Bioresource Nutrient Recycling in the Rice–Wheat Cropping System: Cornerstone of Organic Agriculture
Abstract
1. Introduction
2. Results
2.1. Nutrient Concentration in Farmyard Manure Samples
2.2. Nutrient Concentration in Sesbania Samples
2.3. Elemental Concentration in Rice Residue Samples
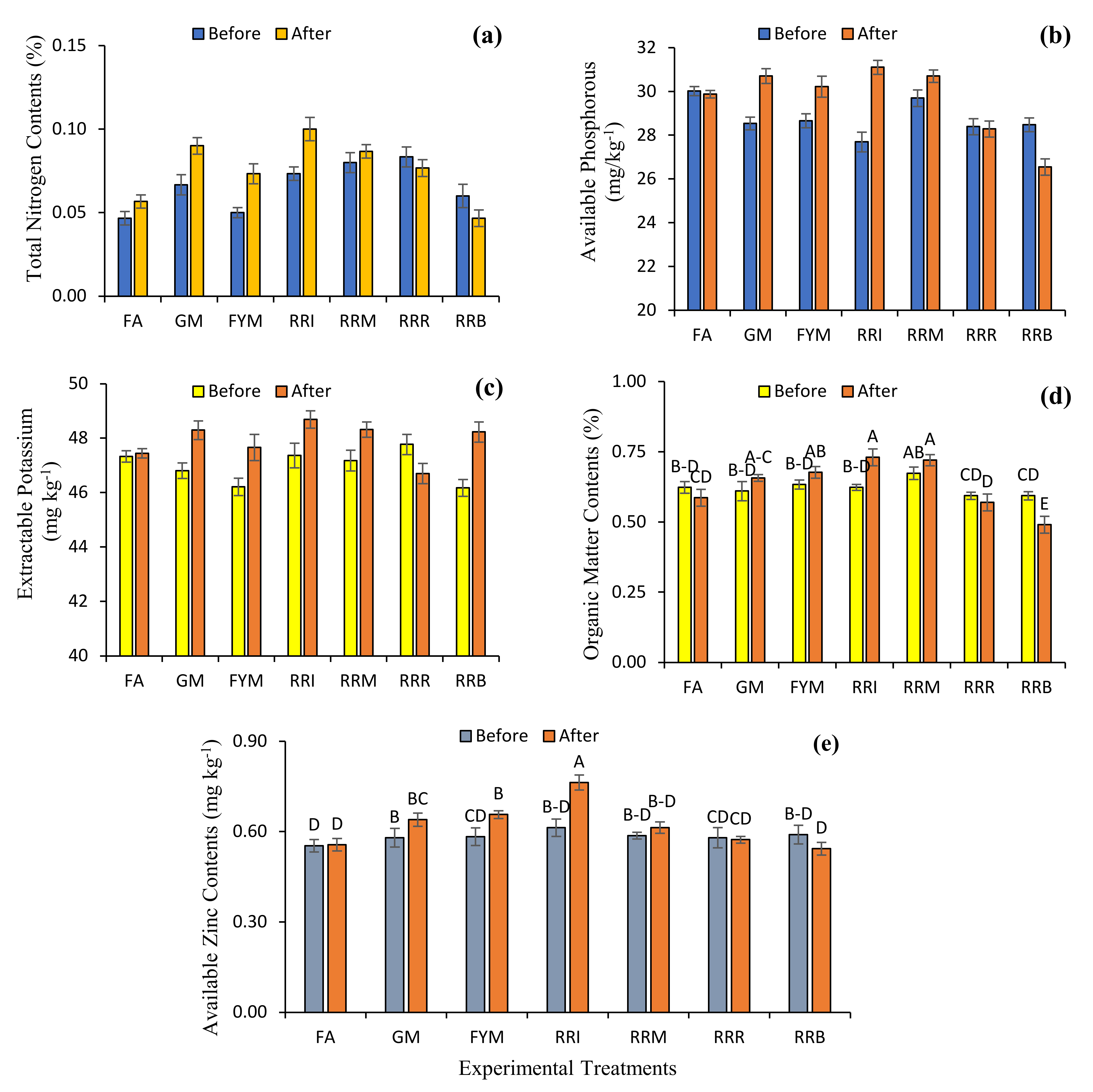
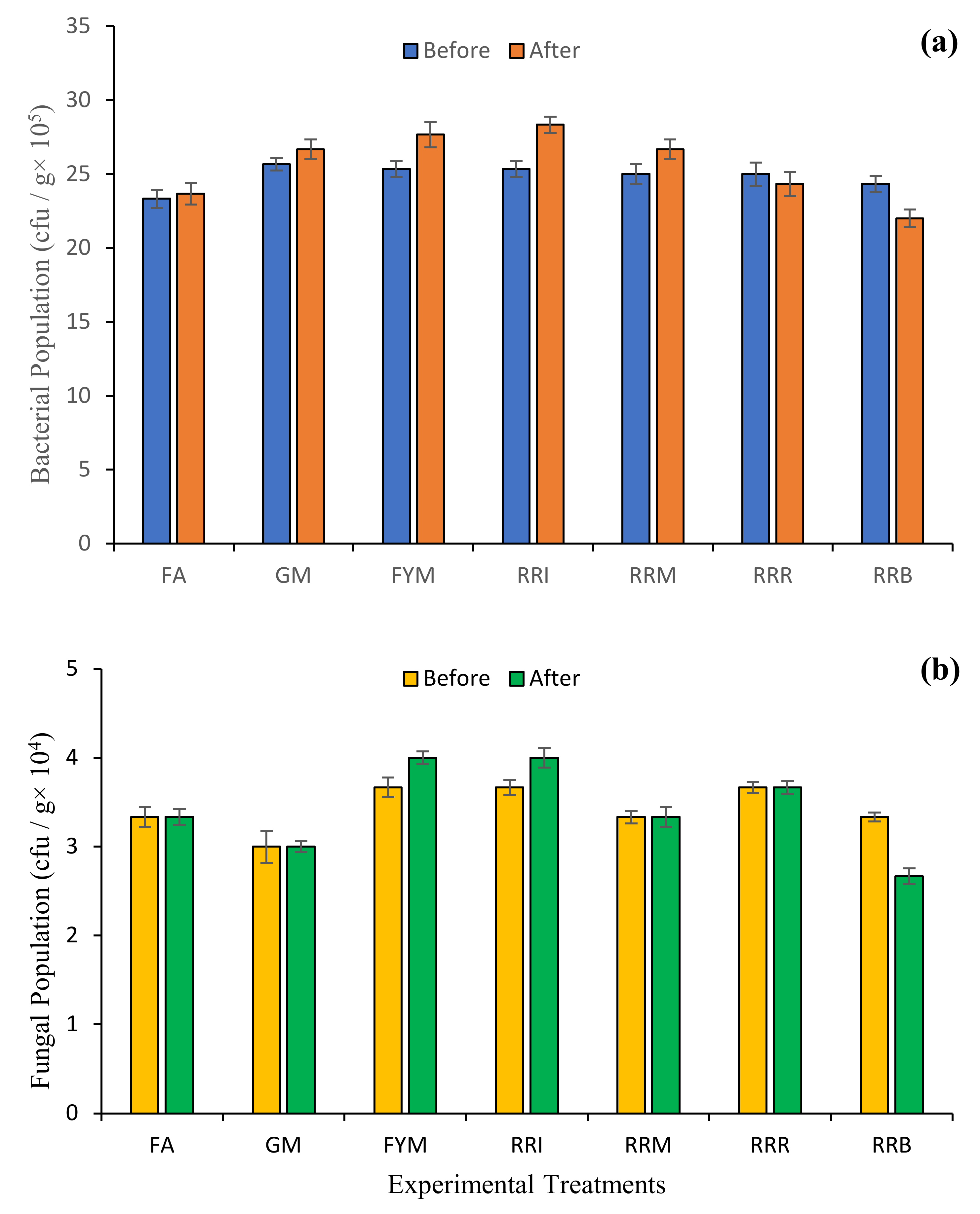
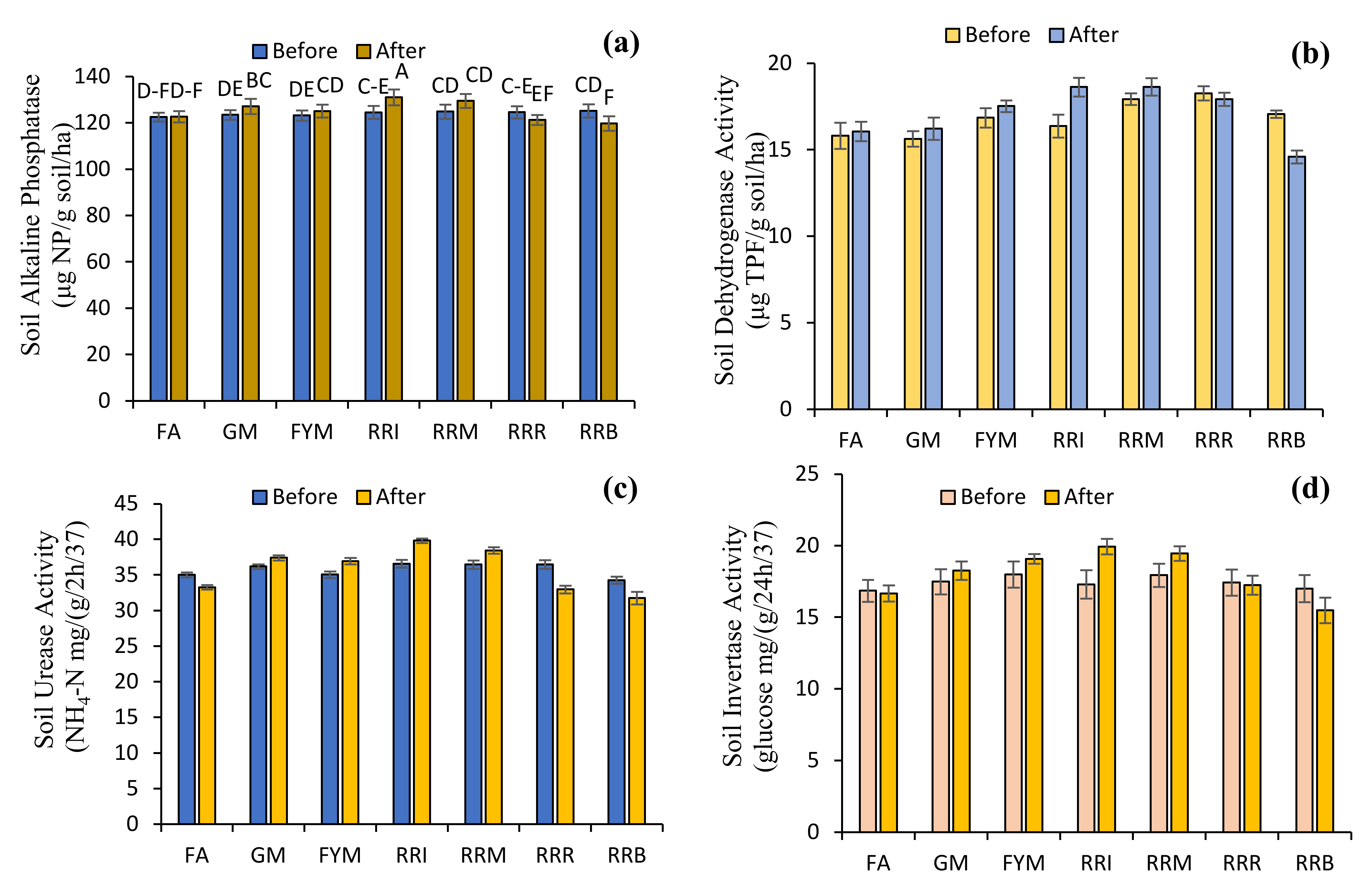
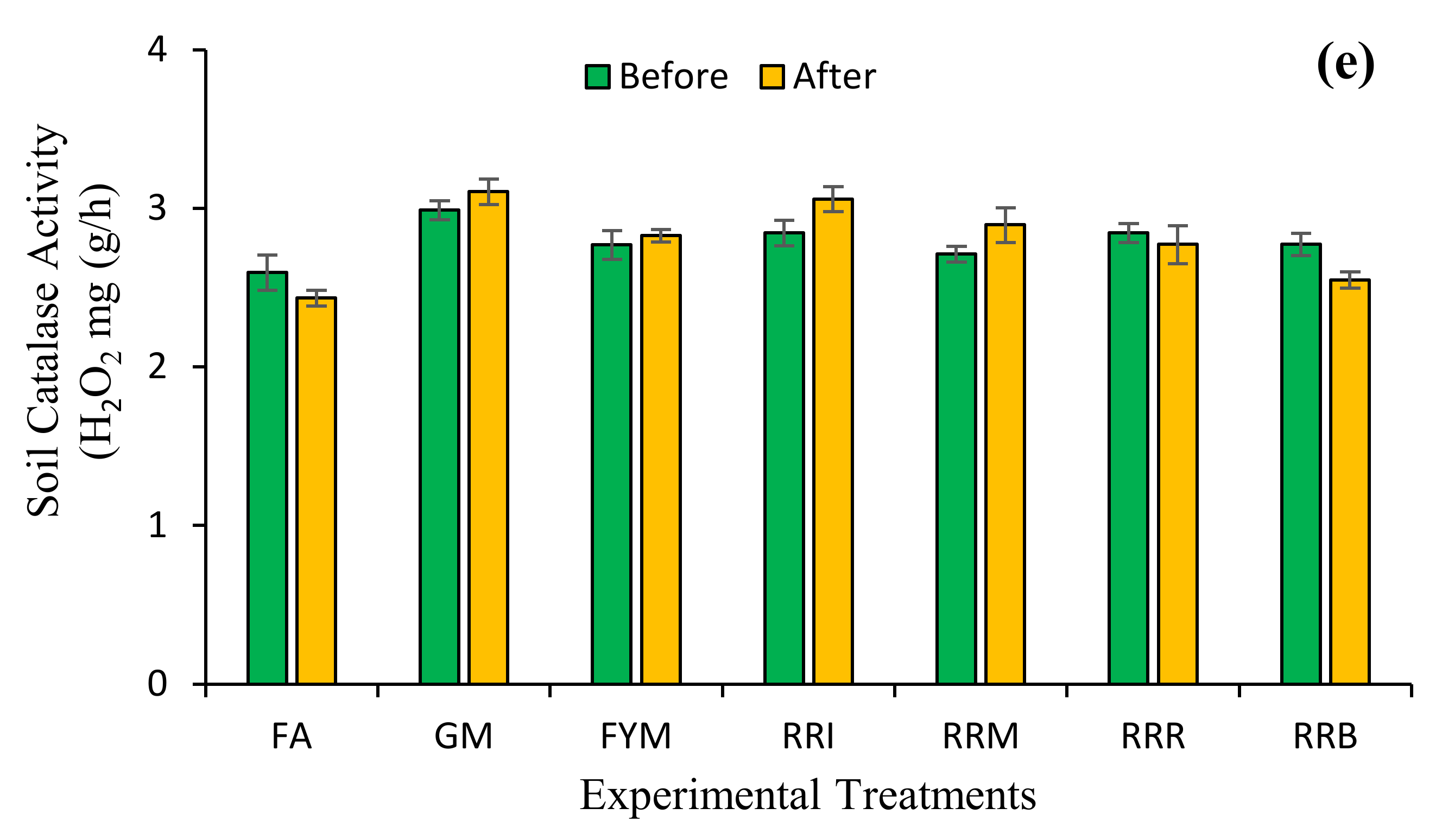
2.4. Diverse Farming Approaches and Soil Health
3. Discussion
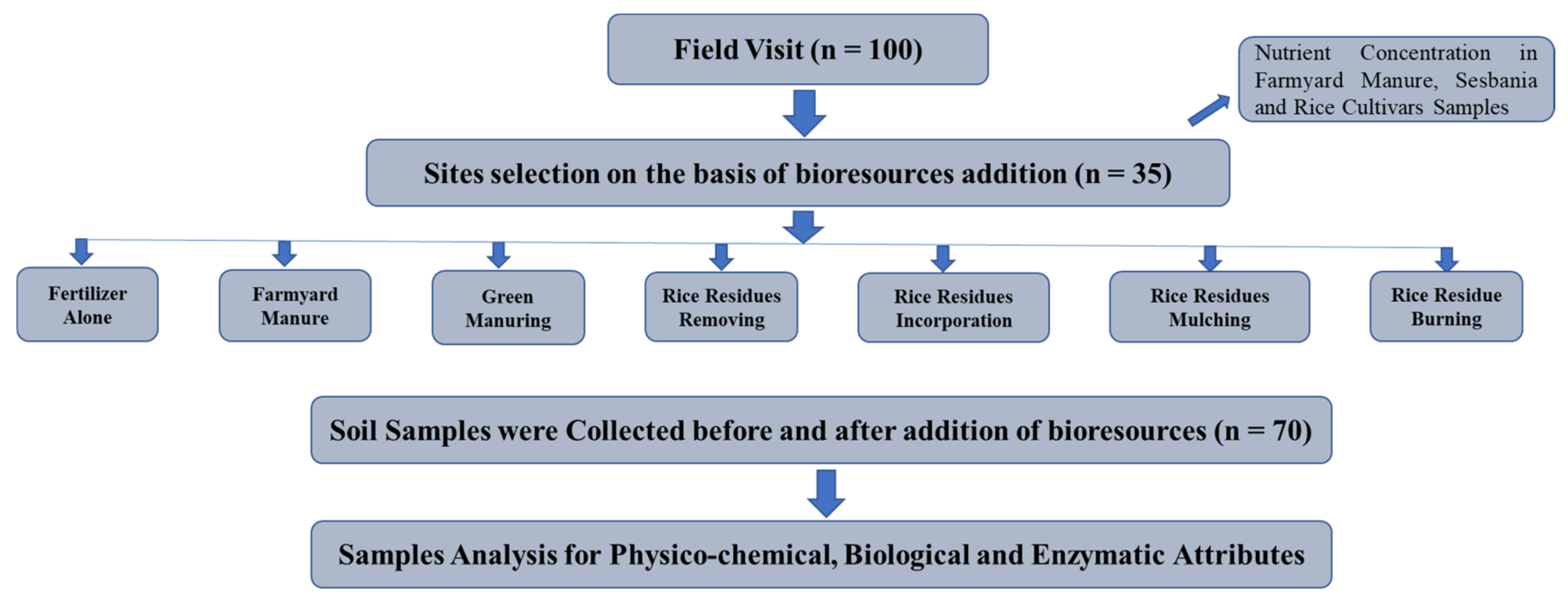
4. Materials and Methods
4.1. Experimental Site
4.2. Experimental Design and Treatments
4.3. Elemental Analysis of Rice Residues, Sesbania and Farmyard Manure
4.4. Measurements of Physicochemical and Biological Properties in Soil
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mairura, F.S.; Mugendi, D.N.; Mwanje, J.I. Integrating scientific and farmers’ evaluation of soil quality indicators in Central Kenya. Geoderma 2007, 139, 134–143. [Google Scholar] [CrossRef]
- Srinivasarao, C.H.; Venkateswarlu, B.; Lal, R.; Singh, A.K.; Sumanta, K. Sustainable management of soils of dryland ecosystems for enhancing agronomic productivity and sequestering carbon. Adv. Agron. 2013, 121, 253–329. [Google Scholar]
- Zaman, S.; Pramanick, P.; Mitra, A. Chemical Fertilizer; Department of Marine Science, University of Calcutta: Kolkata, India, 2019. [Google Scholar]
- Karlen, D.L. Soil health: The concept, its role, and strategies for monitoring. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Behan-Pelletier, V., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 331–336. [Google Scholar]
- Natural Resources Conservation Services (NRCS). Soil Health. 2012. Available online: http://www.nrcs.usda.Gov/wps/portal/nrcs/main/soils/health/ (accessed on 20 January 2019).
