Natural Variation and Domestication Selection of ZmCKX5 with Root Morphological Traits at the Seedling Stage in Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. DNA Isolation, ZmCKX5 Re-Sequencing, and Analysis
2.3. Association Analysis between ZmCKX5 and Root Traits
3. Results
3.1. Nucleotide Diversity of ZmCKX5 in Inbred Lines, Landraces, and Teosintes
3.2. Nucleotide Diversity of ZmCKX5 among Different Populations
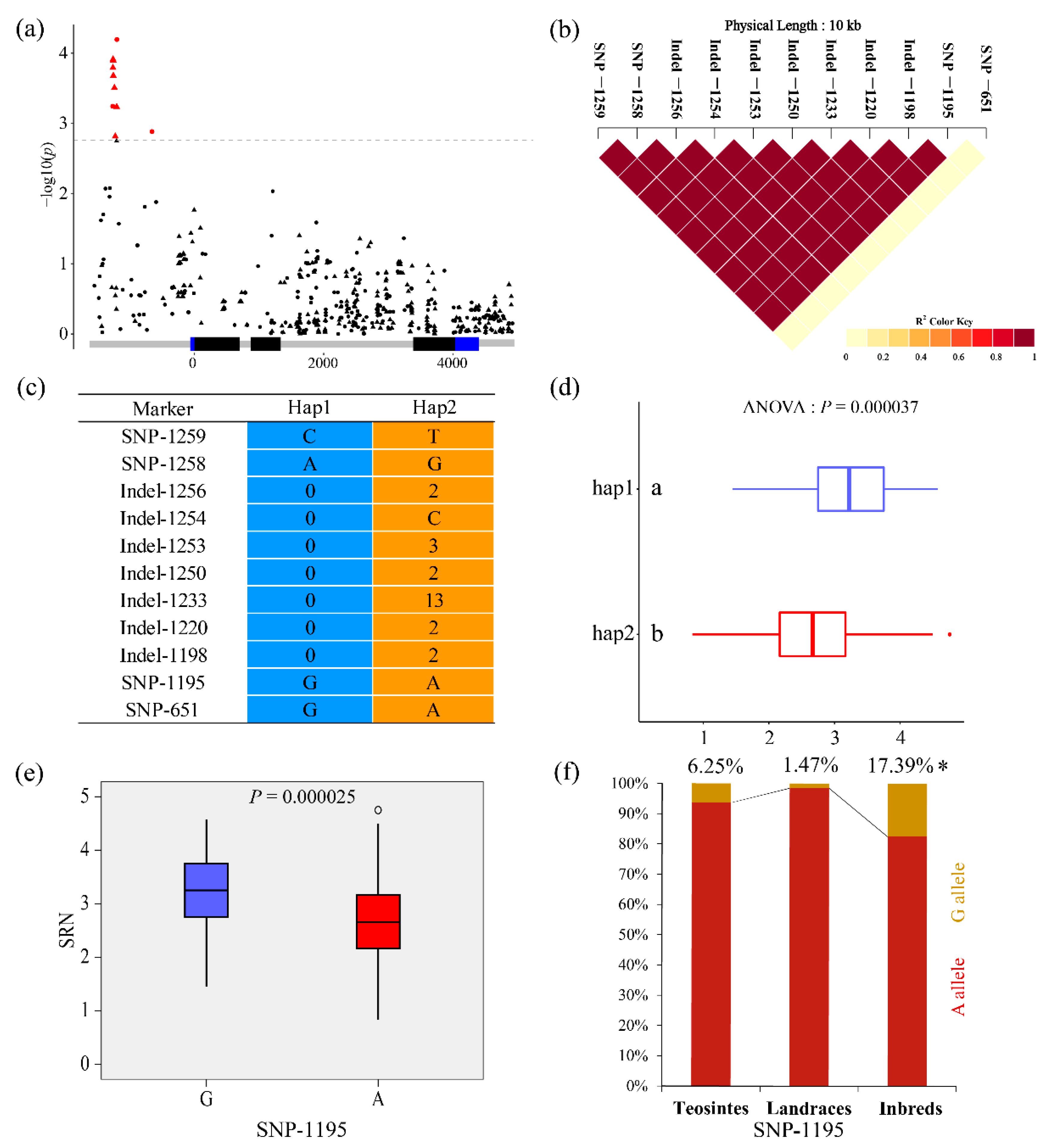
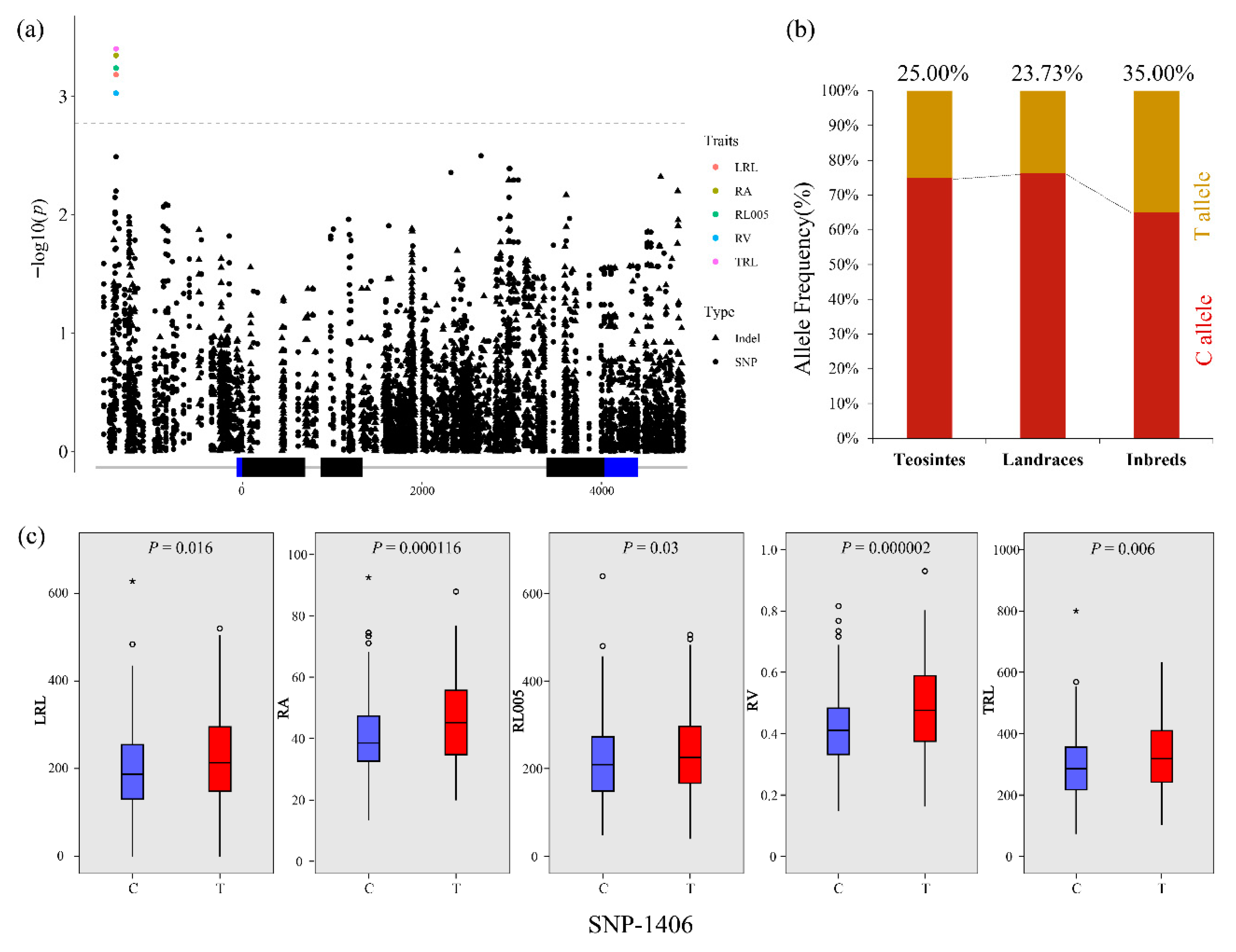
3.3. Association Analysis of Root Traits with ZmCKX5
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Zhang, S.; Siddique, K.H.M.; Chen, Y. Moderate Drought Stress Affected Root Growth and Grain Yield in Old, Modern and Newly Released Cultivars of Winter Wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tollenaar, M.; Lee, E.A. Yield potential, yield stability and stress tolerance in maize. Field Crops Res. 2002, 75, 161–169. [Google Scholar] [CrossRef]
- Den Herder, G.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The roots of a new green revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Hochholdinger, F.; Park, W.J.; Sauer, M.; Woll, K. From weeds to crops: Genetic analysis of root development in cereals. Trends Plant Sci. 2004, 9, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.D.; Giehl, R.F.; Friedel, S.; von Wiren, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Schneider, H.M.; Klein, S.P.; Hanlon, M.T.; Nord, E.A.; Kaeppler, S.; Brown, K.M.; Warry, A.; Bhosale, R.; Lynch, J.P. Genetic control of root architectural plasticity in maize. J. Exp. Bot. 2020, 71, 3185–3197. [Google Scholar] [CrossRef] [Green Version]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Fang, J.; Xu, F.; Wang, W.; Sun, X.; Chu, J.; Cai, B.; Feng, Y.; Chu, C. CYTOKININ OXIDASE/DEHYDROGENASE4 Integrates Cytokinin and Auxin Signaling to Control Rice Crown Root Formation. Plant Physiol. 2014, 165, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Laplaze, L.; Benkova, E.; Casimiro, I.; Maes, L.; Vanneste, S.; Swarup, R.; Weijers, D.; Calvo, V.; Parizot, B.; Herrera-Rodriguez, M.B.; et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 2007, 19, 3889–3900. [Google Scholar] [CrossRef] [Green Version]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmulling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [Green Version]
