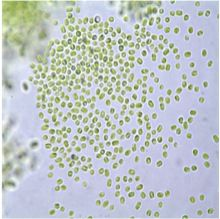
Photobiont Relationships and Phylogenetic History of Dermatocarpon luridum var. luridum and Related Dermatocarpon Species
Abstract
:1. Introduction
2. Results
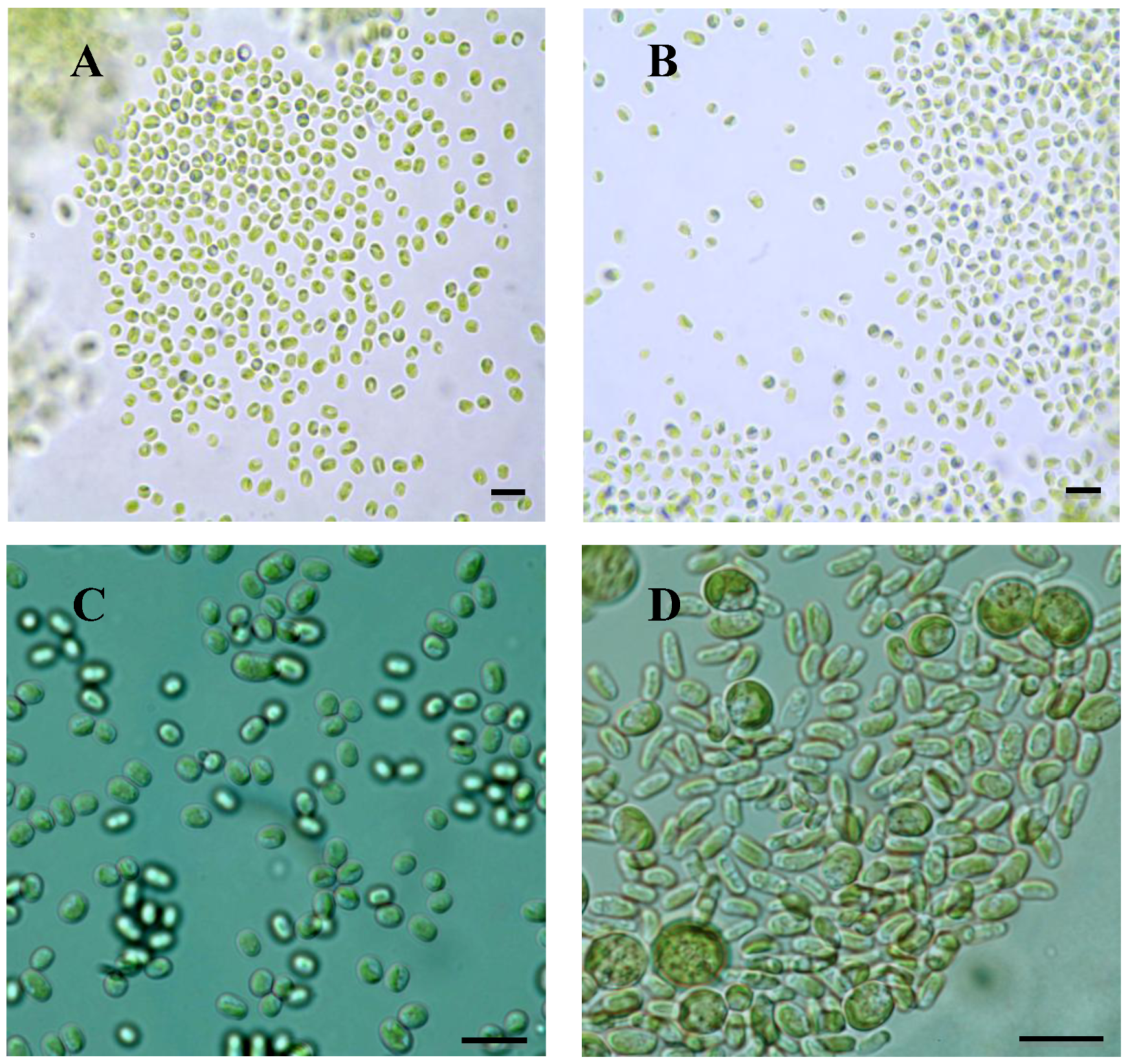
2.1. Microscopy
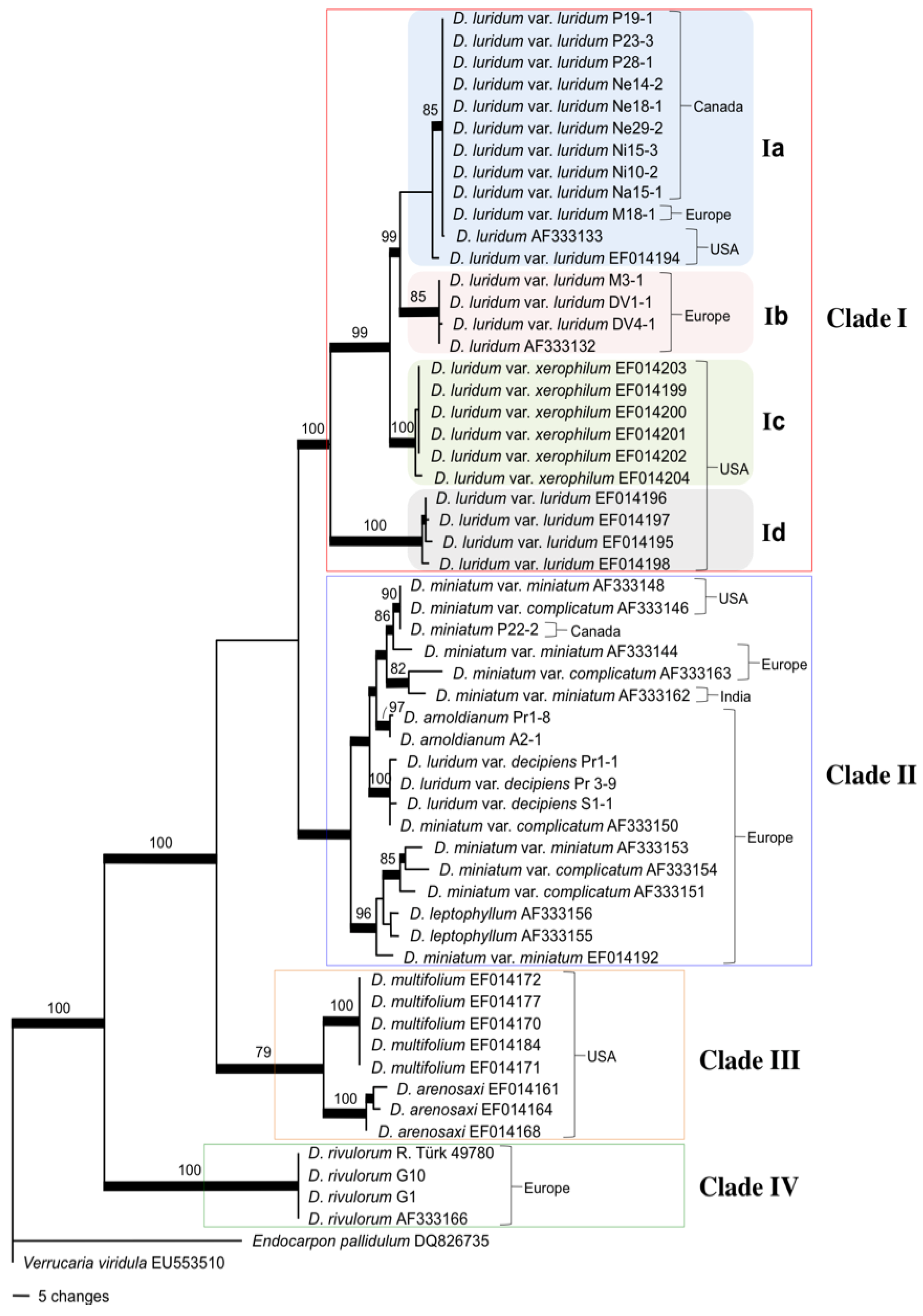
2.2. Mycobiont Internal Transcribed Spacer (ITS) Phylogeny
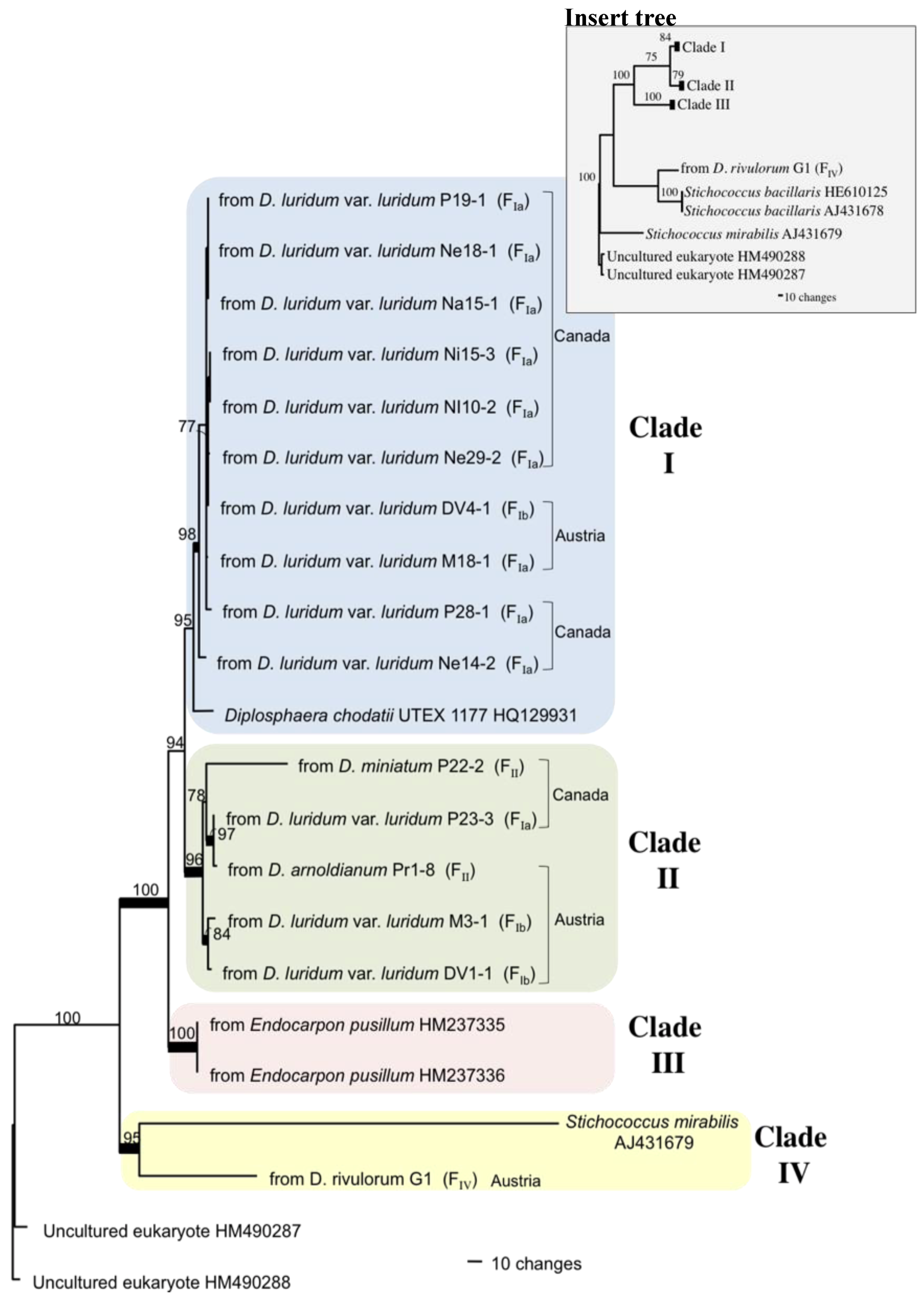
2.3. Photobiont ITS Phylogeny
3. Discussion
3.1. Intercontinental Gene Flow
3.2. Fungal Evolution: D. luridum and D. miniatum Are Paraphyletic
3.3. Algal Identity
3.4. Photobiont Evolution and Algal Selection
4. Experimental Section
4.1. Lichen Material
| Taxon identification | Sample collection number or source | Collection site | Fungal ITS Acc. | Algal ITS Acc. |
|---|---|---|---|---|
| Dermatocarpon arenosaxi | Amtoft et al. [10] | USA, Missouri | EF014161 | NS |
| Dermatocarpon arenosaxi | Amtoft et al. [10] | USA, Missouri | EF014164 | NS |
| Dermatocarpon arenosaxi | Amtoft et al. [10] | USA, Arkansas | EF014168 | NS |
| Dermatocarpon arnoldianum | Fontaine A2-1 | Austria, Salzburg, Preberkessel, 2011; 47°12'47"N; 13°51'10"E | JX645038 | NS |
| Dermatocarpon arnoldianum | Fontaine Pr1-8 | Austria, Salzburg, Preberkessel, 2011; 47°12'37''N; 13°51'05''W | JX645037 | JX645019 |
| Dermatocarpon leptophyllum | Heiđmarsson [12] | Sweden | AF333155 | NS |
| Dermatocarpon leptophyllum | Heiđmarsson [12] | Sweden | AF333156 | NS |
| Dermatocarpon luridum | Heiđmarsson [12] | USA, Minnesota | AF333133 | NS |
| Dermatocarpon luridum | Heiđmarsson [12] | Sweden | AF333132 | NS |
| Dermatocarpon luridum var. decipiens * | Fontaine Pr1-1 | Austria, Salzburg, Preberkessel, 2011; 47°12'37''N; 13°51'05''W | JX645039 | NS |
