Freezing Tolerance Enhancement and Thermographic Observation of Whole Peach Trees Applied with Cellulose Nanocrystals under Realistic Spring Frost Conditions Using a Soil–Fruit–Daylit–System
Abstract
:1. Introduction
2. Results
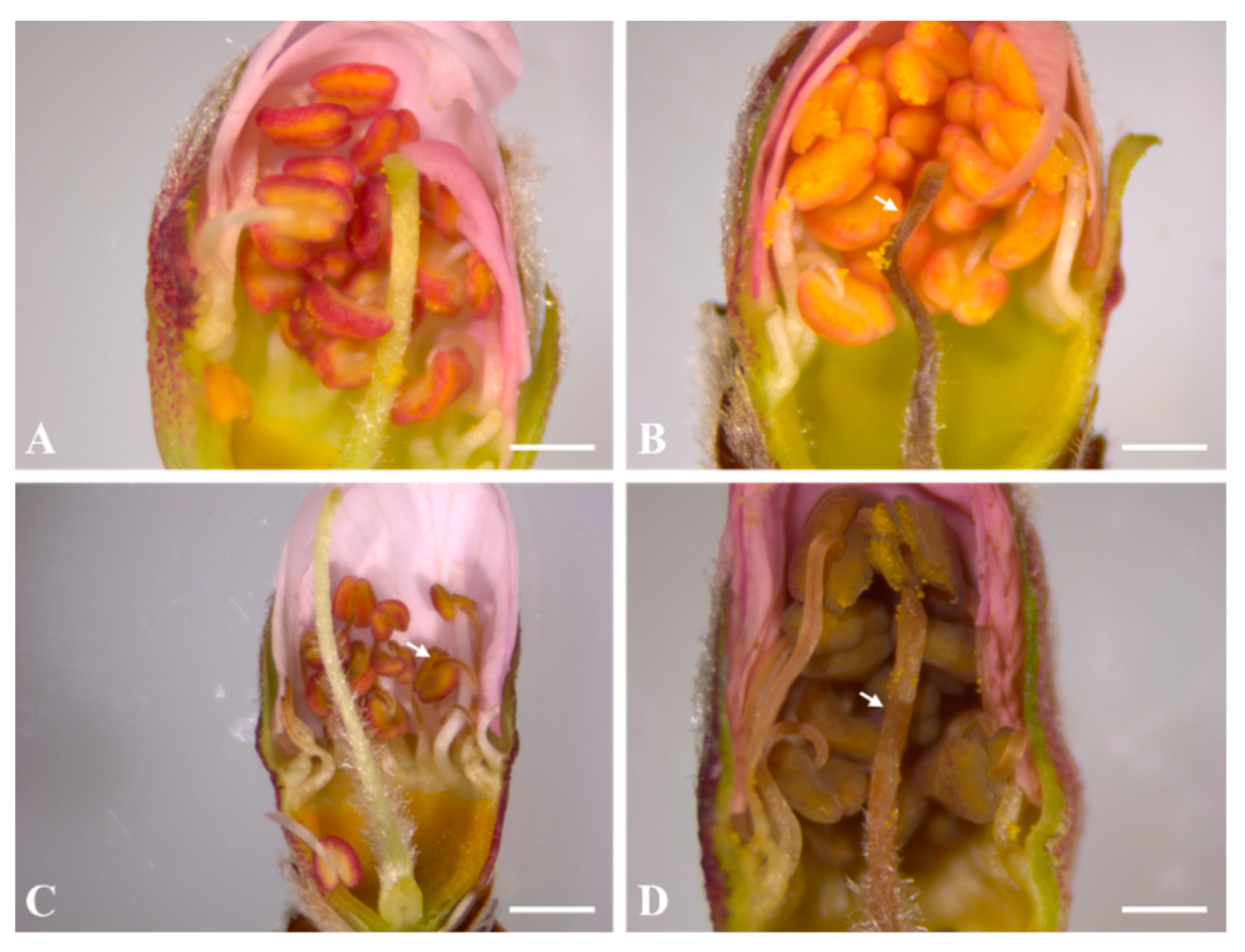
2.1. Microscopic Observation of Frost Damage to Peach Flower Buds after Treatment with 2% CNCs
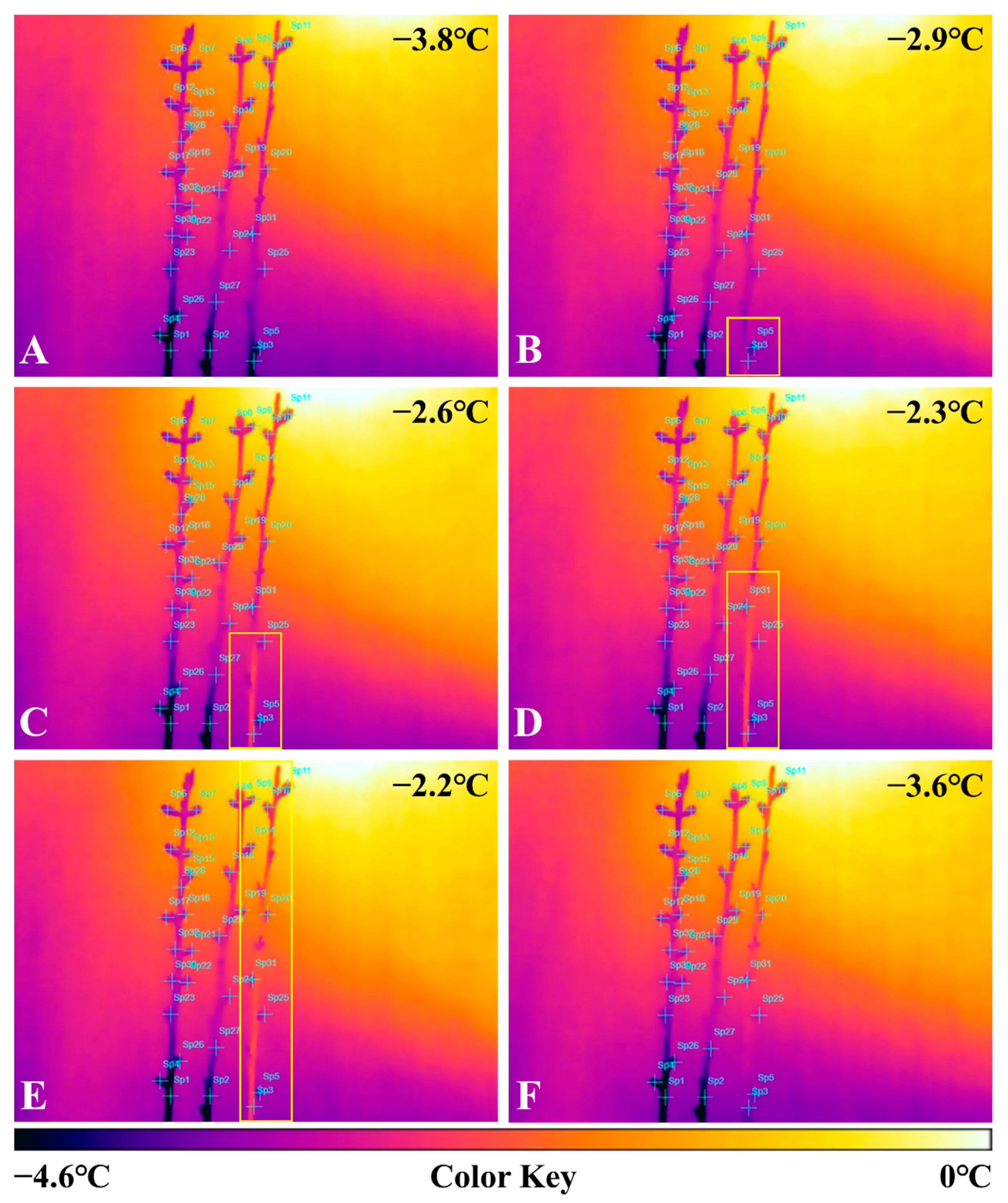
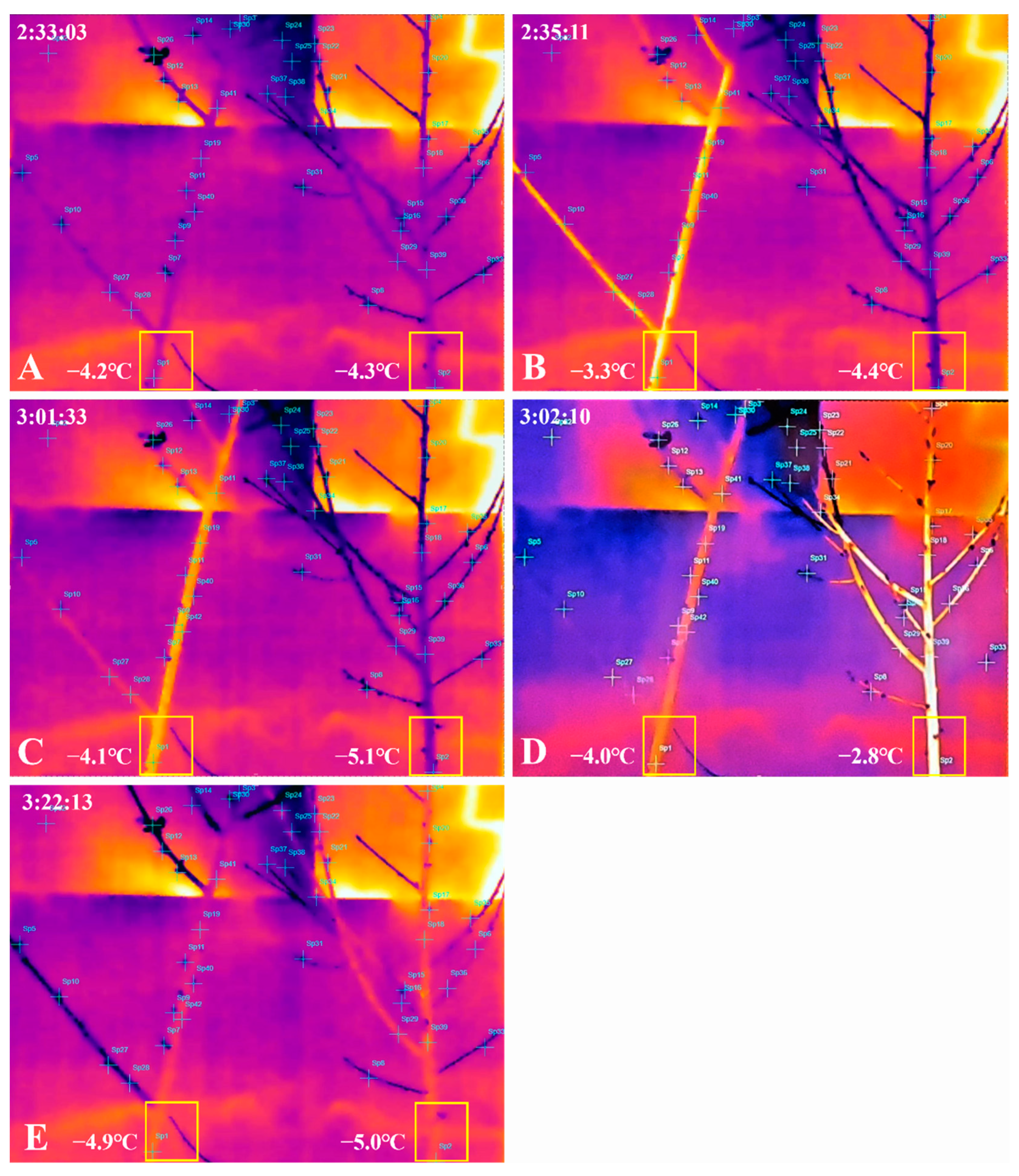
2.2. Thermal Image Analysis of Frost Damage to Peach Flower Buds after Treatment with 2% CNCs
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatment with CNCs
4.2. Observation of 2% CNCs-Treated Excised Shoots Using an IR Camera
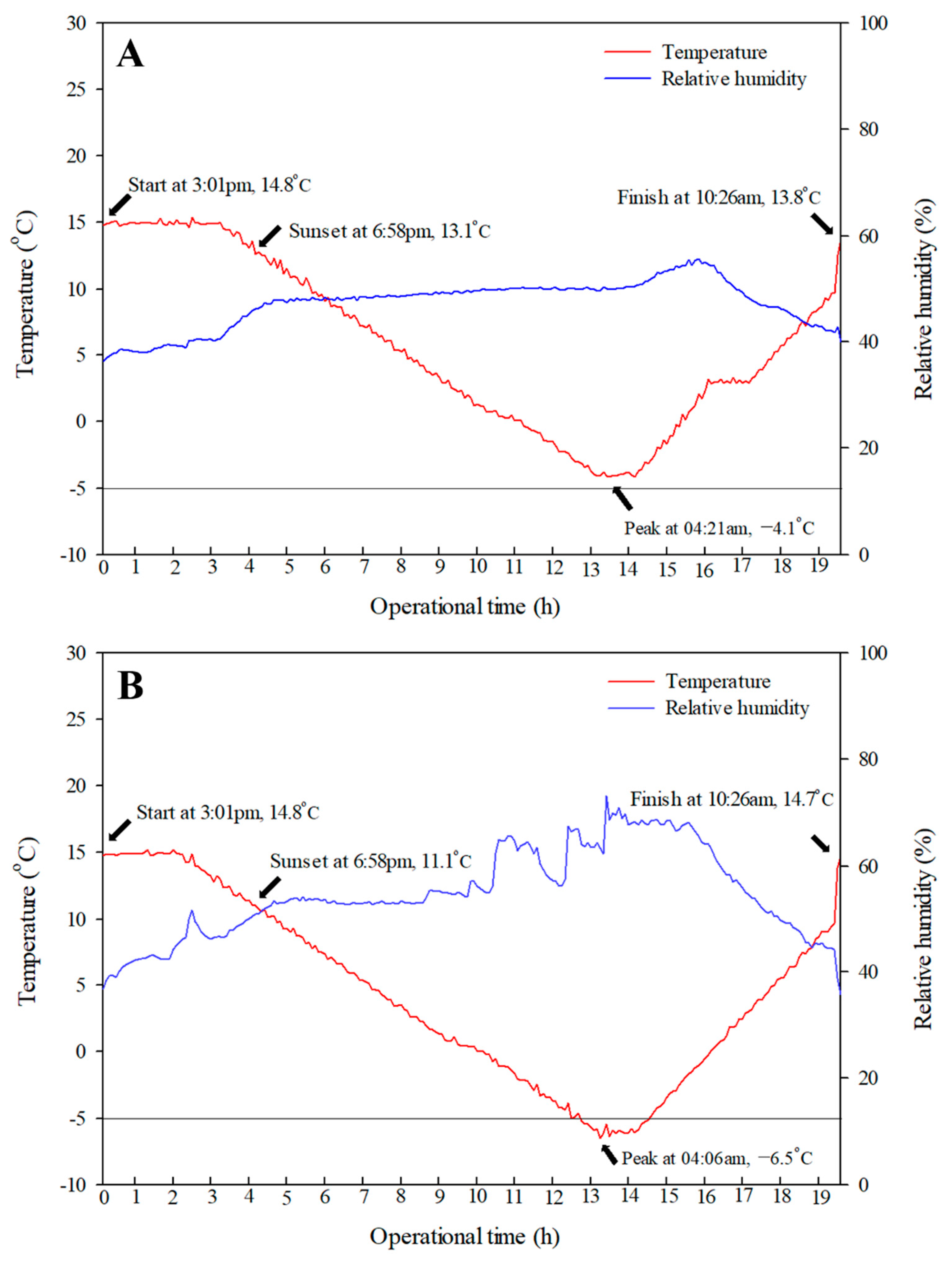
4.3. Simulation of Spring Frost Using SFDS Chambers
4.4. Evaluation of Frost Damage of Peach Flower Buds
4.5. Observation of Frost Events Using an IR Camera
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Wang, L.; Zhu, G.; Fang, W.; Cao, K.; Chen, C.; Wang, X.; Wang, X. Phenological response of peach to climate change exhibits a relatively dramatic trend in China, 1983–2012. Sci. Hortic. 2016, 209, 192–200. [Google Scholar] [CrossRef]