- Jat, S.L.; Parihar, C.M.; Singh, A.K.; Nayak, H.S.; Meena, B.R.; Kumar, B.; Parihar, M.D.; Jat, M.L. Differential Response from Nitrogen Sources with and Without Residue Management under Conservation Agriculture on Crop Yields, Water-Use and Economics in Maize-Based Rotations. Field Crops Res. 2019, 236, 96–110. [Google Scholar] [CrossRef]
- Guerrieri, M.C.; Fanfoni, E.; Fiorini, A.; Trevisan, M.; Puglisi, E. Isolation and screening of extracellular PGPR from the rhizosphere of tomato plants after long-term reduced tillage and cover crops. Plants. 2020, 9, 668. [Google Scholar] [CrossRef]
- Sanchez, P.A. Soil fertility and hunger in Africa. Science 2002, 295, 2019–2020. [Google Scholar] [CrossRef]
- Meena, R.S.; Lal, R. Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 122–132. [Google Scholar]
- Shukla, M.K.; Lal, R.; Ebinger, M. Determining soil quality indicators by factor analysis. Soil Till. Res. 2006, 87, 194–204. [Google Scholar] [CrossRef]
- Riaz, U.; Mehdi, S.M.; Iqbal, S.; Khalid, H.I.; Qadir, A.A.; Anum, W.; Ahmad, M.; Murtaza, G. Bio-fertilizers: Eco-friendly aproach for plant and soil environment. In Bioremediation and Biotechnology; Hakeem, K., Bhat, R., Qadri, H., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020. [Google Scholar]
- Ceja-Navarro, J.A.; Rivera-Orduna, F.N.; Patiño-Zúñiga, L.; Vila-Sanjurjo, A.; Crossa, J.; Govaerts, B.; Dendooven, L. Phylogenetic and multivariate analyses to determine the effects of different tillage and residue management practices on soil bacterial communities. App. Environ. Microbiol. 2010, 76, 3685–3691. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, P.; He, L.; Gao, X.; Li, W.; Zhou, Y.; Yang, L. Effects of tillage managements and maize straw returning on soil microbiome using 16S rDNA sequencing. J. Integ. Plant. Biol. 2019, 61, 765–777. [Google Scholar] [CrossRef]
- Lu, X.A. Meta-Analysis of the Effects of Crop Residue Return on Crop Yields and Water Use Efficiency. PLoS ONE 2020, 15, e0231740. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Poll. Bioavail. 2021, 33, 164–173. [Google Scholar]
- Lal, R. World Crop Residues Production and Implications of Its Use as a Biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef]
- Rathod, P.H.; Bhoyar, S.M.; Katkar, R.N.; Kadu, P.R.; Jadhao, S.D.; Konde, N.M.; Deshmukh, P.W.; Patle, P.N. Recycling and Management of Crop Residues for Sustainable Soil Health in Climate Change Scenario with Farmer’s Profit as Frontline Moto. J. Pharmaco. Phytochem. 2019, 45, 51–55. [Google Scholar]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Global Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, B.Y.; Liu, S.L.; Qi, J.; Wang, X.; Pu, C.; Li, S.; Zhang, X.; Yang, X.; Lal, R. Sustaining Crop Production in China’s Cropland by Crop Residue Retention: A Meta-Analysis. Land Degrad. Develop. 2020, 31, 694–709. [Google Scholar] [CrossRef]
- Efthimiadou, A.; Bilalis, D.; Karkanis, A.; Froud-Williams, B. Combined Organic/Inorganic Fertilization Enhances Soil Quality and Increased Yield, Photosynthesis and Sustainability of Sweet Maize Crop. Aust. J. Crop. Sci. 2010, 4, 722–729. [Google Scholar]
- Dejene, M.; Lemlem, M. Integrated Agronomic Crop Managements to Improve Tef Productivity under Terminal Drought. In Water Stress; Md, I., Rahman, M., Hasegawa, H., Eds.; InTech Open Science: London, UK, 2012; pp. 235–254. [Google Scholar]
- Kumar, S.; Nakajima, T.; Mbonimpa, E.G.; Gautam, S.; Somireddy, U.R.; Kadono, A.; Fausey, N. Long-term tillage and drainage influences on soil organic carbon dynamics, aggregate stability and corn yield. Soil Sci. Plant. Nut. 2014, 60, 108–118. [Google Scholar] [CrossRef]
- Chimouriya, S.; Lamichhane, J.; Gauchan, D.P. Green manure for restoring and improving the soil nutrients quality. Int. J. Res. 2018, 5, 1064–1074. [Google Scholar]
- Melero, S.; Riuz Porrai, J.C.; Herencia, J.F.; Madejon, E. Chemical and biochemical properties in a sylty loam soil under conventional and organic management. Soil Till. Res. 2006, 90, 162–170. [Google Scholar] [CrossRef]
- Talgre, L.; Lauringson, E.; Roostalu, H.; Astover, A.; Makke, A. Green manure as a nutrient source for succeeding crops. Plant Soil Environ. 2012, 58, 275–281. [Google Scholar] [CrossRef]
- Yang, L.; Bai, J.; Liu, J.; Zeng, N.; Cao, W. Green manuring effect on changes of soil nitrogen fractions, maize growth, and nutrient uptake. Agronomy 2018, 8, 261. [Google Scholar] [CrossRef]
- Yadav, G.S.; Lal, R.; Meena, R.S.; Babu, S.; Das, A.; Bhowmik, S.N.; Saha, P. Conservation tillage and nutrient management effects on productivity and soil carbon sequestration under double cropping of rice in north eastern region of India. Ecol. Ind. 2019, 105, 303–315. [Google Scholar] [CrossRef]
- Van Hung, N.; Maguyon-Detras, M.C.; Migo, M.V.; Quilloy, R.; Balingbing, C.; Chivenge, P.; Gummert, M. Rice straw overview: Availability, properties, and management practices. Sust. Rice Straw Manag. 2020, 1, 23–32. [Google Scholar]