- To, J.P.; Kieber, J.J. Cytokinin signaling: Two-components and more. Trends Plant Sci. 2008, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Strnad, M.; Schmulling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, T.; Nehnevajova, E.; Kollmer, I.; Novak, O.; Strnad, M.; Kramer, U.; Schmulling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, S.Y.; Chen, H.W.; Ng, C.Y.; Lin, C.Y.; Tseng, T.H.; Li, W.H.; Ku, M.S. Down-Regulation of Cytokinin Oxidase 2 Expression Increases Tiller Number and Improves Rice Yield. Rice 2015, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Zalabak, D.; Johnova, P.; Plihal, O.; Senkova, K.; Samajova, O.; Jiskrova, E.; Novak, O.; Jackson, D.; Mohanty, A.; Galuszka, P. Maize cytokinin dehydrogenase isozymes are localized predominantly to the vacuoles. Plant Physiol. Biochem. 2016, 104, 114–124. [Google Scholar] [CrossRef]
- Zalabak, D.; Galuszka, P.; Mrizova, K.; Podlesakova, K.; Gu, R.; Frebortova, J. Biochemical characterization of the maize cytokinin dehydrogenase family and cytokinin profiling in developing maize plantlets in relation to the expression of cytokinin dehydrogenase genes. Plant Physiol. Biochem. 2014, 74, 283–293. [Google Scholar] [CrossRef]
- Zheng, Z.; Hey, S.; Jubery, T.; Liu, H.; Schnable, P.S. Shared Genetic Control of Root System Architecture between Zea mays and Sorghum bicolor. Plant Physiol. 2019, 182, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Pace, J.; Gardner, C.; Romay, C.; Ganapathysubramanian, B.; Lubberstedt, T. Genome-wide association analysis of seedling root development in maize (Zea mays L.). BMC Genom. 2015, 16, 47. