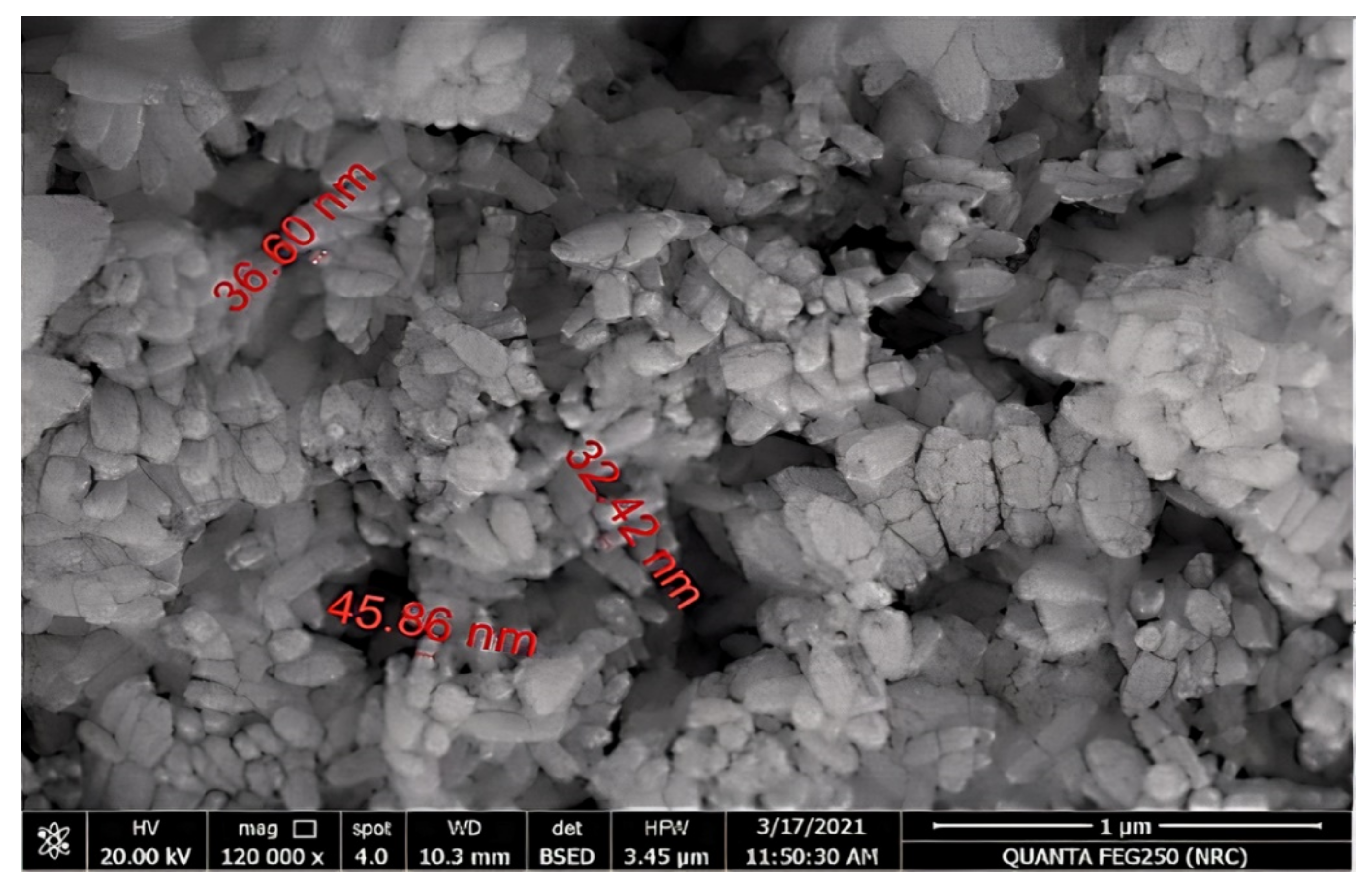
2.2.4. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) Analyses
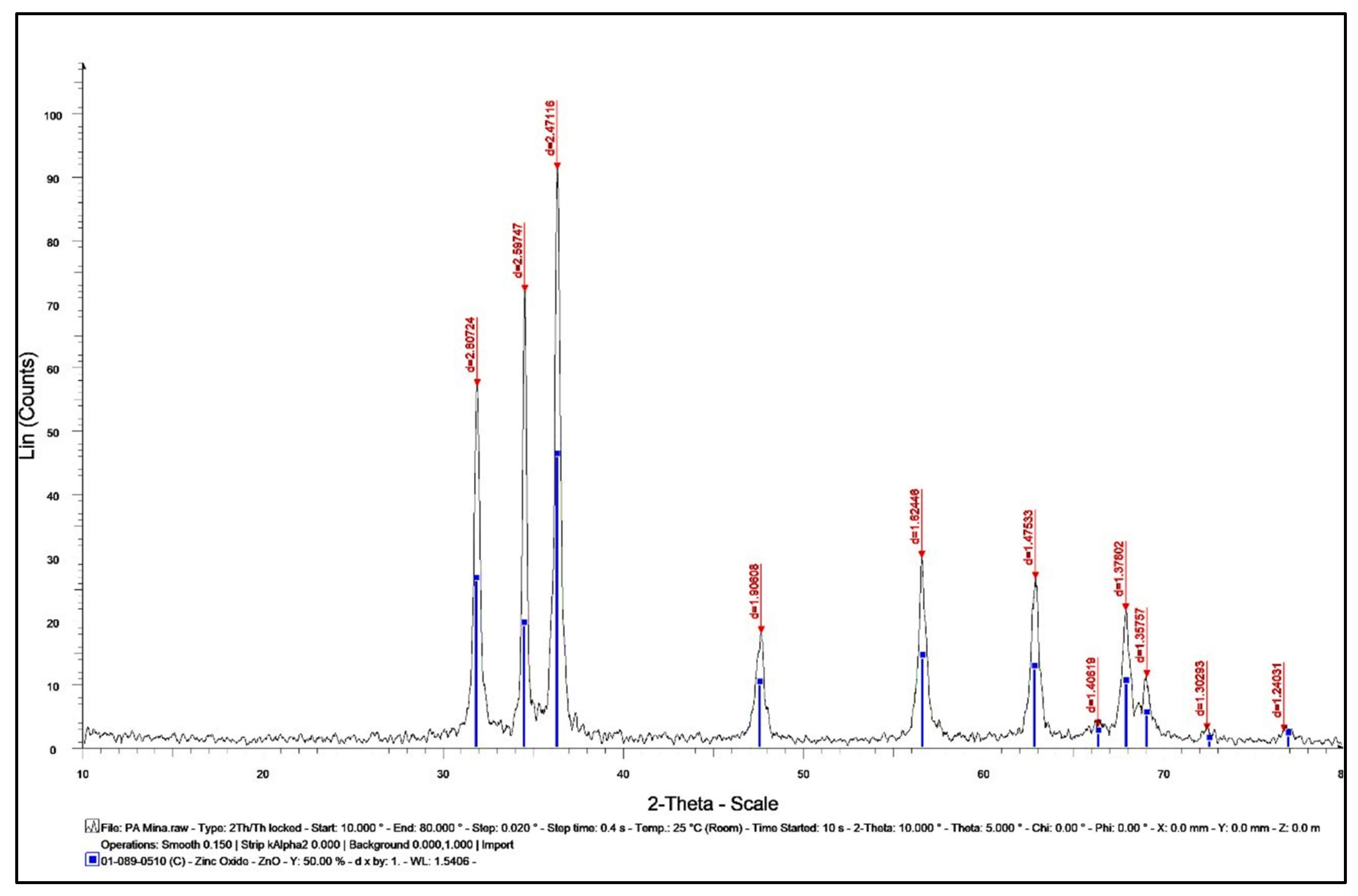
2.2.5. XRD Analysis
2.3. The Cell Viability and Antiviral Activities of P. auriculata Lam. Extract and ZnO NPs
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Plant Active Ingredients
3.3. HPLC Analysis
3.4. Green Synthesis of ZnO NPs
3.5. Characterization of ZnO NPs
3.6. Antiviral Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selim, Y.A.; Azb, M.A.; Ragab, I.; El-Azim, M.H.M.A. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karishma, S.; Naidoo, Y.; Baijnath, H. A comprehensive review on the genus Plumbago with focus on Plumbago auriculata (Plumbaginaceae). Afr. J. Tradit. Complementary Altern. Med. 2018, 15, 199–215. [Google Scholar]
- Kumar, T.S.J.; Balavigneswaran, C.K.; Packiaraj, R.M.; Veeraraj, A.; Prakash, S.; Hassan, Y.N.; Srinivasakumar, K.P. Green Synthesis of Silver Nanoparticles by Plumbago indica and Its Antitumor Activity Against Dalton’s Lymphoma Ascites Model. BioNanoScience 2013, 3, 394–402. [Google Scholar] [CrossRef]
- Jaryal, N.; Kaur, H. Plumbago auriculata leaf extract-mediated AgNPs and its activities as antioxidant, anti-TB and dye degrading agents. J. Biomater. Sci. Polym. Ed. 2017, 28, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Govindan, L.; Anbazhagan, S.; Altemimi, A.B.; Lakshminarayanan, K.; Kuppan, S.; Pratap-Singh, A.; Kandasamy, M. Efficacy