- Gummert, M.; Hung, N.V.; Chivenge, P.; Douthwaite, B. Sustainable Rice Straw Management; Springer Nature: Berlin/Heidelberg, Germany, 2020; p. 192. [Google Scholar]
- Rout, K.K.; Sahoo, S.; Mukhi, S.K.; Mohanty, G.P. Assessment of quality of different organic manures used by the farmers of Khurda district in Orissa and their effect on microbial activity of an acid soil. J. Indian Soc. Soil Sci. 2012, 60, 30–37. [Google Scholar]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995; pp. 15–25. [Google Scholar]
- Kolahchi, Z.; Jalali, M. Kinetics of nutrient release from different organic residues using a laboratory system. Arch. Agron. Soil Sci. 2012, 58, 1013–1031. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Leffelaar, P.A.; Shen, J.; Zhang, F. Characterization of phosphorus in animal manures collected from three (dairy, swine, and broiler) farms in China. PLoS ONE 2014, 9, e102698. [Google Scholar] [CrossRef]
- Latt, Y.K.; Myint, A.K.; Yamakawa, T.; Ogata, K. The effects of green manure (Sesbania rostrata) on the growth and yield of rice. J. Faculty Agri. Kyushu Uni. 2009, 54, 313–319. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhao, B. Arbuscular mycorrhizal fungi mediated uptake of nutrient elements by Chinese milk vetch (Astragalus sinicus L.) grown in lanthanum spiked soil. Biol. Fert. Soil. 2009, 45, 675–678. [Google Scholar] [CrossRef]
- Lee, C.H.; Do Park, K.; Jung, K.Y.; Ali, M.A.; Lee, D.; Gutierrez, J.; Kim, P.J. Effect of Chinese milk vetch (Astragalus sinicus L.) as a green manure on rice productivity and methane emission in paddy soil. Agri. Ecosys. Environ. 2010, 138, 343–347. [Google Scholar] [CrossRef]
- Lu, H.J.; Ye, Z.Q.; Zhang, X.L.; Lin, X.Y.; Ni, W.Z. Growth and yield responses of crops and macronutrient balance influenced by commercial organic manure used as a partial substitute for chemical fertilizers in an intensive vegetable cropping system. Phys. Chem Earth Parts A/B/C 2011, 36, 387–394. [Google Scholar] [CrossRef]
- Ladha, J.K.; Khind, C.S.; Khera, T.S.; Bueno, C.S. Effects of residue decomposition on productivity and soil fertility in rice–wheat rotation. Soil Sci. Am. J. 2004, 68, 854–864. [Google Scholar]
- Sarnklong, C.; Cone, J.W.; Pellikaan, W.; Hendriks, W.H. Utilization of rice straw and different treatments to improve its feed value for ruminants: A review. Asian-Australas. J. Anim. Sci. 2010, 23, 680–692. [Google Scholar] [CrossRef]
- Topno, S.E. Environmental performance and energy recoverable from stored rice straw bales in humid climate. Ph.D. Thesis, University of the Philippines Los Baños, Los Baños, Philippines, 2015. Unpublished. [Google Scholar]
- Jenkins, B.M.; Bakker, R.R.; Wei, J.B. On the properties of washed straw. Biomass Bioenergy 1996, 10, 177–200. [Google Scholar] [CrossRef]
- Barmina, I.; Lickrastina, A.; Valdmanis, R.; Zake, M.; Arshanitsa, A.; Solodovnik, V.; Telysheva, G. Effects of biomass composition variations on gasification and combustion characteristics. Eng. Rural Dev. Jelgava 2013, 5, 23–24. [Google Scholar]
- Li, Y.; Li, Z.; Cui, S.; Jagadamma, S.; Zhang, Q. Residue retention and minimum tillage improve physical environment of the soil in croplands: A global meta-analysis. Soil Till. Res. 2019, 194, 104292. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental factors affecting the mineralization of crop residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Haruna, S.I.; Anderson, S.H.; Udawatta, R.P.; Gantzer, C.J.; Phillips, N.C.; Cui, S.; Gao, Y. Improving soil physical properties through the use of cover crops: A review. Agrosys. Geosci. Environ. 2020, 3, e20105. [Google Scholar] [CrossRef]
- Alskaf, K.; Mooney, S.J.; Sparkes, D.L.; Wilson, P.; Sjögersten, S. Short-term impacts of different tillage practices and plant residue retention on soil physical properties and greenhouse gas emissions. Soil Till. Res. 2021, 206, 104803. [Google Scholar] [CrossRef]
- Jin, Z.; Shah, T.; Zhang, L.; Liu, H.; Peng, S.; Nie, L. Effect of straw returning on soil organic carbon in rice–wheat rotation system: A review. Food Energy Sec. 2020, 9, e200. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Zhao, X.; Xue, J.; Xu, S.; Du, Q. Simulation of Soil Water Evaporation during Freeze–Thaw Periods under Different Straw Mulch Thickness Conditions. Water 2020, 12, 2003. [Google Scholar] [CrossRef]
- Naik, M.A.; Darthiya, M. Crop Residue Management in Multiple Cropping Systems. In Research Trends in Agriculture Science; Naresh, R., Ed.; AkiNik Publications: Delhi, India, 2020; Volume 91, p. 65. [Google Scholar]
- Li, Y.; Li, Z.; Cui, S.; Zhang, Q. Trade-off between soil pH, bulk density and other soil physical properties under global no-tillage agriculture. Geoderma 2020, 361, 114099. [Google Scholar] [CrossRef]
- Brown, J.L.; Stobart, R.; Hallett, P.D.; Morris, N.L.; George, T.S.; Newton, A.C.; McKenzie, B.M. Variable impacts of reduced and zero tillage on soil carbon storage across 4–10 years of UK field experiments. J. Soils Sediment. 2021, 21, 890–904. [Google Scholar] [CrossRef]
