Metabolome Analysis Revealed the Mechanism of Exogenous Glutathione to Alleviate Cadmium Stress in Maize (Zea mays L.) Seedlings
Abstract
1. Introduction
2. Results
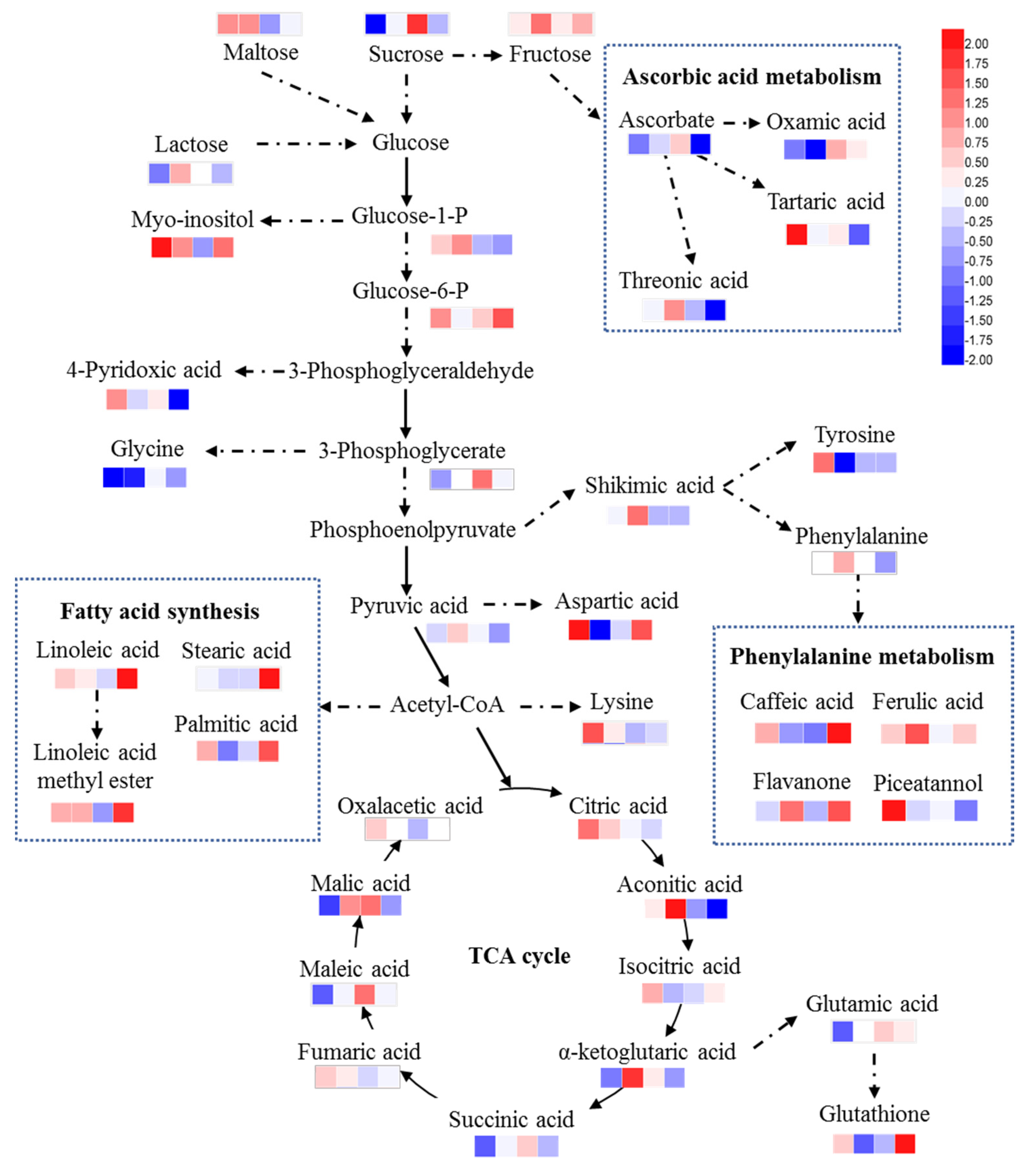
2.1. Metabolite Analysis
2.2. Leaf and Root Metabolite Response to Cd Stress
2.3. Leaf and Root Metabolite Responses to Exogenous GSH Addition
3. Discussion
3.1. GSH Alleviated the Inhibition of Cd Stress on TCA Cycle and Sugar Metabolism in Maize
3.2. GSH Alleviated the Plant Response to Cd Stress with Osmotic Metabolites
3.3. Exogenous GSH Increased the Contents of GSH in Roots
3.4. Exogenous GSH Increased the Contents of Flavonoids Related Metabolites
3.5. Exogenous GSH Decreased the Contents of Ascorbate Related Metabolites
3.6. Exogenous GSH Increased the Contents of Fatty Acids
4. Materials and Methods
4.1. Plant Materials and Plant Growth
4.2. Metabolite Extraction
4.3. Metabolite Derivatization
4.4. GC-MS Analysis
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McBride, M.B. Toxic metals in sewage sludge-amended soils: Has promotion of beneficial use discounted the risks? Adv. Environ. Res. 2003, 8, 5–19. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.M.; Xu, J.M.; Sun, Y.F. Risk assessment for safety of soils and vegetables around a lead/zinc mine. Environ. Geochem. Health 2006, 28, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.A.K.; Kashifuddin, M. Adsorption studies of Cd(II) on ball clay: Comparison with other natural clays. Arab. J. Chem. 2016, 9, S1233–S1241. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Metabolomics in Plant Stress Physiology. Adv. Biochem. Eng. Biol. 2018, 164, 187–236. [Google Scholar]
- Sarry, J.E.; Kuhn, L.; Ducruix, C.; Lafaye, A.; Junot, C.; Hugouvieux, V.; Jourdain, A.; Bastien, O.; Fievet, J.B.; Vailhen, D.; et al. The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics 2006, 6, 2180–2198. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, L.X.; Du, Z.M.; Sun, X.Y.; Amombo, E.; Fan, J.B.; Fu, J.M. Effects of Cadmium Exposure on Growth and Metabolic Profile of Bermudagrass [Cynodon dactylon (L.) Pers.]. PLoS ONE 2014, 9, e115279. [Google Scholar] [CrossRef] [PubMed]
- Durand, T.C.; Sergeant, K.; Planchon, S.; Carpin, S.; Label, P.; Morabito, D.; Hausman, J.F.; Renaut, J. Acute metal stress in Populus tremula x P. alba (717-1B4 genotype): Leaf and cambial proteome changes induced by cadmium(2+). Proteomics 2010, 10, 349–368. [Google Scholar] [CrossRef]
- Villiers, F.; Ducruix, C.; Hugouvieux, V.; Jarno, N.; Ezan, E.; Garin, J.; Junot, C.; Bourguignon, J. Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 2011, 11, 1650–1663. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Gładysz, O.; Szentner, K.; Goliński, P. Role of Glutathione in Abiotic Stress Tolerance. Oxid. Damage Plants Antioxid. Netw. Signal. 2014, 56, 149–181. [Google Scholar]