of antimicrobial and larvicidal activities of green synthesized silver nanoparticles using leaf extract of Plumbago auriculata lam. Plants 2020, 9, 1577. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bharadvaja, N. Silver nanoparticles synthesis from a pharmaceutically important medicinal plant Plumbago zeylanica. MOJ Bioequi. Availa. 2017, 3, 46. [Google Scholar]
- Maheswari, T.; Jayapriya, G.; Prabha, N.; Vennila, M. Synthesis, Characterization and applications of selenium nanoparticle using Plumbago zeylanica leaves extract. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 60–71. [Google Scholar]
- Jamdade, D.A.; Rajpali, D.; Joshi, K.A.; Kitture, R.; Kulkarni, A.S.; Shinde, V.S.; Bellare, J.; Babiya, K.R.; Ghosh, S. Gnidia glauca- and Plumbago zeylanica-Mediated Synthesis of Novel Copper Nanoparticles as Promising Antidiabetic Agents. Adv. Pharmacol. Sci. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chopade, B.A.; Salunke, G.R.; Ghosh, S.; Santosh, R.J.; Khade, S.; Vashisth, P.; Kale, T.; Chopade, S.; Pruthi, V.; Kundu, G.; et al. Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int. J. Nanomed. 2014, 9, 2635–2653. [Google Scholar] [CrossRef] [Green Version]
- Maheswari, T.; Vennila, M. Spectroscopic investigation of green and chemically synthesized zinc oxide nanoparticles and its antimicrobial activities. IJSRR 2018, 8, 3363–3377. [Google Scholar]