- Martsolf, J.D.; Wiltbank, W.J.; Hannah, H.E.; Johnson, F., Jr.; Fernandez, R.T.; Bucklin, R.A.; Harrison, D.S. Modification of temperature and wind by an orchard cover and heaters for freeze protection. Proc. Fla. State Hort. Soc. 1988, 101, 44–48. [Google Scholar]
- Koc, A.B.; Heinemann, P.H.; Crassweller, R.M.; Morrow, C.T. Automated cycled sprinkler irrigation system for frost protection of apple buds. Appl. Eng. Agric. 2000, 16, 231–240. [Google Scholar] [CrossRef]
- Hu, Y.; Asante, E.A.; Lu, Y.; Mahmood, A.; Buttar, N.A.; Yuan, S. Review of air disturbance technology for plant frost protection. Int. J. Agric. Biol. 2018, 11, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.D. Cold hardiness and options for the freeze protection of southern highbush blueberry. Agriculture 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Román-Figueroa, C.; Bravo, L.; Paneque, M.; Navia, R.; Cea, M. Chemical products for crop protection against freezing stress: A review. J. Agron. Crop Sci. 2021, 207, 391–403. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.; Annamalai, P.K.; Martin, D.J. The use of cellulose nanocrystals to enhance the thermal insulation properties and sustainability of rigid polyurethane foam. Ind. Crop. Prod. 2017, 107, 114–121. [Google Scholar] [CrossRef]
