Mediterranean White Lupin Landraces as a Valuable Genetic Reserve for Breeding
Abstract
:1. Introduction
2. Results
2.1. Seed Morphological Characters
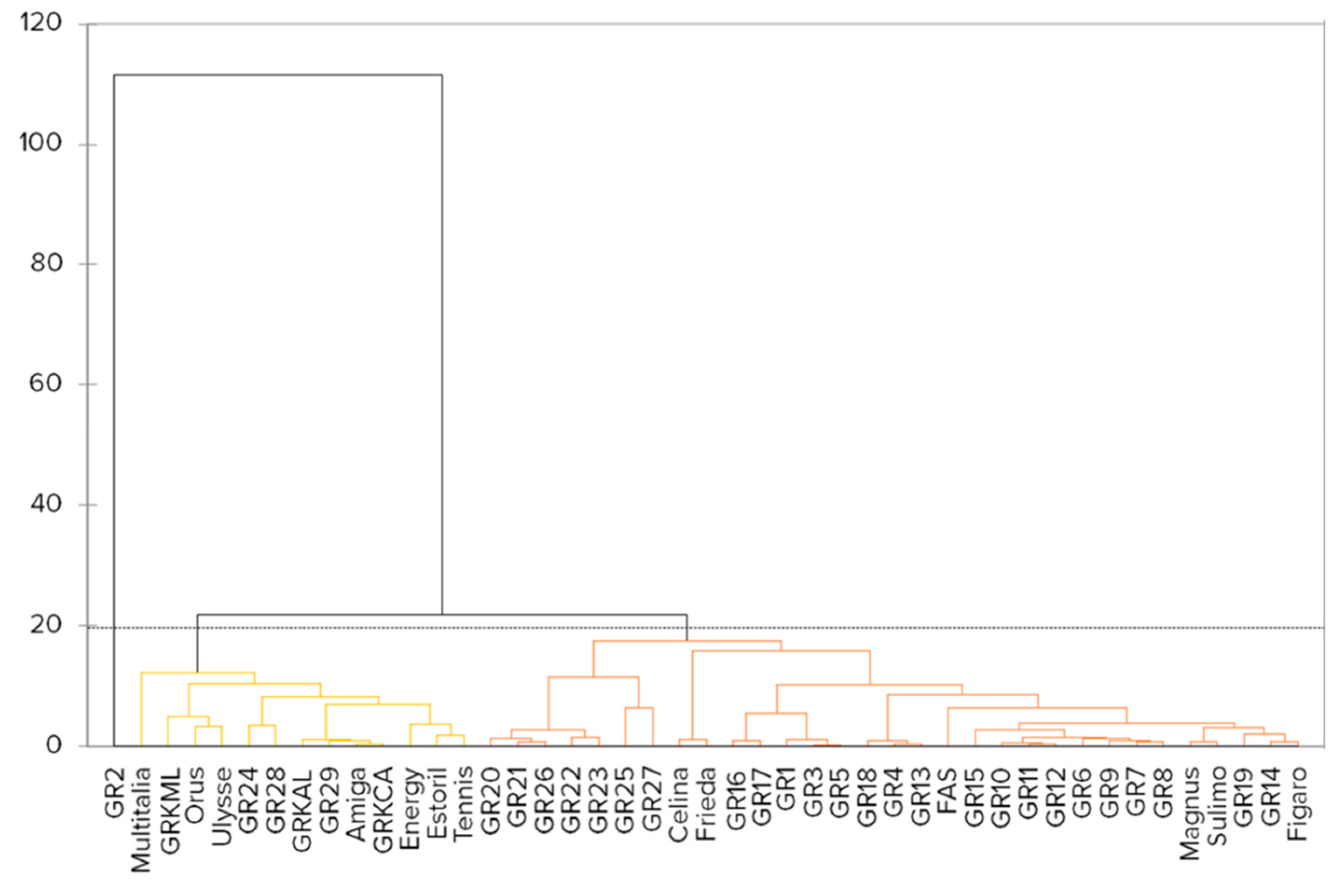
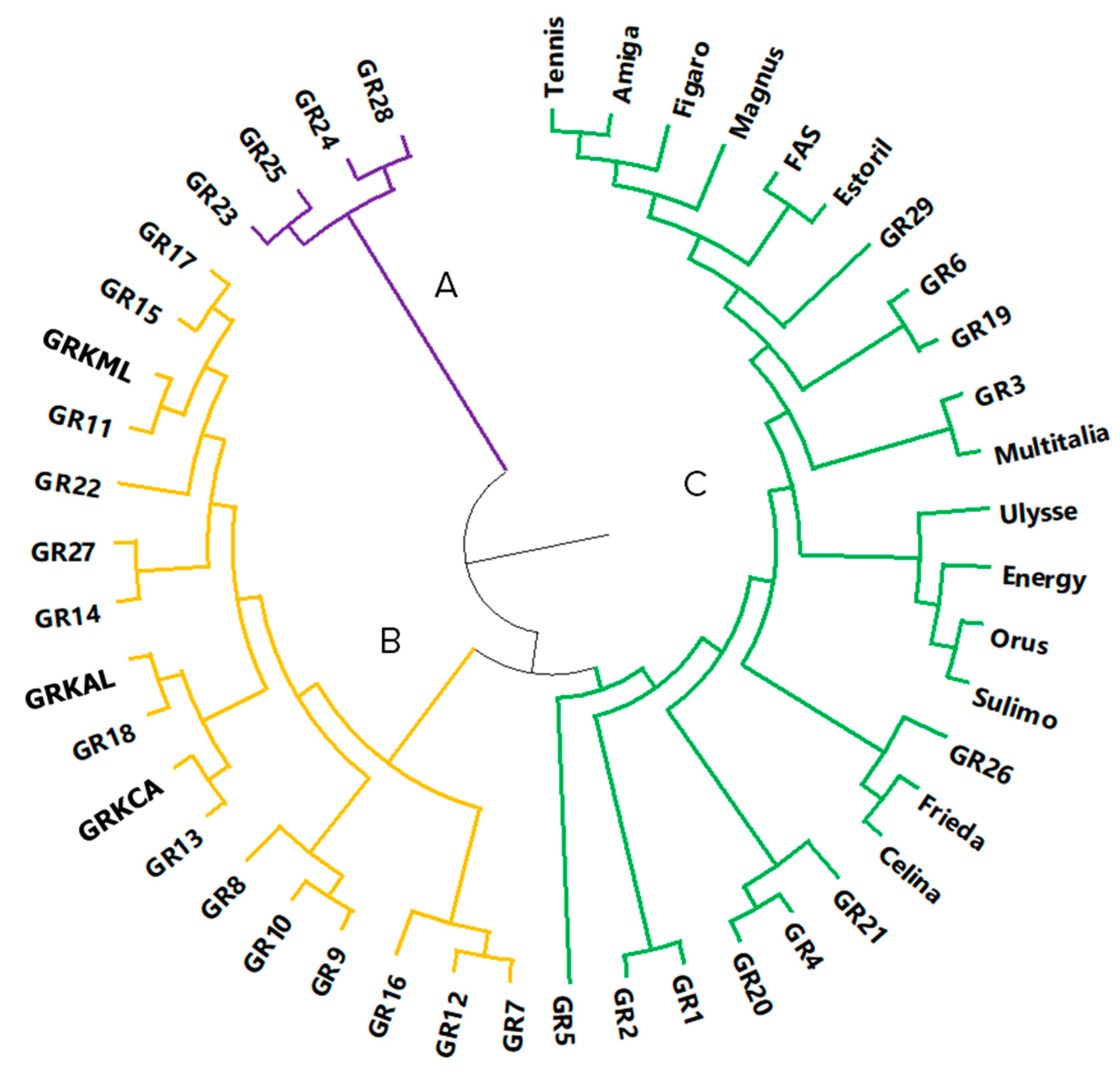
2.2. Markers for Genetic Analysis
2.3. Marker Genotypes Linked to Significant Traits
2.4. Alkaloid Content Profile
3. Discussion
4. Materials and Methods
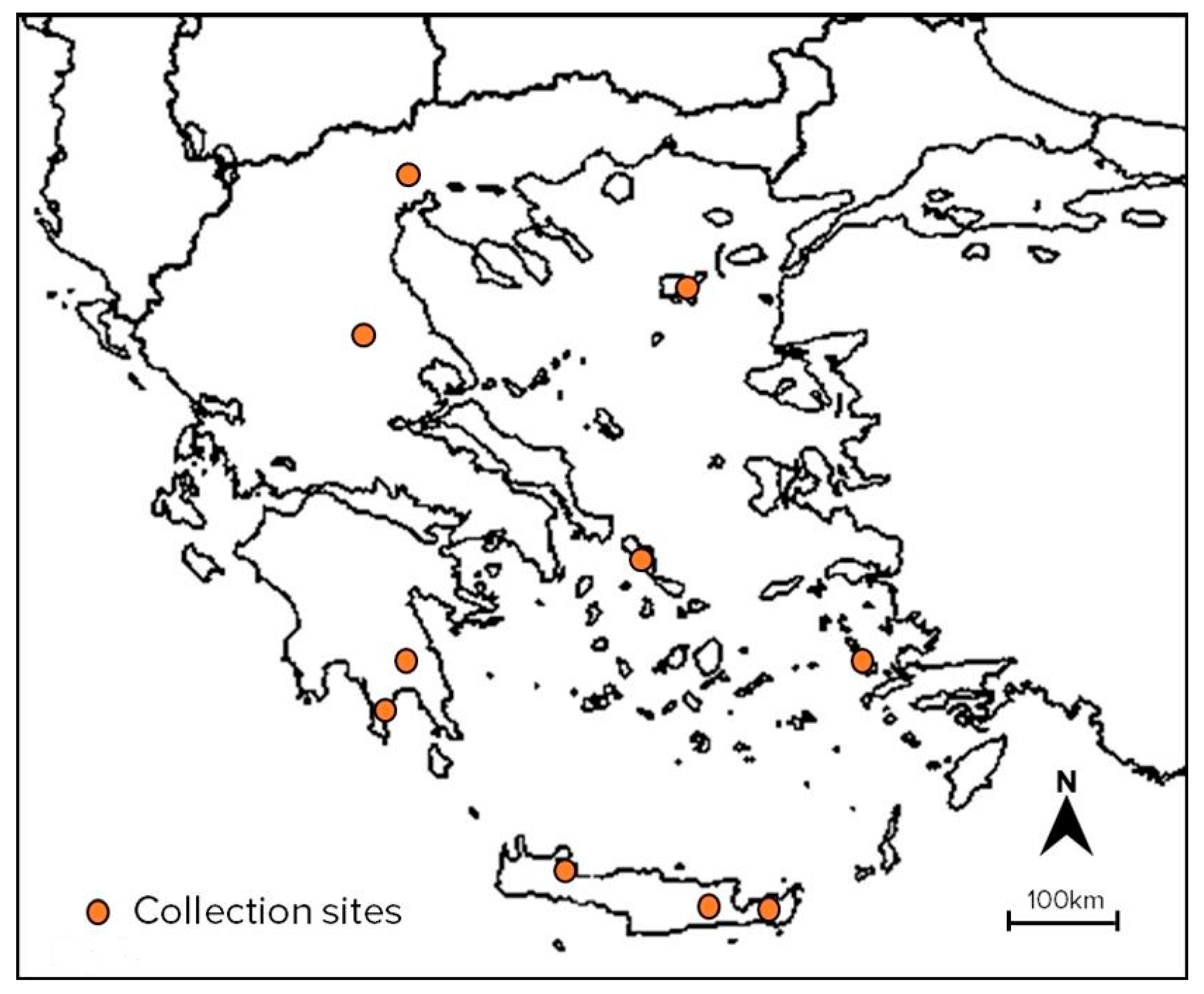
4.1. Plant Material
4.2. Estimation of Genetic Diversity
4.2.1. Seed Morphological Diversity Analysis
4.2.2. PCR and SSR-HRM Analysis
4.3. Analysis of Molecular Genetic Relationships
4.4. Germplasm Molecular Characterization on Anthracnose Resistance, Vernalization Responsiveness amd Alkaloid Biosynthesis
4.5. Extraction of Alkaloids and Profiling by HPLC-HRMS-Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eastwood, R.J.; Drummond, C.S.; Schifino-Wittmann, M.T.; Hughes, C.E. Diversity and Evolutionary History of Lupins–Insights from New Phylogenies. In Lupins for health and wealth, Proceedings of the 12th International Lupin Conference, Fremantle, Australia, 14–18 September 2008; Palta, J.A., Berger, J.B., Eds.; International Lupin Association: Canterbury, New Zealand; pp. 346–354. ISBN 0-86476-153-8.