- Yadavalli, T.; Shukla, D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomedicine 2017, 13, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1–18. [Google Scholar] [CrossRef]
- Catelli, M.; Cecchinato, C.E.; Savage, R.C.; Jones, C.J. Demonstration of loss of attenuation and extended field persistence of a live avian metapneumovirus vaccine. Vaccine 2006, 24, 6476–6482. [Google Scholar] [CrossRef]
- Cook, J.K. Avian rhinotracheitis. Rev. Sci. Et Tech. (Int. Off. Epizoot.) 2000, 19, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Skaar, I.; Jordheim, M.; Byamukama, R.; Mbabazi, A.; Wubshet, S.G.; Kiremire, B.; Andersen, Ø.M. New anthocyanidin and anthocyanin pigments from blue plumbago. J. Agric. Food Chem. 2012, 60, 1510–1515. [Google Scholar] [CrossRef]
- Harborne, J. Plant polyphenols: Occurrence of azalein and related pigments in flowers of Plumbago and Rhododendron species. Arch. Biochem. Biophys 1962, 96, 171–178. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Mehrotra, B.N. Compendium of Indian Medicinal Plants; Central Drug Research Institute: New Delhi, India, 1995. [Google Scholar]
- Srinivasan, N.; Rangasami, C.; Kannan, J.C. Synthesis structure and optical properties of zinc oxide nanoparticles. Int. J. Appl. Eng. Res. 2015, 10, 343–345. [Google Scholar]