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Abdel-Ghani, A.H.; Pace, J.; Reyes-Matamoros, J.; Hochholdinger, F.; Lubberstedt, T. Association analysis of single nucleotide polymorphisms in candidate genes with root traits in maize (Zea mays L.) seedlings. Plant Sci. 2014, 224, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Floro, E.; Bray, A.L.; Laws, B.; Duncan, K.E.; Topp, C.N. Three-Dimensional Time-Lapse Analysis Reveals Multiscale Relationships in Maize Root Systems with Contrasting Architectures. Plant Cell 2019, 31, 1708–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.C.; Pan, T.; Wang, H.M.; Wei, J.; Chen, M.J.; Hu, X.H.; Zhao, Y.; Yang, X.Y.; Yin, S.Y.; Xu, Y.; et al. Natural variation of ZmHKT1 affects root morphology in maize at the seedling stage. Planta 2019, 249, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.L.; Pouleur, S.; Messier, C.; Guay, R. WinRHlZO, a Root-measuring System with a Unique Overlap Correction Method. Hortence Publ. Am. Soc. Hortic. 1995, 30, 906. [Google Scholar] [CrossRef] [Green Version]
- Allen, G.C.; Flores-Vergara, M.A.; Krasnyanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Scholl, U.I.; Ji, W.; Liu, T.; Tikhonova, I.R.; Zumbo, P.; Nayir, A.; Bakkaloglu, A.; Ozen, S.; Sanjad, S.; et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc. Natl. Acad. Sci. USA 2009, 106, 19096–19101. [Google Scholar] [CrossRef] [Green Version]
- Durbin, L.R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Alzohairy, A.M. BioEdit: An important software for molecular biology. GERF Bull. Bioences 2011, 2, 60–61. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Nei, M.; Miller, J.C. A Simple Method for Estimating Average Number of Nucleotide Substitutions within and between Populations from Restriction Data. Genetics 1990, 125, 873–879. [Google Scholar] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [PubMed]
- Fu, Y.X.; Li, W.H. Statistical tests of neutrality of mutations. Genetics 1993, 133, 693–709. [Google Scholar] [PubMed]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, J.; Song, J.; Jameson, P.E. Cytokinin dehydrogenase: A genetic target for yield improvement in wheat. Plant Biotechnol. J. 2020, 18, 614–630. [Google Scholar] [CrossRef]
- Rei, D.E.; Heckmann, A.B.; Nová, O.; Kelly, S.; Stougaar, J. CYTOKININ OXIDASE/DEHYDROGENASE3 Maintains Cytokinin Homeostasis during Root and Nodule Development inLotus japonicus. Plant Physiol. 2015, 170, 1060–1074. [Google Scholar]