| Dermatocarpon luridum var. decipiens | Fontaine Pr3-9 | Austria, Salzburg, Preberkessel, 2011; 47°13'16"N; 13°51'13"E | JX645040 | NS |
| Dermatocarpon luridum var. decipiens | Fontaine S1-1 | Austria, Styria, Schladminger Tauern, 2011; 47°16'17''N; 13°43'47"E | JX645041 | NS |
| Dermatocarpon luridum var. luriudm | Amtoft et al. [10] | USA, North Carolina | EF014194 | NS |
| Dermatocarpon luridum var. luridum | Amtoft et al. [10] | USA, Arkansas | EF014195 | NS |
| Dermatocarpon luridum var. luridum | Amtoft et al. [10] | USA, Missouri | EF014196 | NS |
| Dermatocarpon luridum var. luridum | Amtoft et al. [10] | USA, Alabama | EF014197 | NS |
| Dermatocarpon luridum var. luridum | Amtoft et al. [10] | USA, Missouri | EF014198 | NS |
| Dermatocarpon luridum var. luridum | Fontaine P19-1 | Canada, Manitoba, Payuk Lake, 2010; 54°38'31''N; 101°31'40''W | JX645023 | JX645008 |
| Dermatocarpon luridum var. luridum | Fontaine P23-3 | Canada, Manitoba, Payuk Lake, 2010; 54°38'31''N; 101° 31'40''W | JX645024 | JX645018 |
| Dermatocarpon luridum var. luridum * | Fontaine P28-1 | Canada, Manitoba, Payuk Lake, 2010; 54°38'31''N; 101°31'40''W | JX645025 | JX645015 |
| Dermatocarpon luridum var. luridum | Fontaine Ne14-2 | Canada, Manitoba, Neso Lake, 2010; 54°39'51''N; 101°32'44''W | JX645026 | JX645016 |
| Dermatocarpon luridum var. luridum* | Fontaine Ne18-1 | Canada, Manitoba, Neso Lake, 2010; 54°39'51''N; 101°32'44''W | JX645027 | JX645009 |
| Dermatocarpon luridum var. luridum | Fontaine Ne29-2 | Canada, Manitoba, Neso Lake, 2010; 54°39'51''N; 101°32'44''W | JX645028 | JX645013 |
| Dermatocarpon luridum var. luridum * | Fontaine Ni15-3 | Canada, Manitoba, Nisto Lake, 2010; 54°42'02''N; 101°30'17''W | JX645029 | JX645011 |
| Dermatocarpon luridum var. luridum | Fontaine Ni10-2 | Canada, Manitoba, Nisto Lake, 2010; 54°42'02''N; 101°30'17''W | JX645030 | JX645012 |