- Alhamid, J.O.; Mo, C.; Zhang, X.; Wang, P.; Whiting, M.D.; Zhang, Q. Cellulose nanocrystals reduce cold damage to reproductive buds in fruit crops. Biosyst. Eng. 2018, 172, 124–133. [Google Scholar] [CrossRef]
- Steponkus, P.L.; Lanphear, F.O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967, 42, 1423–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketchie, D.O.; Kammereck, R. Seasonal variation of cold resistance in Malus woody tissue as determined by differential thermal analysis and viability tests. Can. J. Bot. 1987, 65, 2640–2645. [Google Scholar] [CrossRef]
- Arora, R.; Wisniewski, M.E.; Scorza, R. Cold acclimation in genetically related (sibling) deciduous and evergreen peach (Prunus persica [L.] Batsch): I. Seasonal changes in cold hardiness and polypeptides of bark and xylem tissues. Plant Physiol. 1992, 99, 1562–1568. [Google Scholar] [CrossRef] [Green Version]
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017, 12, 143–157. [Google Scholar] [CrossRef]
- Pearce, R.S.; Fuller, M.P. Freezing of barley studied by infrared video thermography. Plant Physiol. 2001, 125, 227–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, M.J.; Gusta, L.V.; Quamme, H.A.; Weiser, C.J.; Li, P.H. Freezing and injury in plants. Annu. Rev. Plant Physiol. 1976, 27, 507–528. [Google Scholar] [CrossRef]
- Simons, R.K.; Doll, C.C. Morphological and anatomical response of apples to a late spring frost in relation to stage of fruit development [Injuries]. J. Am. Soc. Hortic. Sci. 1976, 101, 315–320. [Google Scholar]
- Chen, C.; Okie, W.R.; Beckman, T.G. Peach fruit set and buttoning after spring frost. Hort. Sci. 2016, 51, 816–821. [Google Scholar] [CrossRef] [Green Version]
- Ceccardi, T.L.; Heath, R.L.; Ting, I.P. Low-temperature exotherm measurement using infrared thermography. Hortic. Sci. 1995, 30, 140–142. [Google Scholar] [CrossRef] [Green Version]
- Wisniewski, M.; Lindow, S.E.; Ashworth, E.N. Observations of ice nucleation and propagation in plants using infrared video thermography. Plant Physiol. 1997, 113, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Takeda, F.; Glenn, D.M. Susceptibility of blackberry flowers to freezing temperatures. Eur. J. Hortic. Sci. 2016, 81, 115–121. [Google Scholar] [CrossRef]