- Suryavanshi, T.; Sharma, A.R.; Nandeha, K.L.; Lal, S.; Porte, S.S. Effect of tillage, residue and weed management on soil properties, and crop productivity in greengram (Vigna radiata L.) under conservation agriculture. J. Pharma Phytochem. 2018, 7, 702–712. [Google Scholar]
- Alam, M.K.; Bell, R.W.; Haque, M.E.; Kader, M.A. Minimal soil disturbance and increased residue retention increase soil carbon in rice-based cropping systems on the Eastern Gangetic Plain. Soil Tillage Res. 2018, 183, 28–41. [Google Scholar] [CrossRef]
- Bisen, N.; Rahangdale, C.P. Crop residues management option for sustainable soil health in rice-wheat system: A review. Int. J. Chem. Stud. 2017, 5, 1038–1042. [Google Scholar]
- Naresh, R.K.; Jat, P.C.; Kumar, V.; Singh, S.P.; Kumar, Y. Carbon and nitrogen dynamics, carbon sequestration and energy saving in soils under different tillage, stubble mulching and fertilizer management in rice–wheat cropping system. J. Pharmaco. Phytochem. 2018, 7, 723–740. [Google Scholar]
- Bandyopadhyay, K.K.; Misra, A.K.; Ghosh, P.K.; Hati, K.M. Effect of integrated use of farmyard manure and chemical fertilizers on soil physical properties and productivity of soybean. Soil Tillage Res. 2010, 110, 115–125. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, B.C.; Singh, M.; Gupta, M.; Sharma, R. Crop residue management for productivity enhancement and sustainability under various cropping systems–A Review. J. Soil Water Conser. 2018, 17, 83–91. [Google Scholar] [CrossRef]
- Malik, S.S.; Chauhan, R.C.; Laura, J.S.; Tanvi, K.; Raashee, A.; Natasha, S. Influence of organic and synthetic fertilizers on soil physical properties. Int. J. Current Microbiol. App. Sci. 2014, 3, 802–810. [Google Scholar]
- Bhatt, M.; Singh, A.P.; Singh, V.; Kala, D.C.; Kumar, V. Long-term effect of organic and inorganic fertilizers on soil physico-chemical properties of a silty clay loam soil under rice-wheat cropping system in Tarai region of Uttarakhand. J. Pharmaco. Phytochem. 2019, 8, 2113–2118. [Google Scholar]
- Shirani, H.; Hajabbasi, M.A.; Afyuni, M.; Hemmat, A. Effects of farmyard manure and tillage systems on soil physical properties and corn yield in central Iran. Soil Till. Res. 2002, 68, 101–108. [Google Scholar] [CrossRef]
- Vanzolini, J.I.; Galantini, J.A.; Martínez, J.M.; Suñer, L. Changes in soil pH and phosphorus availability during decomposition of cover crop residues. Arch. Agron. Soil Sci. 2017, 63, 1864–1874. [Google Scholar] [CrossRef]
- Goswami, S.B.; Mondal, R.; Mandi, S.K. Crop residue management options in rice–rice system: A review. Arch. Agron. Soil Sci. 2020, 66, 1218–1234. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, S.K. Ecofriendly Management of Paddy Crop Residues for Sustainable Environment and Development. Bio Sci. Res. Bull. 2018, 34, 59–72. [Google Scholar] [CrossRef]
- Kumar, A.; Kushwaha, K.K.; Singh, S.; Shivay, Y.S.; Meena, M.C.; Nain, L. Effect of paddy straw burning on soil microbial dynamics in sandy loam soil of Indo-Gangetic plains. Environ. Technol. Innov. 2019, 16, 100469. [Google Scholar] [CrossRef]
- Reddy, C.V.; Kashyap, L.; Alok, T. Effect of 14-year long term fertilizer management on soil organic carbon stock, carbon sequestration rate and nutrient balances in Vertisols. Int. J. Current Microbiol. App. Sci. 2017, 6, 895–902. [Google Scholar] [CrossRef][Green Version]
- Ayoola, O.T. Effects of fertilizer treatments on soil chemical properties and crop yields in a cassava-based cropping system. J. App. Sci. Res. 2006, 2, 1112–1116. [Google Scholar]
- Ayyam, V.; Palanivel, S.; Chandrakasan, S. Conservation Agriculture for Rehabilitation of Agro-ecosystems. In Coastal Ecosystems of the Tropics-Adaptive Management; Springer: Singapore, 2019; pp. 407–437. [Google Scholar]
- Karami, A.; Homaee, M.; Afzalinia, S.; Ruhipour, H.; Basirat, S. Organic resource management: Impacts on soil aggregate stability and other soil physico-chemical properties. Agri. Ecosys. Environ. 2012, 148, 22–28. [Google Scholar] [CrossRef]
- Ye, R.; Doane, T.A.; Morris, J.; Horwath, W.R. The effect of rice straw on the priming of soil organic matter and methane production in peat soils. Soil Biol. Biochem. 2015, 81, 98–107. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fert. Soil. 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Schmatz, R.; Recous, S.; Aita, C.; Tahir, M.M.; Schu, A.L.; Chaves, B.; Giacomini, S.J. Crop residue quality and soil type influence the priming effect but not the fate of crop residue. Plant. Soil. 2017, 229–245. [Google Scholar] [CrossRef]
- Kumar, V.; Prasad, R.K. Integrated effect of mineral fertilizers and green manure on crop yield and nutrient availability under rice-wheat cropping system in Calciorthents. J. Indian Soc. Soil Sci. 2008, 56, 209–214. [Google Scholar]
- Pandey, D.K.; Pandey, R.; Mishra, R.P.; Kumar, S.; Kumar, N. Collection of Dhaincha (Sesbania spp.) Variability in Uttar Pradesh, biodiversity and Agriculture (Souvenir); Uttar Pradesh State Biodiversity Board Lucknow: Lucknow, India, 2008; pp. 48–51. Available online: https://upsbdb.org/ (accessed on 16 October 2021).