| Dermatocarpon luridum var. luridum | Fontaine Na15-1 | Canada, Manitoba, Naosap Lake, 2010; 54°50'38''N; 101°26'12''W | JX645031 | JX645010 |
| Dermatocarpon luridum var. luridum * | Fontaine M3-1 | Austria, Waldaist, 2011; 48°19'44''N; 13°52'28''E | JX645033 | JX645020 |
| Dermatocarpon luridum var. luridum * | Fontaine M18-1 | Austria, Waldaist, 2011; 48°23'49''N; 13°35'93''E | JX645032 | JX645045 |
| Dermatocarpon luridum var. luridum | Fontaine DV1-1 | Austria, Schlogener Schlinge, 2011; 48°25'59''N; 13°52'28''E | JX645034 | JX645021 |
| Dermatocarpon luridum var. luridum * | Fontaine DV4-1 | Austria, Schlogener Schlinge, 2011; 48°25'59''N; 13°52'28''E | JX645035 | JX645014 |
| Dermatocarpon luridum var. xerophilum | Amtoft et al. [10] | USA, Missouri | EF014199 | NS |
| Dermatocarpon luridum var. xerophilum | Amtoft et al. [10] | USA, Oklahoma | EF014200 | NS |
| Dermatocarpon luridum var. xerophilum | Amtoft et al. [10] | USA, Arkansas | EF014201 | NS |
| Dermatocarpon luridum var. xerophilum | Amtoft et al. [10] | USA, Arkansas | EF014202 | NS |
| Dermatocarpon luridum var. xerophilum | Amtoft et al. [10] | USA, Arkansas | EF014203 | NS |
| Dermatocarpon luridum var. xerophilum | Amtoft et al. [10] | USA, Arkansas | EF014204 | NS |
| Dermatocarpon miniatum | Fontaine P22-2 | Canada, Manitoba, Payuk Lake, 2010; 54°38'31''N; 101°31'40''W | JX645036 | JX645017 |
| Dermatocarpon miniatum var. complicatum | Heiđmarsson [12] | USA, Minnesota | AF333146 | NS |
| Dermatocarpon miniatum var. complicatum | Heiđmarsson [12] | Sweden | AF333150 | NS |
| Dermatocarpon miniatum var. complicatum | Heiđmarsson [12] | Iceland | AF333151 | NS |
| Dermatocarpon miniatum var. complicatum | Heiđmarsson [12] | Austria | AF333154 | NS |