- Livingston, D.P.; Tuong, T.D.; Murphy, J.P.; Gusta, L.V.; Willick, I.; Wisniewski, M.E. High-definition infrared thermography of ice nucleation and propagation in wheat under natural frost conditions and controlled freezing. Planta 2018, 247, 791–806. [Google Scholar] [CrossRef] [Green Version]
- Wisniewski, M.; Glenn, D.M.; Fuller, M.P. Use of a hydrophobic particle film as a barrier to extrinsic ice nucleation in tomato plants. J. Am. Soc. Hortic. Sci. 2002, 127, 358–364. [Google Scholar] [CrossRef] [Green Version]

| Set Temperature ( °C) | Treatment | Phenological Stage | No Damaged (%) | Only Pistil Damaged (%) | Only Stamen Damaged (%) | Pistil + Stamen Damaged (%) |
|---|---|---|---|---|---|---|
| −4 | Control | Calyx red | 43.0 | 39.5 | 17.5 | 0.0 |
| 2% CNCs | 85.9 | 1.3 | 12.8 | 0.0 | ||
| Control | First pink | 1.8 | 28.9 | 38.0 | 31.3 | |
| 2% CNCs | 29.7 | 0.0 | 70.3 | 0.0 | ||
| −6 | Control | Calyx red | 0.0 | 55.5 | 1.8 | 42.7 |
| 2% CNCs | 9.8 | 57.0 | 6.2 | 27.0 | ||
| Control | First pink | 0.0 | 6.0 | 0.0 | 94.0 | |