- Khandal, H.; Gupta, S.K.; Dwivedi, V.; Mandal, D.; Sharma, N.K.; Vishwakarma, N.K.; Pal, L.; Choudhary, M.; Francis, A.; Malakar, P.; et al. Root-specific expression of chickpea cytokinin oxidase/dehydrogenase 6 leads to enhanced root growth, drought tolerance and yield without compromising nodulation. Plant Biotechnol. J. 2020, 18, 2225–2240. [Google Scholar] [CrossRef] [Green Version]
- Ramireddy, E.; Hosseini, S.A.; Eggert, K.; Gillandt, S.; Gnad, H.; von Wiren, N.; Schmulling, T. Root Engineering in Barley: Increasing Cytokinin Degradation Produces a Larger Root System, Mineral Enrichment in the Shoot and Improved Drought Tolerance. Plant Physiol. 2018, 177, 1078–1095. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.B.; Warburton, M.; Crouch, J. Association Mapping for Enhancing Maize (Zea mays L.) Genetic Improvement. Crop Sci. 2011, 51, 433–449. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Li, W.; Ku, L.; Wang, C.; Ye, J.; Li, K.; Yang, N.; Li, Y.; Zhong, T.; et al. CACTA-like transposable element in ZmCCT attenuated photoperiod sensitivity and accelerated the postdomestication spread of maize. Proc. Natl. Acad. Sci. USA 2013, 110, 16969–16974. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Liu, Q.; Wang, X.; Huang, C.; Xu, G.; Hey, S.; Lin, H.; Li, C.; Xu, D.; Wu, L. ZmMADS69 functions as a flowering activator through the ZmRap2.7-ZCN8 regulatory module and contributes to maize flowering time adaptation. New Phytol. 2019, 221, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.; Zhao, Q.; Ross-Ibarra, J.; Doebley, J. Identification of a functional transposon insertion in the maize domestication gene tb1. Nat. Genet. 2011, 43, 1160–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pengcheng, L.; Fanjun, C.; Hongguang, C.; Jianchao, L.; Qingchun, P.; Zhigang, L.; Riliang, G.; Guohua, M.; Fusuo, Z.; Lixing, Y. A genetic relationship between nitrogen use efficiency and seedling root traits in maize as revealed by QTL analysis. J. Exp. Bot. 2015, 66, 3175–3188. [Google Scholar]