| Dermatocarpon miniatum var. complicatum | Heiđmarsson [12] | Norway | AF333163 | NS |
| Dermatocarpon miniatum var. miniatum | Heiđmarsson [12] | Sweden | AF333144 | NS |
| Dermatocarpon miniatum var. miniatum | Heiđmarsson [12] | USA, Minnesota | AF333148 | NS |
| Dermatocarpon miniatum var. miniatum | Heiđmarsson [12] | Iceland | AF333153 | NS |
| Dermatocarpon miniatum var. miniatum | Heiđmarsson [12] | India | AF333162 | NS |
| Dermatocarpon miniatum var. miniatum | Amtoft et al. [10] | Wales | EF014192 | NS |
| Dermatocarpon multifolium | Amtoft et al. [10] | USA, Missouri | EF014170 | NS |
| Dermatocarpon multifolium | Amtoft et al. [10] | USA, Missouri | EF014171 | NS |
| Dermatocarpon multifolium | Amtoft et al. [10] | USA, Missouri | EF014172 | NS |
| Dermatocarpon multifolium | Amtoft et al. [10] | USA, Virginia | EF014177 | NS |
| Dermatocarpon multifolium | Amtoft et al. [10] | USA, North Carolina | EF014184 | NS |
| Dermatocarpon rivulorum | Heiđmarsson [12] | Sweden | AF333166 | NS |
| Dermatocarpon rivulorum | Türk 49780 | Austria, Carinthia, Hohe Tauern, 2011; 46°59'18''N; 13°15'28''E | JX645042 | NS |
| Dermatocarpon rivulorum | Fontaine G1 | Austria, Carinthia, Hohe Tauern, 2011; 46°56'13''N; 13°00'25''E | JX645044 | JX645022 |
| Dermatocarpon rivulorum | Fontaine G10 | Austria, Carinthia, Hohe Tauern, 2010; 46°56'13''N; 13°00'25''E | JX645043 | NS |
| Diplosphaera chodatii | Zhang and Wei [37] | China | NS | HM237335 |
| Diplosphaera chodatii | Zhang and Wei [37] | China | NS | HM237336 |
| Diplosphaera chodatii | Zhang and Wei [37] | UTEX culture collection #1177 | NS | HQ129931 |
| Stichococcus bacillaris | Marin [53] | Unknown | NS | HE610125 |
| Stichococcus bacillaris | Unpublished | Unknown | NS | AJ431678 |
| Stichococcus mirabilis | Unpublished | Unknown | NS | AJ431679 |