- Tirdiľová, I.; Vollmannová, A.; Siekel, P.; Zetochová, E.; Čéryová, S.; Trebichalský, P. Selected legumes as a source of valuable substances in human nutrition. J. Food Nutr. Res. 2020, 59, 193–201. [Google Scholar]
- Prusinski, J. White lupin (Lupinus albus L.)-Nutritional and health values in human nutrition-A review. Czech J. Food Sci. 2017, 35, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Shu, L.; Shen, J.; Rengel, Z.; Tang, C.; Zhang, F.; Cawthray, G.R. Formation of cluster roots and citrate exudation by Lupinus albus in response to localized application of different phosphorus sources. Plant Sci. 2007, 172, 1017–1024. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kalembasa, S.; Szukała, J.; Faligowska, A.; Kalembasa, D.; Symanowicz, B.; Becher, M.; Gebus-Czupyt, B. Quantification of biologically fixed nitrogen by white lupin (Lupins albus L.) and its subsequent uptake by winter wheat using the 15n isotope dilution method. Agronomy 2020, 10, 1392. [Google Scholar] [CrossRef]
- Lambers, H.; Martinoia, E.; Renton, M. Plant adaptations to severely phosphorus-impoverished soils. Curr. Opin. Plant Biol. 2015, 25, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Lopez, J.C.; Singh, K.B.; Clemente, A.; Nelson, M.N.; Ochatt, S.; Smith, P.M.C. Editorial: Legumes for Global Food Security. Front. Plant Sci. 2020, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The use of lupin as a source of protein in animal feeding: Genomic tools and breeding approaches. Int. J. Mol. Sci. 2019, 20, 851. [Google Scholar] [CrossRef] [Green Version]
- Struti, D.I.; Mierlita, D.; Pop, I.M.; Ladosi, D.; Papuc, T. Evaluation of the chemical composition and nutritional quality of dehulled lupin seed meal (Lupinus spp. L.) and its use for monogastrics animal nutrition: A review. Sci. Pap. Ser. D Anim. Sci. Int. Sess. Sci. Commun. Fac. Anim. Sci. 2020, 63, 92–105. [Google Scholar]
- FAO. FAOSTAT Statistical Database. Crops and Livestock Products. 2019. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 September 2021).