- Fakhari, S.; Jamzad, M.; Fard, H.K. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Suresh, J.; Saravanakumar, B.; Nathanael, A.J.; Hong, S.I.; Rajendran, V. Rambutan peels promoted biomimetic synthesis of bioinspired zinc oxide nanochains for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, F.; Morsali, A.; Retailleau, P. Syntheses and characterization of di_erent zinc (II) oxide nano-structures from direct thermal decomposition of 1D coordination polymers. Polyhedron 2010, 29, 801–806. [Google Scholar] [CrossRef]
- Kashyout, A.B.; Soliman, H.M.A.; Hassan, H.S.; Abousehly, A.M. Fabrication of ZnO and ZnO:Sb Nanoparticles for Gas Sensor Applications. J. Nanomater. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Severson, W.E.; Shindo, N.; Sosa, M.; Fletcher, T., III; White, E.L.; Ananthan, S.; Jonsson, C.B. Development and validation of a high-throughput screen for inhibitors of SARS CoV and its application in screening of a 100,000-compound library. J. Biomol. Screen. 2007, 12, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Maddox, C.; Rasmussen, L.; Hobrath, J.V.; White, L.E. Assay development and high-throughput antiviral drug screening against Bluetongue virus. Antivir. Res. 2009, 83, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Gebre-Mariam, T.; Neubert, R.; Schmidt, P.; Wutzler, P.; Schmidtke, M. Antiviral activities of some Ethiopian medicinal plants used for the treatment of dermatological disorders. J. Ethnopharmacol. 2006, 104, 182–187. [Google Scholar] [CrossRef]