| Uncultured eukaryote | Khan et al. [54] | Antarctica | NS | HM490287 |
| Uncultured eukaryote | Khan et al. [54] | Antarctica | NS | HM490288 |
4.2. Photobiont Isolation for Culturing
4.3. Microscopic Examination
4.4. DNA Extraction, Amplification, and Purification
4.5. DNA Sequencing
4.6. Data Analysis
5. Conclusions
Acknowledgments
References
- Nash, T.H., III. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 1996; p. 303. [Google Scholar]
- Thüs, H. Taxonomie, Verbreitung ond Ökologie silicoler Süßwasserflechten im außeralpinen Mitteleuropa. Bibliotheca Lichenologica 2002, 83, 1–214. [Google Scholar]
- Nascimbene, J.; Thüs, H.; Marini, L.; Nimis, P.L. Freshwater lichens in springs of the eastern Italian Alps: Floristics, ecology and potential for bioindication. Ann. Limnol. Int. J. Lim. 2007, 43, 285–292. [Google Scholar] [CrossRef]
- Gueidan, C.; Roux, C.; Lutzoni, F. Using a multigene analysis to assess generic delineation and character evolution in Verrucariaceae (Verrucariales, Ascomycota). Mycol. Res. 2007, 111, 1147–1170. [Google Scholar]
- Gueidan, C.; Savić, S.; Thüs, H.; Roux, C.; Keller, C.; Tibell, L.; Prieto, M.; Heiđmarsson, S.; Breuss, O.; Orange, A.; et al. Generic classification of the Verrucariaceae (Ascomycota) based on molecular and morphological evidence: Recent progress and remaining challenges. Taxon 2009, 58, 184–208. [Google Scholar]
- Esslinger, T.L. A cumulative checklist for the lichen-forming, lichenicolous and allied fungi of the continental United States and Canada. North Dakota State University. (First Posted on 1 December 1997, Most Recent Version (#17) on 16 May 2011). Fargo, North Dakota, USA, 2011. Available online: http://www.ndsu.edu/pubweb/~esslinge/chcklst/chcklst7.htm/ (accessed on 11 September 2011).