- Gresta, F.; Wink, M.; Prins, U.; Abberton, M.; Capraro, J.; Scarafoni, A.; Hill, G. Lupins in European cropping systems. In Legumes Cropping Systems; Murphy-Bokern, D., Stoddard, F.L., Watson, C.A., Eds.; CABI: Wallingford, UK, 2017; pp. 88–108. [Google Scholar] [CrossRef]
- Rybiński, W.; Święcicki, W.; Bocianowski, J.; Börner, A.; Starzycka-Korbas, E.; Starzycki, M. Variability of fat content and fatty acids profiles in seeds of a Polish white lupin (Lupinus albus L.) collection. Genet. Resour. Crop Evol. 2018, 65, 417–431. [Google Scholar] [CrossRef] [Green Version]
- Ksiazkiewicz, M.; Nazzicari, N.; Yang, H.; Nelson, M.N.; Renshaw, D.; Rychel, S.; Ferrari, B.; Carelli, M.; Tomaszewska, M.; Stawiński, S.; et al. A high-density consensus linkage map of white lupin highlights synteny with narrow-leafed lupin and provides markers tagging key agronomic traits. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Frick, K.M.; Kamphuis, L.G.; Siddique, K.H.M.; Singh, K.B.; Foley, R.C. Quinolizidine Alkaloid Biosynthesis in Lupins and Prospects for Grain Quality Improvement. Front. Plant Sci. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Kroc, M.; Rybiński, W.; Wilczura, P.; Kamel, K.; Kaczmarek, Z.; Barzyk, P.; Święcicki, W. Quantitative and qualitative analysis of alkaloids composition in the seeds of a white lupin (Lupinus albus L.) collection. Genet. Resour. Crop Evol. 2017, 64, 1853–1860. [Google Scholar] [CrossRef] [Green Version]
- Hufnagel, B.; Soriano, A.; Taylor, J.; Divol, F.; Kroc, M.; Sanders, H.; Yeheyis, L.; Nelson, M.; Péret, B. Pangenome of white lupin provides insights into the diversity of the species. Plant Biotechnol. J. 2021. [Google Scholar] [CrossRef]
- Rychel, S.; Książkiewicz, M.; Tomaszewska, M.; Bielski, W.; Wolko, B. Flowering Locus T, Gigantea, Sepallata, and Frigida homologs are candidate genes involved in white lupin (Lupinus albus L.) early flowering. Mol. Breed. 2019, 39, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Rychel-Bielska, S.; Nazzicari, N.; Plewiński, P.; Bielski, W.; Annicchiarico, P.; Książkiewicz, M. Development of PCR-based markers and whole-genome selection model for anthracnose resistance in white lupin (Lupinus albus L.). J. Appl. Genet. 2020, 61, 531–545. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Nazzicari, N.; Ferrari, B.; Harzic, N.; Carroni, A.M.; Romani, M.; Pecetti, L. Genomic prediction of grain yield in contrasting environments for white lupin genetic resources. Mol. Breed. 2019, 39, 1–12. [Google Scholar] [CrossRef]
- Arief, O.M.; Pang, J.; Shaltout, K.H.; Lambers, H. Performance of two Lupinus albus L. cultivars in response to three soil pH levels. Exp. Agric. 2020, 56, 321–330. [Google Scholar] [CrossRef]
- Sloat, L.L.; Davis, S.J.; Gerber, J.S.; Moore, F.C.; Ray, D.K.; West, P.C.; Mueller, N.D. Climate adaptation by crop migration. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brogli, R.; Sørland, S.L.; Kröner, N.; Schär, C. Causes of future Mediterranean precipitation decline depend on the season. Environ. Res. Lett. 2019, 14, 114017. [Google Scholar] [CrossRef]
- Sakellariou, M.; Psiloglou, B.E.; Giannakopoulos, C.; Mylona, P.V. Integration of Abandoned Lands in Sustainable Agriculture: The Case of Terraced Landscape Re-Cultivation in Mediterranean Island Conditions. Land 2021, 10, 457. [Google Scholar] [CrossRef]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth-Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, M.; Guo, C. Crop pollen development under drought: From the phenotype to the mechanism. Int. J. Mol. Sci. 2019, 20, 1550. [Google Scholar] [CrossRef] [Green Version]