- Vivancos, P.D.; Wolff, T.; Markovic, J.; Pallardo, F.V.; Foyer, C.H. A nuclear glutathione cycle within the cell cycle. Biochem. J. 2010, 431, 169–178. [Google Scholar]
- Diaz-Vivancos, P.; De Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione—Linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cao, F.; Cheng, W.; Zhang, G.; Wu, F. Modulation of exogenous glutathione in phytochelatins and photosynthetic performance against cd stress in the two rice genotypes differing in Cd tolerance. Biol. Trace Elem. Res. 2011, 143, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Schnaubelt, D.; Schulz, P.; Hannah, M.A.; Yocgo, R.E.; Foyer, C.H. A phenomics approach to the analysis of the influence of glutathione on leaf area and abiotic stress tolerance in Arabidopsis thaliana. Front. Plant Sci. 2013, 4, 416. [Google Scholar] [CrossRef]
- Singla-Pareek, S.L.; Yadav, S.K.; Pareek, A.; Reddy, M.K.; Sopory, S.K. Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol. 2006, 140, 613–623. [Google Scholar] [CrossRef]
- Iannelli, M.A.; Pietrini, F.; Fiore, L.; Petrilli, L.; Massacci, A. Antioxidant response to cadmium in Phragmites australis plants. Plant Physiol. Biochem. 2002, 40, 977–982. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 2010, 16, 259–272. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar]
- Chen, F.; Wang, F.; Wu, F.B.; Mao, W.H.; Zhang, G.P.; Zhou, M.X. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Hossain, M.A.; Fujita, M.; Tran, L.S.P. Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci. Rep. 2015, 5, 11433. [Google Scholar]
- Li, M.; Hao, P.F.; Cao, F.B. Glutathione-induced alleviation of cadmium toxicity in Zea mays. Plant Physiol. Biochem. 2017, 119, 240–249. [Google Scholar] [PubMed]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Phys. C 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar] [PubMed]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernandez-Zapata, J.C.; Nicolas, J.J.M.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, H.; Maskova, P.; Tarkowska, D.; Masek, T.; Lipavska, H. Carbohydrates and gibberellins relationship in potato tuberization. J. Plant Physiol. 2017, 214, 53–63. [Google Scholar] [PubMed]
- Shahjee, H.M.; Banerjee, K.; Ahmad, F. Comparative analysis of naturally occurring L-amino acid osmolytes and their D-isomers on protection of Escherichia coli against environmental stresses. J. Biosci. 2002, 27, 515–520. [Google Scholar]