- Türk, R.; Hafellner, J. Nachtrag zur Bibliographie der Flechten in Österreich. In Biosystematics and Ecology Series Nr. 27; Ehrendorfer, F., Ed.; Österreichische Akademie der Wissenschaften: Wien, Austria, 2010; p. 381. [Google Scholar]
- Santesson, R. Amphibious pyrenolichens I. Arkiv Botanik 1939, 29, 1–67. [Google Scholar]
- Aptroot, A.; Seaward, M.R.D. Freshwater lichens. Fungal Divers. Res. Ser. 2003, 10, 101–110. [Google Scholar]
- Amtoft, A.; Lutzoni, F.; Miadlikowska, J. Dermatocarpon (Verrucariaceae) in the Ozark Highlands, North America. Bryologist 2008, 111, 1–40. [Google Scholar] [CrossRef]
- Heiđmarsson, S. The genus Dermatocarpon (Verrucariales, lichenized Ascomycotina) in the Nordic countries. Nord. J. Bot. 2000, 20, 605–639. [Google Scholar] [CrossRef]
- Heiđmarsson, S. Molecular study of Dermatocarpon miniatum (Verrucariales) and allied taxa. Mycol. Res. 2003, 107, 459–468. [Google Scholar] [CrossRef]
- Beck, A.; Kasalicky, T.; Rambold, G. Myco-photobiontal selection in a Mediterranean cryptogam community with Fulgensia fulgida. New Phytol. 2002, 153, 317–326. [Google Scholar] [CrossRef]
- Yahr, R.; Vilgalys, R.; DePriest, P.T. Geographic variation in algal partners of Cladonia subtenuis (Cladoniaceae) highlights the dynamic nature of a lichen symbiosis. New Phytol. 2006, 171, 847–860. [Google Scholar] [CrossRef]
- Galun, M.; Bubrick, P. Physiological interactions between partners of the lichen symbiosis. In Encyclopedia of Plant Physiology, New Series; Linskens, H.F., Heslop, H.J., Eds.; Springer-Verlag: Berlin, Germany, 1984; Volume 17, pp. 362–401. [Google Scholar]
- Piercey-Normore, M.D. The lichen-forming ascomycete Evernia mesomorpha associates with multiple genotypes of Trebouxia jamesii. New Phytol. 2006, 169, 331–344. [Google Scholar] [CrossRef]
- 17. Casano, L.M.; del Campo, E.M.; García-Breijo, F.J.; Reig-Armiñana, J.; Gasulla, F.; del Hoyo, A.; Guéra, A.; Barreno, E. Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus Competition? Environ. Microbiol. 2011, 13, 806–818. [Google Scholar] [CrossRef]
- Thüs, H.; Muggia, L; Pérez-Ortega, S.; Favero-Longo, S.; Joneson, S.; O’Brien, H.; Nelsen, M.P.; Duque-Thüs, R.; Grube, M.; Friedl, T.; et al. Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota). Eur. J. Phycol. 2011, 46, 399–415. [Google Scholar] [CrossRef]
- Yahr, R.; Vilgalys, R.; DePriest, P.T. Strong fungal specificity and selectivity for algal symbionts in Florida scrub Cladonia lichens. Mol. Ecol. 2004, 13, 3367–3378. [Google Scholar] [CrossRef]
- Beck, A.; Peršoh, D. Flechten und ihre Stellung in Reich der Pilze. In Rundgespräche der Kommission für Ökologie. Ökologische Rolle der Flechten; der Wissenschaften, B.A., Ed.; Verlag Dr. Friedrich Pfeil: München, Germany, 2009; Issue 36, pp. 13–24. [Google Scholar]
- Honegger, R. Lichen-forming fungi and their photobionts. In Plant relationships V, The Mycota; Deising, H.B., Ed.; Springer-Verlag: Berlin, Germany, 2009; pp. 307–333. [Google Scholar]
- Thüs, H.; Schultz, M. Freshwater Flora of Central Europe, Vol. 21/1, Fungi, Part 1: Lichens; Spektrum: Heidelberg, Germany, 2008; p. 229. [Google Scholar]
- Ahmadjian, V.; Heikkilä, H. The culture and synthesis of Endocarpon pusillum and Staurothele clopima. Lichenologist 1970, 4, 259–267. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E.; Türk, R. The Resynthesis of Thalli of Dermatocarpon miniatum under Laboratory Conditions. Symbiosis 1989, 7, 37–50. [Google Scholar]
- Stocker-Wörgötter, E.; Türk, R. Die Resynthese der Flechte Verrucaria macrostoma unter Laborbedingungen. Nova Hedwigia 1987, 44, 55–68. [Google Scholar]
- Stocker-Wörgötter, E. Stress and Developmental Strategies of Lichens. In Symbioses and Stress: Joint ventures in Biology. Cellular Origins, Life in Extreme Habitats and Astrobiology; Seckbach, J., Grube, M., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2010; Version 17; pp. 525–546. [Google Scholar]
- Buschbom, J. Migration between continents: Geographical structure and long-distance gene flow in Porpidia flavicunda (lichen-forming Ascomycota). Mol. Ecol. 2007, 16, 1835–1846. [Google Scholar] [CrossRef]