- Rering, C.C.; Franco, J.G.; Yeater, K.M.; Mallinger, R.E. Drought stress alters floral volatiles and reduces floral rewards, pollinator activity, and seed set in a global plant. Ecosphere 2020, 11, e03254. [Google Scholar] [CrossRef]
- Descamps, C.; Quinet, M.; Jacquemart, A.L. The effects of drought on plant–pollinator interactions: What to expect? Environ. Exp. Bot. 2021, 182, 104297. [Google Scholar] [CrossRef]
- Brás, T.A.; Seixas, J.; Carvalhais, N.; Jägermeyr, J. Severity of drought and heatwave crop losses tripled over the last five decades in Europe. Environ. Res. Lett. 2021, 16, 065012. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Rychel-Bielska, S.; Surma, A.; Bielski, W.; Kozak, B.; Galek, R.; Książkiewicz, M. Quantitative control of early flowering in white lupin (Lupinus albus L.). Int. J. Mol. Sci. 2021, 22, 3856. [Google Scholar] [CrossRef]
- Falconi, C.E.; Visser, R.G.F.; Van Heusden, S. Influence of plant growth stage on resistance to anthracnose in Andean lupin (Lupinus mutabilis). Crop Pasture Sci. 2015, 66, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Annicchiarico, P.; Barrett, B.; Brummer, E.C.; Julier, B.; Marshall, A.H. Achievements and Challenges in Improving Temperate Perennial Forage Legumes. Crit. Rev. Plant Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Talhinhas, P.; Baroncelli, R.; Le Floch, G. Anthracnose of Lupins Caused by Colletotrichum Lupini: A Recent Disease and a Successful Worldwide Pathogen. J. Plant Pathol. 2016, 98, 5–14. [Google Scholar] [CrossRef]
- Adhikari, K.N.; Buirchell, B.J.; Thomas, G.J.; Sweetingham, M.W.; Yang, H. Identification of anthracnose resistance in Lupinus albus L. and its transfer from landraces to modern cultivars. Crop Pasture Sci. 2009, 60, 472. [Google Scholar] [CrossRef]
- Thomas, G.J.; Sweetingham, M.W.; Yang, H.A.; Speijers, J. Effect of temperature on growth of Colletotrichum lupini and on anthracnose infection and resistance in lupins. Australas. Plant Pathol. 2008, 37, 35–39. [Google Scholar] [CrossRef]
- Msairi, S.; Chliyeh, M.; Touhami, A.O.; El Alaoui, A.; Selmaoui, K.; Benkirane, R.; Filali-Maltouf, A.; El Modafar, C.; Douira, A. First report of colletotrichum lupini causing anthracnose disease on the olive fruits in Morocco. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 1–11. [Google Scholar]
- Phan, H.T.T.; Ellwood, S.R.; Adhikari, K.; Nelson, M.N.; Oliver, R.P. The first genetic and comparative map of white lupin (Lupinus albus L.): Identification of QTLs for anthracnose resistance and flowering time, and a locus for alkaloid content. DNA Res. 2007, 14, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Jacob, I.; Feuerstein, U.; Heinz, M.; Schott, M.; Urbatzka, P. Evaluation of new breeding lines of white lupin with improved resistance to anthracnose. Euphytica 2017, 213, 236. [Google Scholar] [CrossRef]
- Alkemade, J.; Messmer, M.; Arncken, C.; Leska, A.; Annicchiarico, P.; Nazzicari, N.; Książkiewicz, M.; Voegele, R.T.; Finckh, M.; Hohmann, P. A high-throughput phenotyping tool to identify field-relevant anthracnose resistance in white lupin. Plant Dis. 2021, 105, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.N.; Książkiewicz, M.; Rychel, S.; Besharat, N.; Taylor, C.M.; Wyrwa, K.; Jost, R.; Erskine, W.; Cowling, W.A.; Berger, J.D.; et al. The loss of vernalization requirement in narrow-leafed lupin is associated with a deletion in the promoter and de-repressed expression of a Flowering Locus T (FT) homologue. New Phytol. 2017, 213, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Atnaf, M.; Yao, N.; Martina, K.; Dagne, K.; Wegary, D.; Tesfaye, K. Molecular genetic diversity and population structure of Ethiopian white lupin landraces: Implications for breeding and conservation. PLoS ONE 2017, 12, e0188696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroc, M.; Tomaszewska, M.; Czepiel, K.; Bitocchi, E.; Oppermann, M.; Neumann, K.; Guasch, L.; Bellucci, E.; Alseekh, S.; Graner, A.; et al. Towards Development, Maintenance, and Standardized Phenotypic Characterization of Single-Seed-Descent Genetic Resources for Lupins. Curr. Protoc. 2021, 1, e191. [Google Scholar] [CrossRef]