- Chavan, R.D.; Shinde, P.; Girkar, K.; Madage, R.; Chowdhary, A. Assessment of anti-influenza activity and hemagglutination inhibition of Plumbago indica and Allium sativum extracts. Pharmacogn. Res. 2016, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrollahzadeh, M.S.; Ghodsi, R.; Hadizadeh, F.; Maleki, M.F.; Mashreghi, M.; Poy, D. Zinc Oxide Nanoparticles as a Potential Agent for Antiviral Drug Delivery Development; A Systematic Literature Review. Curr. Nanosci. 2021, 17, 1. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, Y.; Ma, Y.; Sun, J.; Liang, X.; Li, J. Zinc Binding Activity of Human Metapneumovirus M2-1 Protein Is Indispensable for Viral Replication and PathogenesisIn Vivo. J. Virol. 2015, 89, 6391–6405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar]
- Attia, G.H.; Alyami, H.S.; Orabi, M.A.; Gaara, A.H.; El Raey, M.A. Antimicrobial Activity of Silver and Zinc Nanoparticles Mediated by Eggplant Green Calyx. Int. J. Pharmacol. 2020, 16, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Kohn, L.; Foglio, M.; Rodrigues, R.; De O Sousa, I.; Martini, M.; Padilla, M.; Neto, D.D.L.; Arns, C. In-Vitro Antiviral Activities of Extracts of Plants of The Brazilian Cerrado against the Avian Metapneumovirus (aMPV). Braz. J. Poult. Sci. 2015, 17, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Kohn, L.K.; Pavam, C.H.; Veronese, D.; Coelho, F.; De Carvalho, J.E.; Almeida, W.P. Antiproliferative effect of Baylis-Hillman adducts and a new phthalide derivative on human tumor cell lines. Eur. J. Med Chem. 2006, 41, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D. Evaluation of a soluble tetrazolium formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
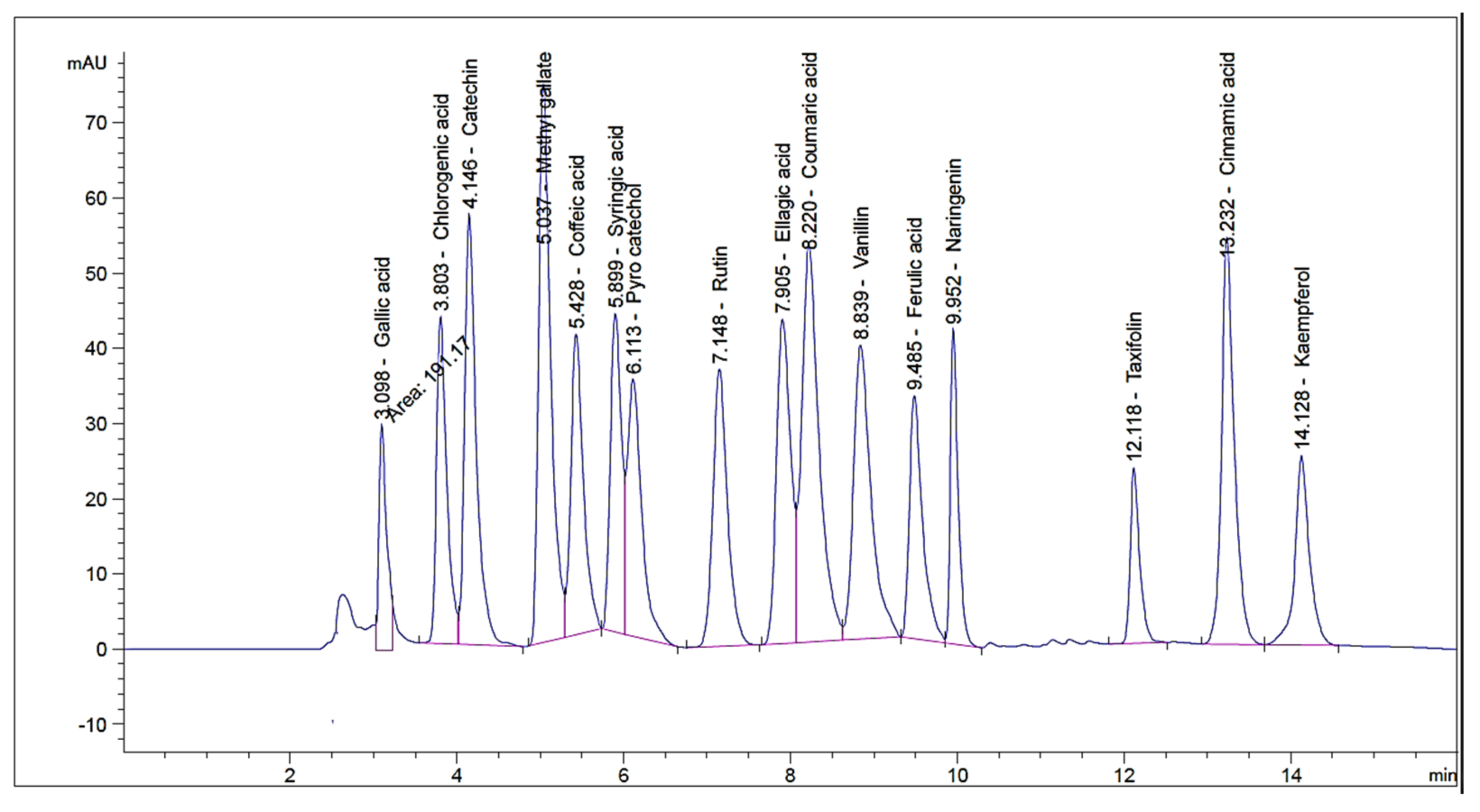
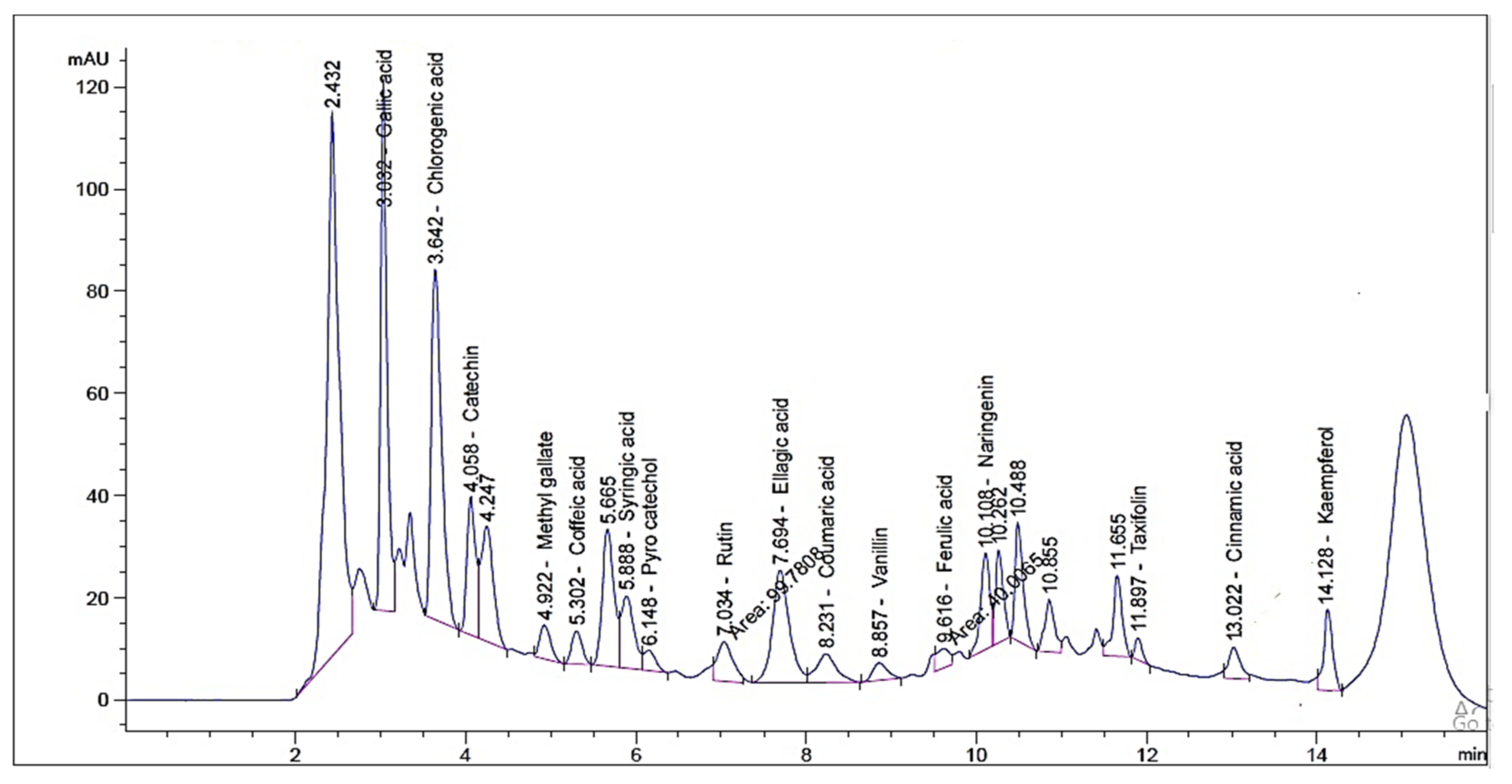


| RT 1 | RRT 2 | Area * | Area% | Conc. (µg/g) | |
|---|---|---|---|---|---|
| Gallic acid | 3.032 | 0.43 | 533.43 ± 2.3 | 21.20 | 1720.26 |
| Chlorogenic acid | 3.642 | 0.52 | 585.81 ± 1.07 | 23.20 | 1600.42 |
| Catechin | 4.058 | 0.58 | 193.40 ± 2.31 | 7.60 | 840.20 |
| Methyl gallate | 4.992 | 0.71 | 58.45 ± 0.84 | 2.36 | 29.57 |
| Coffeic acid | 5.302 | 0.75 | 56.58 ± 0.71 | 2.21 | 87.04 |
| Syringic acid | 5.888 | 0.84 | 150.87 ± 1.38 | 6.03 | 243.48 |
| Pyro catechol | 6.148 | 0.87 | 38.71 ± 0.49 | 1.50 | 97.97 |
| Rutin | 7.034 | 1.00 | 99.781 ± 1.03 | 3.96 | 497.51 |
| Ellagic acid | 7.694 | 1.09 | 30.84 ± 3.66 | 12.05 | 744.63 |
| Coumaric acid | 8.231 | 1.17 | 93.67 ± 2.13 | 3.68 | 57.69 |
| Vanillin | 8.857 | 1.26 | 42.39 ± 1.06 | 1.64 | 34.07 |
| Ferulic acid | 9.616 | 1.37 | 41.03 ± 0.86 | 1.59 | 51.14 |
| Naringenin | 10.108 | 1.44 | 142.74 ± 1.51 | 5.70 | 291.09 |
| Taxifolin | 11.897 | 1.69 | 25.49 ± 0.64 | 1.01 | 67.83 |