- Walser, J.C.; Holderegger, R.; Gugerli, F.; Hoebee, S.E.; Scheidegger, C. Microsatellites reveal regional population differentiation and isolation in Lobaria pulmonaria, an epiphytic lichen. Mol. Ecol. 2005, 14, 457–467. [Google Scholar]
- Högberg, N.; Kroken, S.; Thor, G.; Taylor, J.W. Reproductive mode and genetic variation suggest a North American origin of European Letharia vulpina. Mol. Ecol. 2002, 11, 1191–1196. [Google Scholar] [CrossRef]
- Printzen, C.; Ekman, S.; Tønsberg, T. Phylogeography of Cavernularia hultenii: Evidence for slow genetic drift in a widely disjunct lichen. Mol. Ecol. 2003, 12, 1473–1486. [Google Scholar] [CrossRef]
- Fernández-Mendoza, F.; Domaschke, S.; García, M.A.; Jordan, P.; Martín, M.P.; Printzen, C. Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Mol. Ecol. 2011, 20, 1208–1232. [Google Scholar] [CrossRef]
- Degelius, G. Über Dermatocarpon rivulorum (Arn.) DT. et Sarnth. und D. arnoldianum Degel. n. sp. Nytt Mag. Naturvidensk 1934, 75, 151–163. [Google Scholar]
- Begerow, D.; Nilsson, H.; Unterseher, M.; Maier, W. Current state and perspectives of fungal DNA barcoding and rapid identification procedures. Appl. Microbiol. Biot. 2010, 87, 99–108. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar]
- Lindner, D.L.; Banik, M.T. Intragenomic variation in the ITS rDNA region obscures phylogenetic relationships and inflates estimates of operational taxonomic units in genus Laetiporus. Mycologia 2011, 103, 731–740. [Google Scholar] [CrossRef]
- Goward, T.; McCune, B.; Meidinger, D. The lichens of British Columbia, illustrated keys. Part 1, Foliose and Squamulose species. Brtish Columbia Ministry of Forests Special Report Series 1994, 8, 181. [Google Scholar]
- Zhang, T.; Wei, J.C. Survival analysis of symbionts isolated from Endocarpon pusillum Hedwig to desiccation and starvation stress. Sci. China Life Sci. 2011, 54, 480–489. [Google Scholar] [CrossRef]
- Reháková, H. Lisejníkoví rasy z rodu Trebouxia, Diplosphaeraa Myrmecia (Flechtenalgen der Gattungen Trebouxia, Diplosphaera und Myrmecia). Ph.D. Dissertation, Katedra Bot., University of Karlova, Praha, Czech Republic, 1968. [Google Scholar]
- Grube, M.; Muggia, L. Identifying algal symbionts in lichen symbioses. In Tools for Identifying Biodiversity: Progress and Problems; Nimis, P.L., Lebbe, R.V., Eds.; Edizioni Universitàdi di Trieste: Trieste, Italy, 2010; pp. 295–299. [Google Scholar]
- Ohmura, Y.; Kawachi, M.; Kasaie, F.; Watanabe, M.M.; Takeshita, S. Genetic combinations of symbionts in a vegetatively reproducing lichen, Parmotrema tinctorum, based on ITS rDNA sequences. Bryologist 2006, 109, 43–59. [Google Scholar] [CrossRef]
- Guzow-Krzeminska, B. Photobiont flexibility in the lichen Protoparmeliopsis muralis as revealed by ITS rDNA analyses. Lichenologist 2006, 38, 469–476. [Google Scholar] [CrossRef]
- Bates, S.T.; Berg-Lyons, D.; Lauber, C.L.; Walters, W.A.; Knight, R.; Fierer, N. A preliminary survey of lichen associated eukaryotes using pyrosequencing. Lichenologist 2012, 44, 137–146. [Google Scholar] [CrossRef]
- Beck, A.; Friedl, T.; Rambold, G. Selectivity of photobiont choice in a defined lichen community: Inferences from cultural and molecular studies. New Phytol. 1998, 139, 709–720. [Google Scholar]
- Lukešová, A.; Hoffmann, L. Soil algae from acid rain impacted forest areas of the Krušné hory Mts. 1. Algal communities. Vegetatio 1996, 125, 123–136. [Google Scholar] [CrossRef]
- Flechtner, V.R. Enigmatic desert soil algae. In Enigmatic Microorganisms and Life in Extreme Environments; Seckbach, J., Ed.; Kluwer Academic Publishers: Dordecht, The Netherlands, 1998; pp. 233–241. [Google Scholar]
- Handa, S.; Nakahara, M.; Nakano, T.; Itskovich, V.B.; Masuda, Y. Aerial algae from southwestern area of Lake Baikal. Hikobia 2001, 13, 463–472. [Google Scholar]
- Flechtner, V.R.; Johansen, J.R.; Belnap, J. The biological soil crusts of the San Nicolas Island: Enigmatic algae from a geographically isolated ecosystem. West. N. Am. Nat. 2008, 68, 405–436. [Google Scholar] [CrossRef]