- Beyene, C. Genetic variation among white lupin (Lupinus albus L.) landraces from Northwestern and Southern Ethiopia for agronomic traits and nutrient contents of grain. J. Plant Breed. Crop Sci. 2020, 12, 156–169. [Google Scholar] [CrossRef]
- Plewiński, P.; Książkiewicz, M.; Rychel-Bielska, S.; Rudy, E.; Wolko, B. Candidate domestication-related genes revealed by expression quantitative trait loci mapping of narrow-leafed lupin (Lupinus angustifolius L.). Int. J. Mol. Sci. 2019, 20, 5670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annicchiarico, P.; Romani, M.; Pecetti, L. White lupin (Lupinus albus) variation for adaptation to severe drought stress. Plant Breed. 2018, 137, 782–789. [Google Scholar] [CrossRef]
- Berger, J.D.; Shrestha, D.; Ludwig, C. Reproductive strategies in mediterranean legumes: Trade-offs between phenology, seed size and vigor within and between wild and domesticated lupinus species collected along aridity gradients. Front. Plant Sci. 2017, 8, 548. [Google Scholar] [CrossRef] [Green Version]
- Rychel, S.; Książkiewicz, M. Development of gene-based molecular markers tagging low alkaloid pauper locus in white lupin (Lupinus albus L.). J. Appl. Genet. 2019, 60, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Di Santo, L.N.; Polgar, M.; Nies, S.; Hodgkiss, P.; Canning, C.A.; Wright, J.W.; Hamilton, J.A. Seed morphological traits as a tool to quantify variation maintained in ex situ collections: A case study in Pinus torreyana. AoB Plants 2021, 13, 58. [Google Scholar] [CrossRef]
- Lagunes-Espinoza, L.C.; Huyghe, C.; Papineau, J. Genetic variation for pod wall proportion in Lupinus albus L. Plant Breed. 2000, 119, 421–425. [Google Scholar] [CrossRef]
- González-Andrés, F.; Casquero, P.A.; San-Pedro, C.; Hernández-Sánchez, E. Diversity in White Lupin (Lupinus albus L.) Landraces from Northwest Iberianplateau. Genet. Resour. Crop Evol. 2007, 54, 27–44. [Google Scholar] [CrossRef]
- Raman, R.; Cowley, R.B.; Raman, H.; Luckett, D.J. Analyses Using SSR and DArT Molecular Markers Reveal that Ethiopian Accessions of White Lupin (Lupinus albus L.) Represent a Unique Genepool. Open J. Genet. 2014, 04, 87–98. [Google Scholar] [CrossRef] [Green Version]
- El-Harty, E.; Ashrie, A.; Ammar, M.; Alghamdi, S. Genetic variation among egyptian white lupin (Lupinus albus L.) genotypes. Turk. J. Field Crop. 2016, 21, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an evolved concept of landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Coomes, O.T.; McGuire, S.J.; Garine, E.; Caillon, S.; McKey, D.; Demeulenaere, E.; Jarvis, D.; Aistara, G.; Barnaud, A.; Clouvel, P.; et al. Farmer seed networks make a limited contribution to agriculture? Four common misconceptions. Food Policy 2015, 56, 41–50. [Google Scholar] [CrossRef]
- Fekadu Gemede, H. Antinutritional Factors in Plant Foods: Potential Health Benefits and Adverse Effects. Int. J. Nutr. Food Sci. 2014, 3, 284. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Renshaw, D.; Luckett, D.; Clements, J.; Yan, G.; Adhikari, K.; Buirchell, B.; Sweetingham, M.; Yang, H. Development of a sequence-specific PCR marker linked to the gene “pauper” conferring low-alkaloids in white lupin (Lupinus albus L.) for marker assisted selection. Mol. Breed. 2009, 23, 153–161. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Harzic, N.; Carroni, A.M. Adaptation, diversity, and exploitation of global white lupin (Lupinus albus L.) landrace genetic resources. Field Crop. Res. 2010, 119, 114–124. [Google Scholar] [CrossRef]