- Li, P.C.; Fan, Y.Y.; Yin, S.Y.; Wang, Y.Y.; Wang, H.M.; Xu, Y.; Yang, Z.F.; Xu, C.W. Multi-environment QTL mapping of crown root traits in a maize RIL population. Crop J. 2020, 8, 645–654. [Google Scholar] [CrossRef]


| Parameter | Upstream | 5′-UTR | Exon1 | Exon2 | Exon3 | Intron1 | Intron2 | 3′-UTR | Downstream | Entire Region |
|---|---|---|---|---|---|---|---|---|---|---|
| Total length of amplicons(bp) | 1561 | 64 | 700 | 468 | 650 | 169 | 2048 | 369 | 545 | 6574 |
| Number of all of the sequence variants | 17 | 9 | 55 | 42 | 46 | 28 | 233 | 104 | 32 | 559 |
| Frequency of all of the sequence variants | 0.011 | 0.141 | 0.079 | 0.09 | 0.071 | 0.166 | 0.114 | 0.282 | 0.059 | 0.09 |
| Number of nucleotides substitutions (bp) | 10 | 8 | 47 | 35 | 43 | 25 | 180 | 80 | 22 | 446 |
| Frequency of polymorphic sites per bp | 0.006 | 0.125 | 0.067 | 0.075 | 0.066 | 0.148 | 0.088 | 0.217 | 0.04 | 0.07 |
| Number of Indels | 7 | 1 | 8 | 7 | 3 | 3 | 53 | 24 | 10 | 113 |
| Number of Indel sites | 18 | 1 | 54 | 23 | 15 | 10 | 182 | 49 | 34 | 380 |
| Average Indel length Frequency of Indels per bp | 0.004 | 0.016 | 0.011 | 0.015 | 0.005 | 0.018 | 0.026 | 0.065 | 0.018 | 0.020 |
| π × 1000 | 25.13 | 6.26 | 3.75 | 7.77 | 5.2 | 25.57 | 15.23 | 27.7 | 19.09 | 12.4 |
| θ × 1000 | 54.29 | 37.32 | 19.5 | 16.34 | 12.24 | 45.8 | 35.28 | 46.55 | 23.71 | 26.8 |
| Tajima’s D | −1.199 | −1.727 | −2.269 ** | −1.414 | −1.591 | −1.148 | −1.701 | −1.174 | −0.507 | −1.634 |
| Fu and Li’s D | 0.61 | −3.281 ** | −5.988 ** | −4.651 ** | −4.480 ** | −6.687 ** | −7.126 ** | −6.995 ** | −4.292 ** | −8.305 ** |
| Fu and Li’s F | −0.097 | −3.271 ** | −5.114 ** | −3.891 ** | −3.811 ** | −5.243 ** | −5.010 ** | −4.925 ** | −3.246 ** | −5.397 ** |
| Population | Hd | Dens. | C | π × 1000 | θ × 1000 | Tajima‘s D | D | F |
|---|---|---|---|---|---|---|---|---|
| Teosintes | 1.000 | 88 | 0.779 | 28.47 | 160.41 | −1.338 | −1.965 | −1.901 |
| Landraces | 1.000 | 55 | 0.838 | 14.31 | 78.72 | −0.872 | −1.662 | −1.527 |
| Inbreds | 0.965 | 35 | 0.828 | 10.99 | 37.96 | −0.236 | −2.671 * | −1.548 |
| All | 0.974 | 68 | 0.729 | 12.40 | 26.79 | −1.634 | −8.305 ** | −5.397 ** |
| Traits | Marker | Alleles | p-Value | −log10(p) | r2 (%) | Region |