- Hill, D.J. Asymmetric co-evolution in the lichen symbiosis caused by a limited capacity for adaptation in the photobiont. Bot. Rev. 2009, 75, 326–338. [Google Scholar] [CrossRef]
- Voytsekhovich, A.; Dymytrova, L.; Nadyeina, O. Photobiont composition of some taxa of the genera Micarea and Placynthiella (Lecanoromycetes, lichenized Ascomycota) from Ukraine. Folia Cryptog. Estonica Fasc. 2011, 48, 135–148. [Google Scholar]
- Gilbert, O.; Giavarini, V. The lichen vegetation of lake margins in Britain. Lichenologist 2000, 32, 365–386. [Google Scholar] [CrossRef]
- Ettl, H.; Gärtner. Syllabus der Boden-, Luft- und Flechtenalgen; Gustav Fischer Verlag: Stuttgart, Germany, 1995; p. 722. [Google Scholar]
- Bergsten, J. A review of long-branch attraction. Cladistics 2005, 21, 163–193. [Google Scholar] [CrossRef]
- Marin, B. Nested in the Chlorellales or independent class? Phylogeny and classificationof the Pedinophyceae (Viridiplantae) revealed by molecular phylogenetic analyses of complete nuclear and plastid-encoded rRNA operons. Protist 2012, 163, 778–805. [Google Scholar] [CrossRef]
- Khan, N.; Tuffin, I.M.; Carey, S.C.; Cowan, D.A. Hypolithic microbial communities of quartz rocks from Miers Valley, McMurdo Dry Valleys, Antarctica. Polar Biol. 2011, 34, 1657–1668. [Google Scholar] [CrossRef]
- Yamamoto, Y. Studies of cell aggregates and the production of natural pigments in plant cell culture. Ph.D. Dissertation, Kyoto University, Kyoto, Japan, 1990. [Google Scholar]
- Stocker-Wörgötter, E. Resynthesis of Photosymbiodemes. In Protocols in Lichenology: Culturing, Biochemistry, Ecophysiology and Use in Biomonitoring; Springer Lab Manual; Kramer, I., Beckett, R.P., Varma, A.K., Eds.; Springer-Verlag: Berlin, Germany, 2002; pp. 47–60. [Google Scholar]
- Stocker-Wörgötter, E.; Hager, A. Culture methods for lichens and lichen symbionts. In Lichen Biology; Nash, T.H., III, Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 353–363. [Google Scholar]
- Nichols, H.W. Growth media, Freshwater. In Handbook of Phycological Methods, Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: Cambridge, UK, 1973; pp. 8–23. [Google Scholar]
- Grube, M.; DePriest, P.T.; Gargas, A.; Hafellner, J. DNA isolation from lichen ascomata. Mycol. Res. 1995, 99, 1321–1324. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- O’Rourke, J.A.; Nelson, R.T.; Grant, D.; Schmutz, J.; Grimwood, J.; Cannon, S.; Vance, C.P.; Graham, M.A.; Shoemaker, R.C. Integrating microarray analysis and the soybean genome to understand the soybeans iron deficiency response. BMC Genomics 2009, 10, 376. [Google Scholar] [CrossRef]
- Rambaut, A. Se-Al (Sequence Alignment Editor Version 1); Department of Zoology, University of Oxford: Oxford, UK, 2001. [Google Scholar]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4; Sinauer Associates: Sunderland, MA, USA, 2003. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F.; Nielsen, R.; Bollback, J.P. Bayesian inference of phylogeny and its impact on evolutionary biology. Science 2001, 294, 2310–2314. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fontaine, K.M.; Beck, A.; Stocker-Wörgötter, E.; Piercey-Normore, M.D. Photobiont Relationships and Phylogenetic History of Dermatocarpon luridum var. luridum and Related Dermatocarpon Species. Plants 2012, 1, 39-60. https://doi.org/10.3390/plants1020039
Fontaine KM, Beck A, Stocker-Wörgötter E, Piercey-Normore MD. Photobiont Relationships and Phylogenetic History of Dermatocarpon luridum var. luridum and Related Dermatocarpon Species. Plants. 2012; 1(2):39-60. https://doi.org/10.3390/plants1020039
Chicago/Turabian StyleFontaine, Kyle M., Andreas Beck, Elfie Stocker-Wörgötter, and Michele D. Piercey-Normore. 2012. "Photobiont Relationships and Phylogenetic History of Dermatocarpon luridum var. luridum and Related Dermatocarpon Species" Plants 1, no. 2: 39-60. https://doi.org/10.3390/plants1020039
APA StyleFontaine, K. M., Beck, A., Stocker-Wörgötter, E., & Piercey-Normore, M. D. (2012). Photobiont Relationships and Phylogenetic History of Dermatocarpon luridum var. luridum and Related Dermatocarpon Species. Plants, 1(2), 39-60. https://doi.org/10.3390/plants1020039