- Noffsinger, S.L.; Van Santen, E. Evaluation of Lupinus albus L. germplasm for the southeastern USA. Crop Sci. 2005, 45, 1941–1950. [Google Scholar] [CrossRef]
- Tsanakas, G.F.; Mylona, P.V.; Koura, K.; Gleridou, A.; Polidoros, A.N. Genetic diversity analysis of the Greek lentil (Lens culinaris) landrace “Eglouvis” using morphological and molecular markers. Plant Genet. Resour. Charact. Util. 2018, 16, 469–477. [Google Scholar] [CrossRef]
- Nelson, M.N.; Phan, H.T.T.; Ellwood, S.R.; Moolhuijzen, P.M.; Hane, J.; Williams, A.; O’Lone, C.E.; Fosu-Nyarko, J.; Scobie, M.; Cakir, M.; et al. The first gene-based map of Lupinus angustifolius L.-location of domestication genes and conserved synteny with Medicago truncatula. Theor. Appl. Genet. 2006, 113, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Tajima, F.; Tateno, Y. Accuracy of estimated phylogenetic trees from molecular data-II. Gene frequency data. J. Mol. Evol. 1983, 19, 153–170. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Ben Hassine, A.; Rocchetti, G.; Zhang, L.; Senizza, B.; Zengin, G.; Mahomoodally, M.F.; Ben-Attia, M.; Rouphael, Y.; Lucini, L.; El-Bok, S. Untargeted Phytochemical Profile, Antioxidant Capacity and Enzyme Inhibitory Activity of Cultivated and Wild Lupin Seeds from Tunisia. Molecules 2021, 26, 3452. [Google Scholar] [CrossRef] [PubMed]


| Variable | Observations | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|---|
| TSW (g) | 45 | 70.8 | 968.2 | 357.2 | 116.7 |
| Area (mm2) | 45 | 628.0 | 2317.2 | 963.2 | 260.5 |
| Perimeter (mm) | 45 | 304.5 | 578.8 | 374.1 | 42.2 |
| Circularity † | 45 | 0.076 | 0.090 | 0.085 | 0.004 |
| Height (mm) | 45 | 66.7 | 105.9 | 81.1 | 9.4 |
| Width (mm) | 45 | 82.3 | 140.5 | 102.8 | 12.2 |
| Gray Value † (Min.) | 45 | 0.000 | 102. | 46.1 | 27.5 |
| Gray Value † (Max.) | 45 | 128.0 | 255. | 205.6 | 34.8 |
| Gray Value † (Mean) | 45 | 70.4 | 166.6 | 136.1 | 28.2 |
| Gray Value † (Median) | 45 | 65.0 | 169.0 | 137.0 | 28.7 |
| Integrated Density † | 45 | 576,855.0 | 23,398,105.0 | 6,649,080.0 | 6,775,359.0 |
| Accession | Anthracnose Resistance | Early Flowering | |||
|---|---|---|---|---|---|
| TP222136 | TP338761 | GI-F1 | SEP3-F1 | FTa1-F1 | |
| GR1 | − | + | − | − | + |
| GR2 | − | + | − | − | + |
| GR3 | − | − | − | + | + |
| GR4 | + | − | − | − | + |
| GR5 | − | − | + | − | + |
| GR6 | − | − | − | − | + |
| GR7 | + | − | − | + | + |
| GR8 | + | − | − | − | + |
| GR9 | + | − | − | − | + |
| GR10 | + | − | + | − | + |
| GR11 | + | − | − | − | + |
| GR12 | − | − | − | + | + |
| GR13 | + | − | − | − | + |
| GR14 | + | − | − | − | + |
| GR15 | + | − | − | − | + |
| GR16 | + | − | − | + | + |
| GR17 | + | − | − | − | + |
| GR18 | + | − | − | − | + |
| GR19 | − | − | − | + | + |
| GR20 | − | − | − | * | + |
| GR21 | + | − | − | + | + |
| GR22 | + | − | − | − | + |
| GR23 | + | + | − | − | + |
| GR24 | + | * | − | * | * |
| GR25 | + | + | − | − | * |
| GR26 | + | − | − | + | + |
| GR27 | + | − | − | − | + |
| GR28 | − | * | − | − | * |
| GR29 | − | − | − | + | + |
| GRKML | + | − | − | − | + |
| GRKAL | + | − | − | − | + |
| GRKCA | + | − | − | − | + |
| Energy | + | − | − | + | + |
| Magnus | − | − | − | + | + |
| Orus | − | − | − | + | + |
| FAS | − | − | − | + | + |
| Estoril | − | − | − | + | + |
| Ulysse | − | − | − | − | + |
| Sulimo | − | + | − | + | + |
| Figaro | − | − | − | + | + |
| Multitalia | − | − | − | + | + |
| Tennis | − | − | − | + | + |
| Amiga | − | − | − | + | + |
| Frieda | + | − | + | + | * |
| Celina | + | − | − | + | * |
| Accession | Collection Site/Origin | Genotype | Sample Type | Sample Site |