- Ravindra, K.; Seweta, S.; Manisha, S.; Asha, S. Effect of organic amendments on soil mycoflora. Asian J. Plant. Pathol. 2010, 4, 73–81. [Google Scholar]
- Dhakal, Y.; Meena, R.S.; Kumar, S. Effect of INM on nodulation, yield, quality and available nutrient status in soil after harvest of greengram. Legume Res. Int. J. 2016, 39, 590–594. [Google Scholar] [CrossRef]
- Müjdeci, M.; Demircioğlu, A.C.; Alaboz, P. The effects of farmyard manure and green manure applications on some soil physical properties. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Derg. 2020, 30, 9–17. [Google Scholar] [CrossRef][Green Version]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; Ksenofontova, N.; Zinyakova, N.B.; van Bruggen, A.H. Does fresh farmyard manure introduce surviving microbes into soil or activate soil-borne microbiota? J. Environ. Manag. 2021, 294, 113018. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol. Biochem. 2010, 42, 516–520. [Google Scholar] [CrossRef]
- Seymour, N. Impacts of pesticides and fertilizers on soil biota. Ph.D. Thesis, University of Southern Queensland, Darling Heights, Australia, 2002. [Google Scholar]
- Cheng, F.; Peng, X.; Zhao, P.; Yuan, J.; Zhong, C.; Cheng, Y.; Zhang, S. Soil microbial biomass, basal respiration and enzyme activity of main forest types in the Qinling Mountains. PLoS ONE 2013, 8, e67353. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef]
- Turmel, M.S.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaerts, B. Crop residue management and soil health: A systems analysis. Agricult. Sys. 2015, 134, 6–16. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Certini, G.; Moya, D.; Lucas-Borja, M.E.; Mastrolonardo, G. The impact of fire on soil-dwelling biota: A review. Forest Ecol. Manag. 2021, 488, 118989. [Google Scholar] [CrossRef]
- Jamali, M.; Bakhshandeh, E.; Yaghoubi Khanghahi, M.; Crecchio, C. Metadata Analysis to Evaluate Environmental Impacts of Wheat Residues Burning on Soil Quality in Developing and Developed Countries. Sustainability 2021, 13, 6356. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, Y.; Weng, Y.; Huang, K.; Wang, J.; Zhao, J.; Wu, L. Effects of burning harvested residues on the archaeal and bacterial communities of Eucalyptus urophylla substituting native vegetation. App. Soil Ecol. 2021, 158, 103796. [Google Scholar] [CrossRef]
- Goyal, S.; Mishra, M.M.; Dhankar, S.S.; Kapoor, K.K.; Batra, R. Microbial biomass turnover and enzyme activities following the application of farmyard manure to field soils with and without previous long-term applications. Biol. Fert. Soil. 1993, 15, 60–64. [Google Scholar] [CrossRef]
- Kirchner, M.J.; Wollum, A.G.; King, L.D. Soil microbial populations and activities in reduced chemical input agroecosystems. Soil Sci. Soc. Am. J. 1993, 57, 1289–1295. [Google Scholar] [CrossRef]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Mikhailouskaya, N.; Bogdevitch, I. Relations of enzyme activities with different fractions of soil organic matter. Polish J. Soil Sci. 2009, 42, 175–182. [Google Scholar]
- Ma, Q.; Wen, Y.; Ma, J.; Macdonald, A.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Long-term farmyard manure application affects soil organic phosphorus cycling: A combined metagenomic and 33P/14C labelling study. Soil Biol. Biochem. 2020, 149, 107959. [Google Scholar] [CrossRef]
- Song, D.; Chen, L.; Zhang, S.; Zheng, Q.; Ullah, S.; Zhou, W.; Wang, X. Combined biochar and nitrogen fertilizer change soil enzyme and microbial activities in a 2-year field trial. Eur. J. Soil Biol. 2020, 99, 103212. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef] [PubMed]
- Kotroczó, Z.; Juhos, K.; Biró, B.; Kocsis, T.; Pabar, S.A.; Varga, C.; Fekete, I. Effect of detritus manipulation on different organic matter decompositions in temperate deciduous forest soils. Forests 2020, 11, 675. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, P.; Kumar, S. Responses of Soil Carbon Pools, Enzymatic Activity, and Crop Yields to Nitrogen and Straw Incorporation in a Rice-Wheat Cropping System in North-Western India. Front. Sustain. Food Syst. 2020, 4, 203. [Google Scholar] [CrossRef]
- Foster, E.J.; Baas, P.; Wallenstein, M.D.; Cotrufo, M.F. Precision biochar and inoculum applications shift bacterial community structure and increase specific nutrient availability and maize yield. App. Soil Ecol. 2020, 151, 103541. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar]
- Chen, X.; Condron, L.M.; Dunfield, K.E.; Wakelin, S.A.; Chen, L. Impact of grassland afforestation with contrasting tree species on soil phosphorus fractions and alkaline phosphatase gene communities. Soil Biol. Biochem. 2021, 159, 108274. [Google Scholar] [CrossRef]
- Criquet, S.; Ferre, E.; Farnet, A.M.; Le petit, J. Annual dynamics of phosphatase activities in an evergreen oak litter: Influence of biotic and abiotic factors. Soil Biol. Biochem. 2004, 36, 1111–1118. [Google Scholar] [CrossRef]
- Norton, G.J.; Shafaei, M.; Travis, A.J.; Deacon, C.M.; Danku, J.; Pond, D.; Price, A.H. Impact of alternate wetting and drying on rice physiology, grain production, and grain quality. Field Crops Res. 2017, 205, 1–13. [Google Scholar] [CrossRef]
- Modi, C.D.; Smith, H.W.; Mccreer, A. Laboratory Manual for Soil Fertility; Department of Agronomy, State College of Washington: Pullman, WA, USA, 1957; pp. 1–75. [Google Scholar]