|---|---|---|---|---|---|---|
| TRL | SNP-1406 | T/C | 0.000398 | 3.40 | 4.49 | Upstream |
| SRN | SNP-1259 | T/C | 0.000571 | 3.24 | 4.53 | Upstream |
| SRN | SNP-1258 | G/A | 0.000571 | 3.24 | 4.53 | Upstream |
| SRN | Indel-1256 | AC/-- | 0.000161 | 3.79 | 5.15 | Upstream |
| SRN | Indel-1254 | C/- | 0.000122 | 3.91 | 5.36 | Upstream |
| SRN | Indel-1253 | TCA/--- | 0.000127 | 3.90 | 5.31 | Upstream |
| SRN | Indel-1250 | CC/-- | 0.000209 | 3.68 | 4.96 | Upstream |
| SRN | Indel-1233 | AAGTGTTAGACTT/------------- | 0.000311 | 3.51 | 4.70 | Upstream |
| SRN | Indel-1220 | TT/-- | 0.001530 | 2.82 | 3.81 | Upstream |
| SRN | Indel-1198 | CA/-- | 0.000582 | 3.24 | 4.53 | Upstream |
| SRN | SNP-1195 | A/G | 0.000064 | 4.19 | 6.01 | Upstream |
| SRN | SNP-651 | A/G | 0.001310 | 2.88 | 3.68 | Upstream |
| RV | SNP-1406 | T/C | 0.000942 | 3.03 | 3.90 | Upstream |
| RL005 | SNP-1406 | T/C | 0.000578 | 3.24 | 4.18 | Upstream |
| RA | SNP-1406 | T/C | 0.000452 | 3.34 | 4.43 | Upstream |
| LRL | SNP-1406 | T/C | 0.000656 | 3.18 | 4.18 | Upstream |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Sun, H.; Xia, H.; Wu, T.; Li, P.; Xu, C.; Yang, Z. Natural Variation and Domestication Selection of ZmCKX5 with Root Morphological Traits at the Seedling Stage in Maize. Plants 2021, 10, 1. https://doi.org/10.3390/plants10010001
Wang H, Sun H, Xia H, Wu T, Li P, Xu C, Yang Z. Natural Variation and Domestication Selection of ZmCKX5 with Root Morphological Traits at the Seedling Stage in Maize. Plants. 2021; 10(1):1. https://doi.org/10.3390/plants10010001
Chicago/Turabian StyleWang, Houmiao, Hui Sun, Haofeng Xia, Tingting Wu, Pengcheng Li, Chenwu Xu, and Zefeng Yang. 2021. "Natural Variation and Domestication Selection of ZmCKX5 with Root Morphological Traits at the Seedling Stage in Maize" Plants 10, no. 1: 1. https://doi.org/10.3390/plants10010001
APA StyleWang, H., Sun, H., Xia, H., Wu, T., Li, P., Xu, C., & Yang, Z. (2021). Natural Variation and Domestication Selection of ZmCKX5 with Root Morphological Traits at the Seedling Stage in Maize. Plants, 10(1), 1. https://doi.org/10.3390/plants10010001