|---|---|---|---|---|
| GR1 | Crete | Landrace | Cultivated area | Cultivated |
| GR2 | Crete | Landrace | Cultivated area | Cultivated |
| GR3 | Crete | Landrace | Farm storage | Cultivated |
| GR4 | Crete | Landrace | Cultivated area | Cultivated |
| GR5 | Leros | Landrace | Farm storage | Cultivated |
| GR6 | Leros | Landrace | “ | “ |
| GR7 | Lemnos | Landrace | “ | “ |
| GR8 | Lemnos | Landrace | “ | “ |
| GR9 | Lemnos | Landrace | “ | “ |
| GR10 | Lemnos | Landrace | “ | “ |
| GR11 | Lemnos | Landrace | “ | “ |
| GR12 | Lemnos | Landrace | “ | “ |
| GR13 | Macedonia | Landrace | “ | “ |
| GR14 | Macedonia | Landrace | “ | “ |
| GR15 | Mani | Landrace | “ | “ |
| GR16 | Mani | Landrace | “ | “ |
| GR17 | Mani | Landrace | “ | “ |
| GR18 | Mani | Landrace | “ | “ |
| GR19 | Crete | Landrace | “ | “ |
| GR20 | Crete | Landrace | “ | “ |
| GR21 | Lakonia | Landrace | “ | “ |
| GR22 | Lakonia | Landrace | “ | “ |
| GR23 | Andros | Landrace | Uncultivated disturbed area | Wild |
| GR24 | Andros | Landrace | Uncultivated and undisturbed area | Wild |
| GR25 | Andros | Landrace | “ | “ |
| GR26 | Andros | Landrace | Uncultivated and disturbed area | Wild |
| GR27 | Andros | Landrace | “ | “ |
| GR28 | Andros | Landrace | “ | “ |
| GR29 | Thessaly | Breeding Line | Agricultural institute | Breeder’s line |
| GRKML | Thessaly | Breeding Line | “ | “ |
| GRKAL | Thessaly | Breeding Line | “ | “ |
| GRKCA | Thessaly | Breeding Line | “ | “ |
| Energy | France | Cultivar * | Company | Cultivated |
| Magnus | France | Cultivar | “ | “ |
| Orus | France | Cultivar | “ | “ |
| FAS | EU | Cultivar | “ | “ |
| Estoril | Portugal | Cultivar | “ | “ |
| Ulysse | France | Cultivar | “ | “ |
| Sulimo | France | Cultivar | “ | “ |
| Figaro | France | Cultivar | “ | “ |
| Multitalia | Italy | Cultivar | “ | “ |
| Tennis | Italy | Cultivar | “ | “ |
| Amiga | France/Czech Republic | Cultivar | “ | “ |
| Frieda | Germany | Cultivar | “ | “ |
| Celina | Germany | Cultivar | “ | “ |
| Trait | Molecular Marker | Validated Enzyme | Phenotype: Allele (bp) |
|---|---|---|---|
| QA content | LAGI01_35805_F1_R1 | BclI | low QA content: 197 |
| high QA content: 108, 89 | |||
| Anthracnose resistance | TP222136 | CviKI-1 | Anthr. susceptible: 168, 27, 15 |
| Anthr. resistant: 183.27 | |||
| TP338761 | SchI | Anthr. susceptible: 83, 28 | |
| Anthr. resistant: 64, 47 | |||
| Vernalization requirement | GI-F1 | AciI | vernalization unresponsive: 127, 30 |
| vernalization responsive: 157 | |||
| FTa1-F1 | - | vernalization unresponsive: 2036 | |
| vernalization responsive: 1353 | |||
| SEP3-F1 | TaqI | vernalization unresponsive: 122, 23 | |
| vernalization responsive: 145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafeiriou, I.; Polidoros, A.N.; Baira, E.; Kasiotis, K.M.; Machera, K.; Mylona, P.V. Mediterranean White Lupin Landraces as a Valuable Genetic Reserve for Breeding. Plants 2021, 10, 2403. https://doi.org/10.3390/plants10112403
Zafeiriou I, Polidoros AN, Baira E, Kasiotis KM, Machera K, Mylona PV. Mediterranean White Lupin Landraces as a Valuable Genetic Reserve for Breeding. Plants. 2021; 10(11):2403. https://doi.org/10.3390/plants10112403
Chicago/Turabian StyleZafeiriou, Ioannis, Alexios N. Polidoros, Eirini Baira, Konstantinos M. Kasiotis, Kyriaki Machera, and Photini V. Mylona. 2021. "Mediterranean White Lupin Landraces as a Valuable Genetic Reserve for Breeding" Plants 10, no. 11: 2403. https://doi.org/10.3390/plants10112403
APA StyleZafeiriou, I., Polidoros, A. N., Baira, E., Kasiotis, K. M., Machera, K., & Mylona, P. V. (2021). Mediterranean White Lupin Landraces as a Valuable Genetic Reserve for Breeding. Plants, 10(11), 2403. https://doi.org/10.3390/plants10112403