| Cinnamic acid | 13.022 | 1.85 | 58.95 ± 0.42 | 2.38 | 22.18 |
| Kaempferol | 14.128 | 2.01 | 97.23 ± 0.26 | 3.90 | 141.09 |
| I.P | CC50 μg/mL * | IC50 μg/mL * | SI | |
|---|---|---|---|---|
| P. auriculata | 99% | 87.00 ± 3.76 | 67.97 ± 4.63 | 1.28 |
| ZnO NPs | 99% | 52.48 ± 1.57 | 42.67 ± 4.08 | 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melk, M.M.; El-Hawary, S.S.; Melek, F.R.; Saleh, D.O.; Ali, O.M.; El Raey, M.A.; Selim, N.M. Nano Zinc Oxide Green-Synthesized from Plumbago auriculata Lam. Alcoholic Extract. Plants 2021, 10, 2447. https://doi.org/10.3390/plants10112447
Melk MM, El-Hawary SS, Melek FR, Saleh DO, Ali OM, El Raey MA, Selim NM. Nano Zinc Oxide Green-Synthesized from Plumbago auriculata Lam. Alcoholic Extract. Plants. 2021; 10(11):2447. https://doi.org/10.3390/plants10112447
Chicago/Turabian StyleMelk, Mina Michael, Seham S. El-Hawary, Farouk Rasmy Melek, Dalia Osama Saleh, Omar M. Ali, Mohamed A. El Raey, and Nabil Mohamed Selim. 2021. "Nano Zinc Oxide Green-Synthesized from Plumbago auriculata Lam. Alcoholic Extract" Plants 10, no. 11: 2447. https://doi.org/10.3390/plants10112447
APA StyleMelk, M. M., El-Hawary, S. S., Melek, F. R., Saleh, D. O., Ali, O. M., El Raey, M. A., & Selim, N. M. (2021). Nano Zinc Oxide Green-Synthesized from Plumbago auriculata Lam. Alcoholic Extract. Plants, 10(11), 2447. https://doi.org/10.3390/plants10112447