- Abbas, F.; Zahra, S.I.; Ibrahim, M.; Akram, B.; Ishaq, W.; Hammad, H.M.; Salik, M.R. Carbon sequestration potential of soils under maize production in irrigated agriculture of the Punjab province of Pakistan. J. Anim. Plant. Sci. 2016, 26, 706–715. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt: New Delhi, India, 1967; pp. 205–226. [Google Scholar]
- Gammon, J.R.N. Determination of total potassium and sodium in sandy soils by flame photometer. Soil Sci. 1951, 71, 211–214. [Google Scholar] [CrossRef]
- Konen, M.E.; Jacobs, P.M.; Burras, C.L.; Talaga, B.J.; Mason, J.A. Equations for predicting soil organic carbon using loss-on-ignition for north central US soils. Soil Sci. Soc. Am. J. 2002, 66, 1878–1881. [Google Scholar] [CrossRef]
- Navarro, A.F.; Cegarra, J.; Roig, A.; Garcia, D. Relationships between organic matter and carbon contents of organic wastes. Biores. Technol. 1993, 44, 203–207. [Google Scholar] [CrossRef]
- Min, H.; Ye, Y.F.; Chen, Z.Y.; Wu, W.X.; Yufeng, D. Effects of butachlor on microbial populations and enzyme activities in paddy soil. J. Environ. Sci. Health Part B 2001, 36, 581–595. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Dick, R.P.; Burns, R.G. A brief history of soil enzymology research. Meth. Soil Enzymol. 2011, 9, 1–34. [Google Scholar]
- Frankeberger, W.T.; Johanson, J.B. Method of measuring invertase activity in soils. Plant Soil. 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Johnson, K.W. Implications of adopting soil health practices on the economic performance of Minnesota row crop operations. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2021. [Google Scholar]
- Janssen, P.H.; Yates, P.S.; Grinton, B.E.; Taylor, P.M.; Sait, M. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. App. Environ. Microbiol. 2002, 68, 2391–2396. [Google Scholar] [CrossRef]
- Wei-xiang, W.; Qing-fu, Y.; Hang, M.; Xue-jun, D.; Wen-ming, J. Bt-transgenic rice straw affects the culturable microbiota and dehydrogenase and phosphatase activities in a flooded paddy soil. Soil Biol. Biochem. 2004, 36, 289–295. [Google Scholar] [CrossRef]
- Aslam, Z.; Yasir, M.; Jeon, C.O.; Chung, Y.R. Lysobacter oryzae sp. nov., isolated from the rhizosphere of rice (Oryza sativa L.). Int. J. System. Evol. Microbiol. 2009, 59, 675–680. [Google Scholar] [CrossRef]
- Martin, J.P. Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]





| Nutrient | Unit | Farmyard Manure | Sesbania |
|---|---|---|---|
| Nitrogen | % | 0.91 ± 0.04 | 3.32 ± 1.04 |
| Phosphorous | % | 0.36 ± 0.02 | 0.73 ± 0.09 |
| Potassium | % | 0.89 ± 0.05 | 1.32 ± 0.15 |
| Calcium | % | 0.90 ± 0.03 | 1.34 ± 0.13 |
| Magnesium | % | 0.20 ± 0.04 | 208.00 ± 3.74 |
| Sulphur | % | 0.02 ± 0.03 | 0.20 ± 0.12 |
| Sodium | % | 0.09 ± 0.03 | - |
| Zinc | mg g−1 | 56 ± 1.23 | - |
| Iron | mg g−1 | 140.30 ± 3.42 | - |
| Boron | mg g−1 | 2.30 ± 0.97 | - |
| Copper | mg g−1 | 2.80 ± 1.11 | - |
| Manganese | mg g−1 | 69.00 ± 1.32 | - |
| Treatments | pH | EC | N | P | K | OM | Zn | Bacteria | Fungi |
|---|---|---|---|---|---|---|---|---|---|
| FA | 1.85 | 11.42 | 21.43 | −0.47 | 0.25 | −5.88 | 0.60 | 1.43 | 0.00 |
| GM | 1.78 | −6.91 | 35.00 | 7.59 | 3.18 | 7.65 | 10.34 | 3.90 | 0.00 |
| FYM | 1.40 | −5.28 | 46.67 | 5.43 | 3.13 | 6.84 | 12.57 | 9.21 | 9.09 |
| RRI | −5.61 | −8.33 | 36.36 | 12.34 | 2.79 | 17.11 | 24.46 | 11.84 | 9.09 |
| RRM | −2.40 | −2.17 | 8.33 | 3.39 | 2.42 | 6.93 | 4.55 | 6.67 | 0.00 |
| RRR | −1.74 | −3.11 | −8.00 | −0.38 | −2.24 | −3.93 | −1.15 | −2.67 | 0.00 |
| RRB | −2.50 | 11.05 | −22.22 | −6.79 | 4.44 | −17.42 | −7.91 | −9.59 | −20.00 |
| Treatments | AP | DH | Urease | Invertase | Catalase |
|---|---|---|---|---|---|
| FA | 0.05 | 1.58 | −4.95 | −1.09 | −6.16 |
| GM | 2.98 | 3.80 | 3.38 | 4.44 | 3.90 |
| FYM | 1.51 | 4.00 | 5.39 | 6.00 | 2.17 |
| RRI | 5.19 | 13.76 | 8.82 | 15.22 | 7.49 |
| RRM | 3.75 | 3.96 | 5.41 | 8.44 | 6.76 |
| RRR | −2.66 | −1.84 | −9.59 | −1.09 | −2.58 |
| RRB | −4.31 | −14.51 | −7.37 | −9.00 | −8.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, S.; Zaman, Q.u.; Abbasi, A.; Komal, N.; Riaz, U.; Ashraf, K.; Ahmad, N.; Agarwal, S.; Nasir, R.; Chen, Y. Bioresource Nutrient Recycling in the Rice–Wheat Cropping System: Cornerstone of Organic Agriculture. Plants 2021, 10, 2323. https://doi.org/10.3390/plants10112323
Nazir S, Zaman Qu, Abbasi A, Komal N, Riaz U, Ashraf K, Ahmad N, Agarwal S, Nasir R, Chen Y. Bioresource Nutrient Recycling in the Rice–Wheat Cropping System: Cornerstone of Organic Agriculture. Plants. 2021; 10(11):2323. https://doi.org/10.3390/plants10112323
Chicago/Turabian StyleNazir, Saba, Qamar uz Zaman, Asim Abbasi, Nayab Komal, Umair Riaz, Kamran Ashraf, Nabeel Ahmad, Shweta Agarwal, Rabiya Nasir, and Yinglong Chen. 2021. "Bioresource Nutrient Recycling in the Rice–Wheat Cropping System: Cornerstone of Organic Agriculture" Plants 10, no. 11: 2323. https://doi.org/10.3390/plants10112323
APA StyleNazir, S., Zaman, Q. u., Abbasi, A., Komal, N., Riaz, U., Ashraf, K., Ahmad, N., Agarwal, S., Nasir, R., & Chen, Y. (2021). Bioresource Nutrient Recycling in the Rice–Wheat Cropping System: Cornerstone of Organic Agriculture. Plants, 10(11), 2323. https://doi.org/10.3390/plants10112323