- Murakeozy, E.P.; Nagy, Z.; Duhaze, C.; Bouchereau, A.; Tuba, Z. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J. Plant Physiol. 2003, 160, 395–401. [Google Scholar]
- Sarraf, N.S.; Saboury, A.A.; Ranjbar, B.; Nemat-Gorgani, M. Effect of some amino acids on the structure and activity of carbonic anhydrase. Asian J. Chem. 2005, 17, 2385–2394. [Google Scholar]
- Sun, X.M.; Zhang, J.X.; Zhang, H.J.; Ni, Y.W.; Zhang, Q.; Chen, J.P.; Guan, Y.F. The responses of Arabidopsis thaliana to cadmium exposure explored via metabolite profiling. Chemosphere 2010, 78, 840–845. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, Z.L.; Zhang, J.W.; Chen, X.J.; Cui, J.X.; Xu, W.; Liu, H.Y. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. Amst. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Mianabadi, M.; Hoshani, M.; Salmanian, S. Antimicrobial and Anti-oxidative Effects of Methanolic Extract of Dorema aucheri Boiss. J. Agric. Sci. Technol. Iran. 2015, 17, 623–634. [Google Scholar]
- Quartacci, M.F.; Argilla, A.; Baker, A.J.M.; Navari-Izzo, F. Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere 2006, 63, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Castagna, A.; Ranieri, A.; Sanita di Toppi, L. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. 2012, 57, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Reig, M.; Jaumot, J.; Pina, B.; Moyano, E.; Galceran, M.T.; Tauler, R. Metabolomic analysis of the effects of cadmium and copper treatment in Oryza sativa L. using untargeted liquid chromatography coupled to high resolution mass spectrometry and all-ion fragmentation. Metallomics 2017, 9, 660–675. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.K.; Wang, Y.B.; Liu, Z.X.; Cheng, H.; Xue, Y. HemI: A Toolkit for Illustrating Heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef]


| Heading | Metabolites | FC (CdL) | FC (CdR) | FC (GSHL) | FC (GSHR) |
|---|---|---|---|---|---|
| Sugars | Fructose 1 | 0.41 | 1.34 | 0.39 | 0.86 |
| Gentiobiose 1 | −0.09 | −0.75 | −0.15 | 1.27 | |
| D-Glucoheptose 1 | −1.74 | 0.23 | 1.16 | 1.75 | |
| Maltose | 1.24 | 1.02 | −0.55 | 0.05 | |
| Sophorose 2 | −1.00 | −0.23 | 1.20 | 0.26 | |
| Sucrose | −2.23 | 0.20 | 1.84 | −0.28 | |
| Turanose 1 | 1.31 | −0.03 | −0.24 | −0.22 | |
| Alcohols | Myo-inositol | 2.37 | 1.19 | −0.53 | 1.27 |
| Sorbitol | 2.38 | - | −3.01 | - | |
| Allo-inositol | −1.04 | - | 1.64 | - | |
| Palatinitol 1 | 1.07 | 0.12 | 0.29 | 0.12 | |
| Amino acids | Aspartic acid 1 | 3.38 | −2.54 | −0.13 | 1.56 |
| Aspartic acid 2 | 1.21 | −1.38 | −0.16 | 1.63 | |
| β-Alanine 2 | 0.74 | −1.03 | −0.66 | −0.23 | |
| Glutamic acid | 0.48 | −1.31 | 0.16 | 0.19 | |
| Glycine 2 | −1.85 | −1.56 | 0.21 | −0.70 | |