| 2% CNCs | 1.4 | 22.8 | 2.4 | 73.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Jeong, J.H.; Kim, S.H.; Shin, H. Freezing Tolerance Enhancement and Thermographic Observation of Whole Peach Trees Applied with Cellulose Nanocrystals under Realistic Spring Frost Conditions Using a Soil–Fruit–Daylit–System. Plants 2021, 10, 2301. https://doi.org/10.3390/plants10112301
Lee S, Jeong JH, Kim SH, Shin H. Freezing Tolerance Enhancement and Thermographic Observation of Whole Peach Trees Applied with Cellulose Nanocrystals under Realistic Spring Frost Conditions Using a Soil–Fruit–Daylit–System. Plants. 2021; 10(11):2301. https://doi.org/10.3390/plants10112301
Chicago/Turabian StyleLee, Seongho, Jae Hoon Jeong, Seung Heui Kim, and Hyunsuk Shin. 2021. "Freezing Tolerance Enhancement and Thermographic Observation of Whole Peach Trees Applied with Cellulose Nanocrystals under Realistic Spring Frost Conditions Using a Soil–Fruit–Daylit–System" Plants 10, no. 11: 2301. https://doi.org/10.3390/plants10112301
APA StyleLee, S., Jeong, J. H., Kim, S. H., & Shin, H. (2021). Freezing Tolerance Enhancement and Thermographic Observation of Whole Peach Trees Applied with Cellulose Nanocrystals under Realistic Spring Frost Conditions Using a Soil–Fruit–Daylit–System. Plants, 10(11), 2301. https://doi.org/10.3390/plants10112301