| L-Glutamic acid | −1.21 | - | 0.73 | 0.34 | |
| Lysine | 1.67 | 0.40 | −0.46 | −0.12 | |
| Oxamic acid | −0.81 | −1.91 | 0.81 | 0.25 | |
| Tyrosine 1 | 1.40 | −2.20 | −0.28 | −0.36 | |
| Glycolysis | 3-Phosphoglycerate | −0.61 | - | 1.35 | 0.06 |
| Fructose-6-phosphate | −0.04 | 0.40 | 0.38 | −0.41 | |
| D-(glycerol 1-phosphate) | 1.74 | 0.22 | −0.72 | 0.47 | |
| Fructose 2,6-biphosphate degr. prod | 0.34 | 0.63 | −0.27 | 1.71 | |
| Glucose-1-phosphate | 0.51 | 1.02 | −0.44 | −0.54 | |
| Glucose-6-phosphate 1 | 1.13 | 0.22 | 0.66 | 1.72 | |
| TCA cycle | α-Ketoglutaric acid | −0.78 | 1.75 | 0.28 | −0.61 |
| Citric acid | 1.34 | 0.59 | 0.10 | −0.15 | |
| L-Malic acid | −1.49 | 1.14 | 1.35 | −0.52 | |
| Succinic acid | −1.24 | 0.22 | 0.65 | −0.39 | |
| Aconitic acid | 0.31 | 3.69 | −0.61 | −3.30 | |
| Maleic acid | −1.06 | 0.16 | 1.46 | 0.09 | |
| Phenylalanine metabolism | Shikimic acid | 0.24 | 1.32 | −0.43 | −0.26 |
| Caffeic acid | 0.86 | −0.64 | −0.76 | 2.27 | |
| Ferulic acid | 0.66 | 1.68 | 0.05 | 0.60 | |
| Piceatannol 1 | 2.32 | −0.01 | 0.08 | −0.86 | |
| Flavanone 1 | −0.19 | 1.49 | −0.40 | 1.66 | |
| Fatty acids | Linoleic acid | 0.74 | 0.27 | −0.15 | 3.42 |
| Linoleic acid methyl ester | 0.90 | 0.84 | −0.74 | 1.99 | |
| Fatty acids | Palmitic acid | 0.90 | −0.77 | −0.02 | 1.65 |
| Stearic acid | 0.12 | −0.09 | −0.23 | 2.23 | |
| Ascorbate metabolism | Ascorbate | −0.89 | −0.24 | 0.54 | −2.22 |
| Tartaric acid | 2.28 | 0.14 | 0.33 | −1.07 | |
| Threonic acid | 0.21 | 1.00 | −0.41 | −2.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Lin, K.; Chen, H.; Qi, Z.; Liu, B.; Cao, F.; Chen, H.; Wu, F. Metabolome Analysis Revealed the Mechanism of Exogenous Glutathione to Alleviate Cadmium Stress in Maize (Zea mays L.) Seedlings. Plants 2021, 10, 105. https://doi.org/10.3390/plants10010105
Wang R, Lin K, Chen H, Qi Z, Liu B, Cao F, Chen H, Wu F. Metabolome Analysis Revealed the Mechanism of Exogenous Glutathione to Alleviate Cadmium Stress in Maize (Zea mays L.) Seedlings. Plants. 2021; 10(1):105. https://doi.org/10.3390/plants10010105
Chicago/Turabian StyleWang, Runfeng, Kaina Lin, Huabin Chen, Zhenyu Qi, Bohan Liu, Fangbin Cao, Hao Chen, and Feibo Wu. 2021. "Metabolome Analysis Revealed the Mechanism of Exogenous Glutathione to Alleviate Cadmium Stress in Maize (Zea mays L.) Seedlings" Plants 10, no. 1: 105. https://doi.org/10.3390/plants10010105
APA StyleWang, R., Lin, K., Chen, H., Qi, Z., Liu, B., Cao, F., Chen, H., & Wu, F. (2021). Metabolome Analysis Revealed the Mechanism of Exogenous Glutathione to Alleviate Cadmium Stress in Maize (Zea mays L.) Seedlings. Plants, 10(1), 105. https://doi.org/10.3390/plants10010105








